Key points
Increased appetite and weight gain occurs during pregnancy, associated with development of leptin resistance, and satiety responses to the anorectic peptide α‐melanocyte stimulating hormone (α‐MSH) are suppressed.
This study investigated hypothalamic responses to α‐MSH during pregnancy, using c‐fos expression in specific hypothalamic nuclei as a marker of neuronal signalling, and in vivo electrophysiology in supraoptic nucleus (SON) oxytocin neurons, as a representative α‐MSH‐responsive neuronal population that shows a well‐characterised α‐MSH‐induced inhibition of firing.
While icv injection of α‐MSH significantly increased the number of c‐fos‐positive cells in the paraventricular, supraoptic, arcuate and ventromedial hypothalamic nuclei in non‐pregnant rats, this response was suppressed in pregnant rats. Similarly, SON oxytocin neurons in pregnant rats did not demonstrate characteristic α‐MSH‐induced inhibition of firing that was observed in non‐pregnant animals.
Given the known functions of α‐MSH in the hypothalamus, the attenuated responses are likely to facilitate adaptive changes in appetite regulation and oxytocin secretion during pregnancy.
Abstract
During pregnancy, a state of positive energy balance develops to support the growing fetus and to deposit fat in preparation for the subsequent metabolic demands of lactation. As part of this maternal adaptation, the satiety response to the anorectic peptide α‐melanocyte stimulating hormone (α‐MSH) is suppressed. To investigate whether pregnancy is associated with changes in the response of hypothalamic α‐MSH target neurons, non‐pregnant and pregnant rats were treated with α‐MSH or vehicle and c‐fos expression in hypothalamic nuclei was then examined. Furthermore, the firing rate of supraoptic nucleus (SON) oxytocin neurons, a known α‐MSH responsive neuronal population, was examined in non‐pregnant and pregnant rats following α‐MSH treatment. Intracerebroventricular injection of α‐MSH significantly increased the number of c‐fos‐positive cells in the paraventricular, arcuate and ventromedial hypothalamic nuclei in non‐pregnant rats, but no significant increase was observed in any of these regions in pregnant rats. In the SON, α‐MSH did induce expression of c‐fos during pregnancy, but this was significantly reduced compared to that observed in the non‐pregnant group. Furthermore, during pregnancy, SON oxytocin neurons did not demonstrate the characteristic α‐MSH‐induced inhibition of firing rate that was observed in non‐pregnant animals. Melanocortin receptor mRNA levels during pregnancy were similar to non‐pregnant animals, suggesting that receptor down‐regulation is unlikely to be a mechanism underlying the attenuated responses to α‐MSH during pregnancy. Given the known functions of α‐MSH in the hypothalamus, the attenuated responses will facilitate adaptive changes in appetite regulation and oxytocin secretion during pregnancy.
Key points
Increased appetite and weight gain occurs during pregnancy, associated with development of leptin resistance, and satiety responses to the anorectic peptide α‐melanocyte stimulating hormone (α‐MSH) are suppressed.
This study investigated hypothalamic responses to α‐MSH during pregnancy, using c‐fos expression in specific hypothalamic nuclei as a marker of neuronal signalling, and in vivo electrophysiology in supraoptic nucleus (SON) oxytocin neurons, as a representative α‐MSH‐responsive neuronal population that shows a well‐characterised α‐MSH‐induced inhibition of firing.
While icv injection of α‐MSH significantly increased the number of c‐fos‐positive cells in the paraventricular, supraoptic, arcuate and ventromedial hypothalamic nuclei in non‐pregnant rats, this response was suppressed in pregnant rats. Similarly, SON oxytocin neurons in pregnant rats did not demonstrate characteristic α‐MSH‐induced inhibition of firing that was observed in non‐pregnant animals.
Given the known functions of α‐MSH in the hypothalamus, the attenuated responses are likely to facilitate adaptive changes in appetite regulation and oxytocin secretion during pregnancy.
Abbreviations
- AgRP
agouti‐related peptide
- α‐MSH
α‐melanocyte stimulating hormone
- CCK
cholecystokinin
- MC3‐R
melanocortin 3 receptor
- MC4‐R
melanocortin 4 receptor
- MRAP
melanocortin receptor accessory protein
- POMC
pro‐opiomelanocortin
- PVN
paraventricular nucleus of the hypothalamus
- SON
supraoptic nucleus
- VMH
ventromedial nucleus of the hypothalamus
Introduction
During pregnancy, it is physiologically appropriate to gain weight. This allows the mother to supply the energy required for the growth and development of the fetus, as well as preparing adequate energy reserves for the subsequent, metabolically demanding period of lactation. In non‐pregnant adults, body weight is maintained at a relatively stable level due to complex interactions between short‐ and long‐term regulators of energy balance. The adipocyte‐derived hormone leptin plays a vital role in this system, providing a long‐term negative feedback signal to the hypothalamus to suppress appetite and increase metabolic rate, thus maintaining relatively stable body fat levels. Despite elevated leptin concentrations, hyperphagia is maintained throughout pregnancy (Ladyman & Grattan, 2004). In addition, central administration of exogenous leptin, which significantly decreases food intake in non‐pregnant rats, fails to do so in day 14 pregnant rats (Ladyman & Grattan, 2004). This indicates that pregnancy is a physiological state of leptin resistance, allowing a period of positive energy balance and weight gain. Pregnant rats also become insensitive to the acute satiety effects of cholecystokinin (CCK), a short‐term regulator of appetite (Ladyman et al. 2011), indicating that both long‐ and short‐term regulation of appetite are modulated during pregnancy to facilitate a state of positive energy balance.
The melanocortin system is a key downstream pathway mediating the actions of both leptin (Balthasar et al. 2005) and CCK (Fan et al. 2004; Blevins et al. 2009), and involves activation of pro‐opiomelanocortin (POMC) neurons in the arcuate nucleus. Alpha‐melanocyte stimulating hormone (α‐MSH) is a derivative of the POMC precursor protein and, once released in response to leptin or CCK, acts on melanocortin 3 (MC3‐R) and melanocortin 4 receptors (MC4‐R) in the hypothalamus (Mountjoy, 2010) to inhibit food intake and increase energy expenditure. Melanocortin receptors are also targeted by an endogenous antagonist, agouti‐related peptide (AgRP), which is produced in neuropeptide‐Y (NPY) neurons of the arcuate nucleus. AgRP promotes food intake, and AgRP mRNA is upregulated in response to fasting (Mizuno & Mobbs, 1999; Korner et al. 2001). Using c‐fos as a neuronal marker to assess cellular responses, the paraventricular nucleus of the hypothalamus (PVN) has been identified as a key site of action of α‐MSH (Thiele et al. 1998; McMinn et al. 2000). The PVN, in particular, has a high density of MC4‐R and receives neuronal inputs from POMC and AgRP neurons located in the arcuate nucleus (Bagnol et al. 1999; Cowley et al. 1999). Indeed, MC4‐Rs within the PVN are critical for MC4‐R‐mediated regulation of appetite (Balthasar et al. 2005; Garza et al. 2008). Within the hypothalamus, MC3‐R‐expressing neurons are found in nuclei associated with energy homeostasis, including the arcuate nucleus and the ventromedial nucleus of the hypothalamus (VMH) (Roselli‐Rehfuss et al. 1993), although the exact nature of the role of MC3‐R in energy homeostasis remains to be determined (Butler et al. 2000; Begriche et al. 2013).
While leptin resistance induced by a high fat diet can be bypassed by administration of the downstream regulator α‐MSH (Pierroz et al. 2002; Hansen et al. 2005), we have shown that pregnant rats do not show a satiety response to α‐MSH (Ladyman et al. 2009). Given the critical role of the melanocortin system in the anorectic response to leptin (Marsh et al. 1999), this suggests that the downstream impairment of the melanocortin response may underlie pregnancy‐induced leptin resistance. Therefore, the aim of the current study was to examine hypothalamic responses to α‐MSH during pregnancy to determine if the lack of satiety response to α‐MSH during pregnancy is associated with an impaired activation of hypothalamic cells by α‐MSH, using two independent markers of cellular response: (1) α‐MSH‐induced expression of c‐fos in key hypothalamic nuclei (McMinn et al. 2000), and (2) the well‐characterised inhibitory action of α‐MSH on the firing rate of oxytocin neurons in vivo (Sabatier et al. 2003). To address the possible mechanism behind a loss of response to α‐MSH, the expression of mRNA for melanocortin receptors and levels of the endogenous antagonist AgRP were examined during pregnancy.
Methods
Animals
Ten‐week‐old female Sprague Dawley rats were obtained from our colony at the University of Otago and housed under a 14:10 h light–dark cycle (lights on at 05.00 h) for anatomical studies or a 12:12 h light–dark cycle (lights on at 07.00 h) for electrophysiology studies. Temperature was maintained at 21 ± 1°C. All animals had free access to food and water, except during fasting when only water was available. The oestrous cycle was monitored by daily cytological examination of vaginal smears. To generate timed pregnancies, pro‐oestrous females were housed overnight with a male rat and impregnation was confirmed by the presence of sperm in the vaginal smear the following morning (day 0 of pregnancy). All experimental protocols were approved by the University of Otago Animal Ethics Committee.
α‐MSH induced c‐fos in the hypothalamus during pregnancy
To assess the c‐fos response of hypothalamic nuclei to intracerebroventricular (icv) administration of α‐MSH, non‐pregnant and pregnant animals (day 6 or 7 of pregnancy) underwent stereotaxic surgery to implant guide cannula (Plastics One, Roanoke, VA, USA) into the right lateral ventricle as previously described (Ladyman & Grattan, 2004). After surgery, rats were housed individually and food intake, water intake and weight gain were monitored daily for approximately 1 week. To determine correct cannula placement, rats were given a 2 μl icv injection of angiotensin‐II (25 ng μl−1; Bachem, Torrance, CA, USA). Cannula placement was considered correct if rats consumed more than 5 ml of water in the 30 min after injection.
On day 13 of pregnancy or metoestrus for the non‐pregnant rats, food was removed from the cages 1 h prior to the start of the dark phase (5–6 per group). The following morning, between 09.00 and 11.00 h, rats received a 2 μl injection of either 10 μg α‐MSH (Bacham), or saline into the right lateral ventricle. Ninety minutes after icv injection, animals were anaesthetised with sodium pentobarbitone (17 mg/100 g body weight for pregnant rats or 6 mg/100 g for dioestrous rats, ip injection) and transcardially perfused with heparinised saline, followed by 4% paraformaldehyde in 0.1 m PBS. Brains were removed and post‐fixed in the same fixative overnight at room temperature. Brains were then immersed in 30% sucrose in 0.1 m PBS until they sank, then rapidly frozen and stored until further processing. Serial coronal sections (30 μm) from the hypothalamus were cut on a cryostat then processed for immunohistochemistry using a c‐fos‐specific antibody (1:10,000, Calbiochem, San Diego, CA, USA) as previously described (Ladyman et al. 2011).
In vivo electrophysiology
On the day of electrophysiology, rats were anaesthetised by intraperitoneal injection of urethane (ethyl carbamate; 1.25 g kg−1). The pituitary stalk and the right supraoptic nucleus (SON) were exposed by the transpharyngeal approach. Following removal of the meninges, a U‐shaped microdialysis probe (in‐house design, supplied by Prof. M. Ludwig, University of Edinburgh; total membrane length 2.0 mm, permeable to 10 kDa; Spectra/Por RC Hollow Fibres, Spectrum Medical Inc., Houston, TX, USA) was bent (between 100 and 200 deg) and the loop of the membrane was positioned to lie flat over the exposed ventral surface of the brain over the SON. A glass recording pipette (15–40 MΩ, filled with 0.9% NaCl) was lowered into the SON through the centre of the microdialysis loop. A side‐by‐side SNEX‐200 bipolar stimulating electrode (Science Products GmbH, Hofheim, Germany) was placed on the pituitary stalk to elicit antidromic action potentials in SON neurons. The SON was continuously dialysed with artificial cerebrospinal fluid (composition in mm: NaCl 138, KCl 3.36, NaHCO3 9.52, Na2HPO4 0.49, urea 2.16, CaCl2 1.26, MgCl2 1.18) at 3 μl min−1 and the dialysate was changed to include α‐MSH [2.5 μg μl−1 (1.5 mm) for 30–60 min] during recording. At the end of experiments, rats were killed by an overdose of intravenous urethane.
SON neuronal activity was recorded onto a personal computer using Spike2 software (Cambridge Electronic Design, Cambridge, UK) and analysed off‐line. Neurons were characterised as oxytocin neurons on the basis of a transient excitation following intravenous CCK injection (20 μg kg−1, 0.5 ml kg−1 in 0.9% saline; Sigma) (Brown et al. 1996).
Melanocortin receptor expression in the hypothalamus during pregnancy
The hypothalamic location of MC3‐R mRNA and MC4‐R mRNA expression was determined by in situ hybridization. Female rats were anaesthetised with sodium pentobarbital and transcardially perfused with 2% paraformaldhyde in 0.1 m PBS. Brains were post‐fixed in the same fixative overnight at room temperature then transferred to 30% sucrose in 0.1 m PBS until they sank (<5 days) and stored at −80°C until coronal sections (16 μm) were collected through the hypothalamus. Antisense and sense riboprobes complementary to fragments of human MC4‐R were transcribed from a cloned cDNA template (donated by Julian G. Mercer, Rowett Research Institute, Aberdeen). MC3‐R riboprobes were synthesized from RT‐PCR products generated using an oligonucleotide primer set (F: TCTTCCTGTGCAGCCTGGCTA, R: TGCTGTGGTAACGGAGGGCA, gene accession number NM_001025270.3) that contained SP6 (forward) and T7 (reverse) RNA polymerase promoter sequences ligated to the 5′ ends.
In situ hybridization for MC4‐R was carried out as previously described (Ladyman et al. 2009). Briefly, slides were fixed with 4% paraformaldehyde in 0.1 m phosphate buffer and acetylated as described. Sections were then hybridized overnight at 58°C using [35S] UTP radioactive riboprobes for MC4‐R mRNA (1.2×107 c.p.m. per section). Sections were then treated with RNase A, desalted and given a final high stringency wash in 0.1× SSC. Following dehydration and drying, sections were exposed to Kodak BioMas MR film (Radiographic Supplies Ltd, Christchurch, New Zealand) for 3 weeks to generate autoradiographs.
For MC3‐R mRNA, in situ hybridization using [35S] UTP radioactive riboprobes was carried out as previously described (Phillipps et al. 2013). Briefly, slides were fixed with 2% paraformaldehyde in 0.1 m phosphate buffer, treated with proteinase K (2 μg ml−1, Roche, Manneheim, Germany), and acetylated by incubation in 0.25% (v/v) acetic anhydride in TEA buffer. Sections were dehydrated, immersed in chloroform and then rehydrated. Sections were then hybridized overnight at 55°C using [35S] UTP radioactive riboprobes for MC3‐R mRNA (1.2×106 c.p.m. per section). Slides were treated with RNase A (Sigma‐Aldrich, St Louis, MO, USA), and desalted, then given a final high stringency wash in 0.1× SSC (containing 10 mm β‐mercaptoethanol and 1 mm EDTA). Following dehydration and drying, sections were exposed to Kodak BioMas MR film (Radiographic Supplies Ltd) for 2 weeks to generate autoradiographs.
To quantify levels of MC3‐R and MC4‐R mRNA, brains from dioestrous, non‐pregnant (n = 6) and day 14 pregnant rats (n = 6) underwent laser capture microdissection and quantitative RT‐PCR (qPCR) as previously described (Phillipps et al. 2013). Based on the in situ data, the PVN was selected for analysis of MC4‐R, while the VMH was selected to assess MC3‐R. Coronal cryosections (10 μm thickn) were taken at 20 and 40 μm intervals through the PVN and VMH, respectively. Slides were placed upside down in a Leica laser capture microdissector (LMD) (Leica, Wetzlar, Germany) and the entire PVN or VMH isolated from appropriate sections within 1 h. Cells were collected into lysis buffer (with 1%, v/v, β‐mercaptoethanol) (Qiagen RNeasy Micro kit, Valencia, CA, USA) and total RNA extracted with an on column DNase digestion step according to the manufacturer's instructions for the Qiagen RNeasy Micro kit. RNA quality and quantity was determined using an Agilent Bioanalyser (Agilent Technologies, Inc., Waldbronn, Germany). Only RNA samples with distinct 18S and 28S ribosomal bands and RNA integrity number (RIN) values >6.00 were used for qPCR analysis.
Total RNA (1–4 ng) was reversed transcribed using the Superscript III First Strand Synthesis System (Invitrogen New Zealand Limited, Victoria, Australia) in accordance with the manufacturer's instructions. Resultant cDNA was stored at −20°C prior to PCR amplification. Oligonucleotide primer/probe sets were designed using PrimerQuest software (Integrated DNA Technologies, Coralville, IA, USA) and purchased from Integrated DNA Technologies: MC3‐R (accession no.NM_001025270.3): F: 5′‐CCACAGCATCATGACGGTTAGGA‐3′; R: 5′‐TCTTGCTCTCGGAGTAGACGATGAAC‐3′; P: 5′‐/56FAM/AGCCCTCTC/ZEN/CTTGATCGTGGCCATCTGGGT/3IABkFQ/‐3′; and MC4‐R (accession no. NM_013099.2): F: 5′‐TGGCTGTGGCGGACATG‐3′; R: 5′‐TGTTTAGCAGGGTGATGACGAT‐3′; P: 5′‐/56‐FAM/TGGTGAGCG/ZEN/TTTCGAACGGGTCA/3IABkFQ/‐3′. Β‐actin (accession no. NM_031144) was used as a reference gene: F: 5′‐AGATGACCCAGATCATGTTTGAGA‐3′; R: 5′‐ACCAGAGGCATACAGGGACAA‐3′; P: 5′‐/56FAM/TCAACACCC/ZEN/CAGCCATGTACGTAGCC/3IABkFQ/‐3′.
Single‐plex qPCRs (20 μl) were set up by addition of PCR‐grade water, optimized final concentrations of primers (600 nm: β‐actin forward and reverse primers, MC3‐R reverse primer, MC4‐R reverse primer, 900 nm: MC3‐R forward primer, 300 nm: MC4‐R forward primer), and probe (200 nm for all genes) and LightCycler 480 Probes Master (1× concentration, Roche Applied Science, Mannheim, Germany). Template cDNA (2 μl) was added to appropriate wells in duplicate. Each plate contained negative wells in which RNase‐free water was added in place of template cDNA.
All plates were run using the LightCycler 480 Instrument II Real time PCR system (Roche Applied Science). The following protocol was utilised: initial preincubation at 95°C for 10 min, followed by 45 cycles of 95°C for 10 s, 60°C for 30 s and 72°C for 1 s, and a final cooling step at 40°C for 10 s. The raw data were run through an absolute quantification/2nd derivative maximum analysis using the associated LightCycler 480 software to calculate Cq values for each reaction. Relative gene expression analysis was undertaken using the comparative Cq method as described previously (Phillipps et al. 2013).
AgRP immunoreactivity in the PVN during pregnancy
Day 14 pregnant (n = 3) and non‐pregnant rats in the dioestrous phase of the oestrous cycle (n = 3) were perfused with 4% paraformaldehyde in 0.1 m PBS and brains processed as described for c‐fos immunohistochemistry above. Coronal sections (35 μm) were sliced throughout the PVN and collected in three series. Sections were washed five times with PBS/0.1% triton for 10 min per wash and then incubated in blocking solution (PBS/0.1% triton/5% normal goat serum/0.25% BSA) for 1 h. Following this, sections were incubated for 48 h at 4°C in block solution containing the guinea pig polyconal anti‐AgRP antibody (dilution 1:10,000; Antibodies Australia, Clayton, Australia). Sections were then washed in PBS/0.1% triton as described above, then incubated for 2 h at room temperature with a goat anti‐guinea pig Alexa fluor 555 antibody (dilution 1:500). Following further washes, sections were mounted on slides and cover‐slipped using vectamount. Sections were viewed and photographed on an Olympus AX70 research microscope (Olympus, Tokyo, Japan). The extent of fibre staining in the PVN was determined using ImageJ/Fiji software in at least two sections per animal and the mean for each animal was used for statistical comparisons. Briefly, the region of interest was selected, images were binarized and then skeletonized, and total voxels were counted as a measure of the area of region of interest stained positive for AgRP (with background levels subtracted).
Statistics
For the c‐fos immunohistochemistry analysis, at least three sections per area from each animal were counted and all analysis was performed blind to treatment group. The number of cells positive for c‐fos immunoreactivity was assessed in the PVN and SON (approximately −1.30 to −2.12 mm, relative to Bregma), as well as the arcuate nucleus and the VMH (approximately −2.12 to −3.60 mm relative to bregma) (Paxinos & Watson, 2005). For each rat, the average number of positive cells per section was calculated and used for statistical comparisons between treatment groups. For both c‐fos immunohistochemistry data and electrophysiological data, differences between treatment groups were analysed by two‐way ANOVA, and where a significant difference was obtained, post hoc comparisons were made using Holm–Sidak analysis. To determine the presence of any significant difference in MC3‐R and MC4‐R mRNA expression a Student's t test was used, while to compare AgRP fibre density in PVN between non‐pregnant and day 14 pregnant rats a non‐parametric Mann–Whitney U test was used. All data are presented as mean ± SEM and P < 0.05 was considered statistically significant. Graphpad Prism (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analyses.
Results
α‐MSH induced c‐fos in the hypothalamus during pregnancy
The number of c‐fos‐positive cells after α‐MSH or vehicle treatment was determined in the PVN (Fig. 1), arcuate nucleus (Fig. 2), VMH (Fig. 2) and SON (Fig. 3) in both pregnant and non‐pregnant rats. Statistical analysis by two‐way ANOVA revealed significant interactions between reproductive state and treatment (PVN P = 0.016, arcuate nucleus P = 0.001, VMH P = 0.018, SON P = 0.025). In all four hypothalamic regions examined, α‐MSH treatment significantly increased the expression of c‐fos‐positive cells in the dioestrous rats (Figs 1, 2, 3). In contrast, there was no significant increase in c‐fos‐positive cell number after α‐MSH treatment in the PVN (Fig. 1), arcuate nucleus (Fig. 2) or VMH (Fig. 2) in the pregnant group. In the SON, α‐MSH treatment significantly increased c‐fos‐positive cell number in the pregnant animals, but the increase was significantly attenuated compared to the α‐MSH‐treated dioestrous group (Fig. 3).
Figure 1. Expression of c‐fos in representative coronal sections of the paraventricular nucleus 90 min after icv injection of α‐MSH or vehicle in non‐pregnant and day 14 pregnant rats .
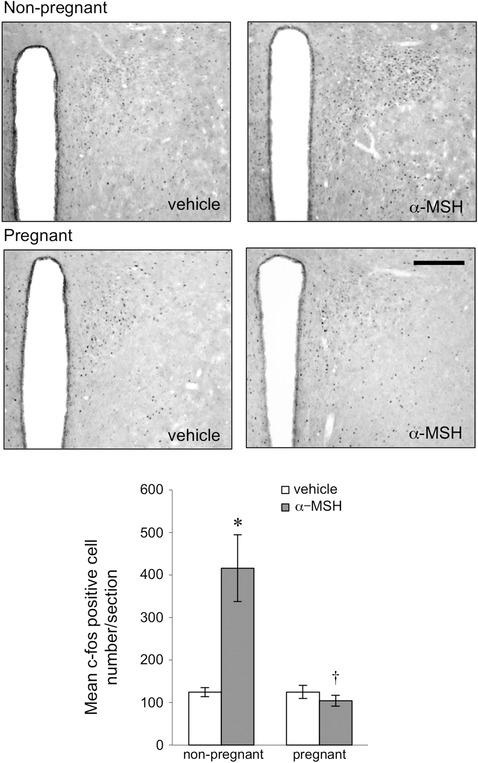
Scale bar = 200 μm. Bars represent the mean ± SEM number of c‐fos‐positive cells in the paraventricular nucleus of the hypothalamus after α‐MSH or vehicle treatment in non‐pregnant and day 14 pregnant rats (n = 5–6 per group). *Significant with respect to vehicle‐treated group of the same physiological state (P < 0.05). †Significant with respect to α‐MSH‐treated non‐pregnant group (P < 0.05).
Figure 2. Expression of c‐fos in representative coronal sections of the arcuate nucleus and ventromedial nucleus of the hypothalamus (VMH) 90 min after α‐MSH or vehicle icv injection in non‐pregnant and day 14 pregnant rats .
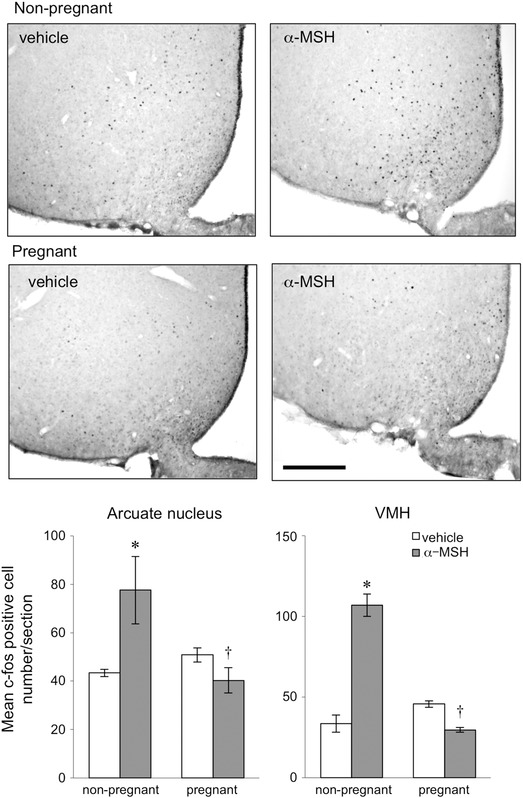
Scale bar = 200 μm. Bars represent the mean ± SEM number of c‐fos‐positive cells in the arcuate nucleus and the VMH after α‐MSH or vehicle treatment in non‐pregnant and day 14 pregnant rats (n = 5–6 per group). *Significant with respect to vehicle‐treated group of the same physiological state (P < 0.05). †Significant with respect to α‐MSH‐treated non‐pregnant group (P < 0.05).
Figure 3. Expression of c‐fos in representative coronal sections of the supraoptic nucleus 90 min after α‐MSH or vehicle icv injection in non‐pregnant and day 14 pregnant rats .
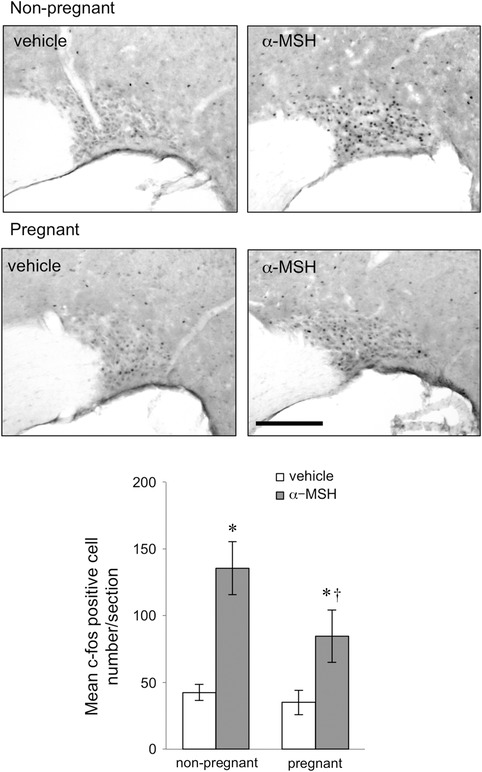
Scale bar = 200 μm. Bars represent the mean ± SEM number of c‐fos‐positive cells in the supraoptic nucleus after α‐MSH or vehicle treatment in non‐pregnant and day 14 pregnant rats (n = 5–6 per group). *Significant with respect to vehicle‐treated group of the same physiological state. †Significant with respect to α‐MSH treated non‐pregnant group (P < 0.05).
Effect of α‐MSH on electrical activity of oxytocin neurons during pregnancy
In vivo extracellular single‐unit recordings were made from 12 oxytocin neurons from nine female rats during microdialysis application of α‐MSH (1.5 mm for 30–60 min). Similar to previous findings (Sabatier et al. 2003), the mean spontaneous firing rate of all six oxytocin neurons recorded from non‐pregnant rats decreased progressively by approximately 50% during microdialysis application of α‐MSH (Fig. 4). In contrast to this inhibitory effect in non‐pregnant rats, on average oxytocin neuron firing rate was not affected by α‐MSH administration in mid‐pregnant (pregnancy day 10–16) rats, with most neurons showing no response and two cells even showing an increase in firing after α‐MSH (Fig. 4). Overall, the effect of α‐MSH on the firing rate of oxytocin neurons was significantly different in pregnant compared with non‐pregnant rats (P < 0.05).
Figure 4. Pregnancy‐induced changes in the in vivo electrophysiological response of oxytocin neurons to α‐MSH .
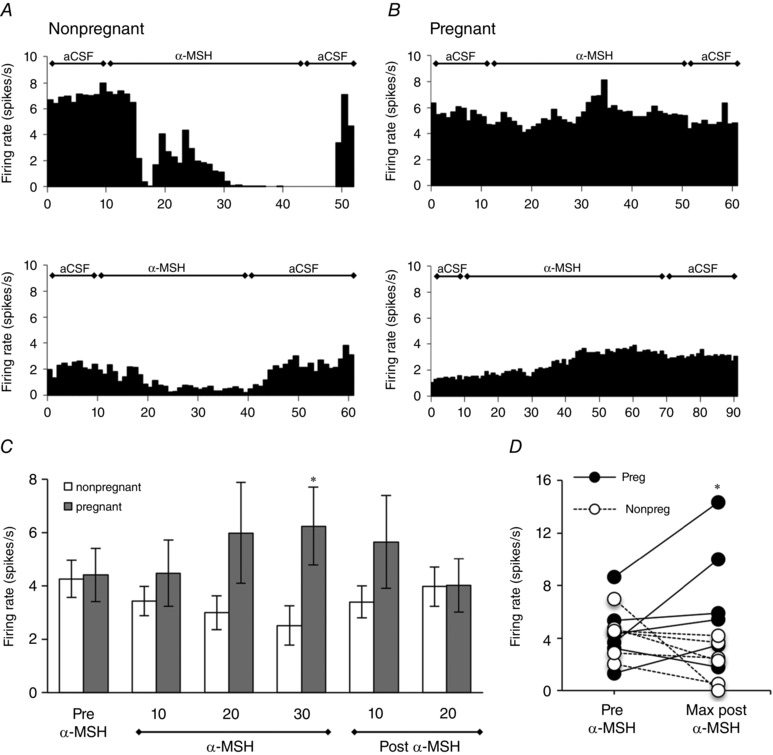
A and B, representative rate‐meter records (in 1 min bins) showing changes in firing rate in response to continuous α‐MSH of an oxytocin neuron in two representative urethane‐anesthetised non‐pregnant, virgin (A) or pregnant rats (B) (please note the x‐axis differs for some of these individual recordings, due to variability in basal firing rate of oxytocin neurons in vivo). C, mean firing rate of identified oxytocin neurons (averaged in 10 min bins) before, during and after continuous dialysis of α‐MSH in non‐pregnant and pregnant rats (days 10–16). α‐MSH decreased firing rate in oxytocin neurons in non‐pregnant rats, but had no effect or slightly increased firing rate in pregnancy. *Significant with respect to firing rate in pregnant animals (P < 0.05) (n = 6 rats per group). D. scatter plot illustrating the individual changes in firing from all recorded cells, showing the change from the period prior to α‐MSH injection (Pre‐αMSH) to the maximum response during administration of α‐MSH (Max response αMSH). Note that there is considerable inter‐cell variability in firing in both groups, but the α‐MSH‐induced decrease in firing seen in non‐pregnant animals is lost in pregnant animals, with some animals even showing an increase in firing *Response in pregnant animals is significantly different from that in non‐pregnant animals (P < 0.05).
Melanocortin receptor expression in the hypothalamus during pregnancy
MC3‐R mRNA expression within the hypothalamus was predominantly confined to the VMH and the arcuate nucleus (Fig. 5). MC4‐R mRNA expression was localized to the PVN, with the SON and arcuate nucleus displaying a weaker signal for this riboprobe (Fig. 5). Additional non‐hypothalamic sites also showed strong signal for MC4‐R with the medial amygdala (Fig. 5) and caudate putamen (not shown) displaying dark staining on the autoradiogram. Within the VMH, positive hybridization of MC3‐R was limited to the dorsomedial areas of this nucleus (Fig. 5). There was no significant difference between levels of MC3‐R mRNA in the VMH (P = 0.4) or MC4‐R mRNA in the PVN (P = 0.073) in day 14 pregnant and non‐pregnant rats, as determined by qPCR following laser microdissection of these nuclei (Fig. 5).
Figure 5. MC3‐R and MC4‐R mRNA expression in the rat hypothalamus .
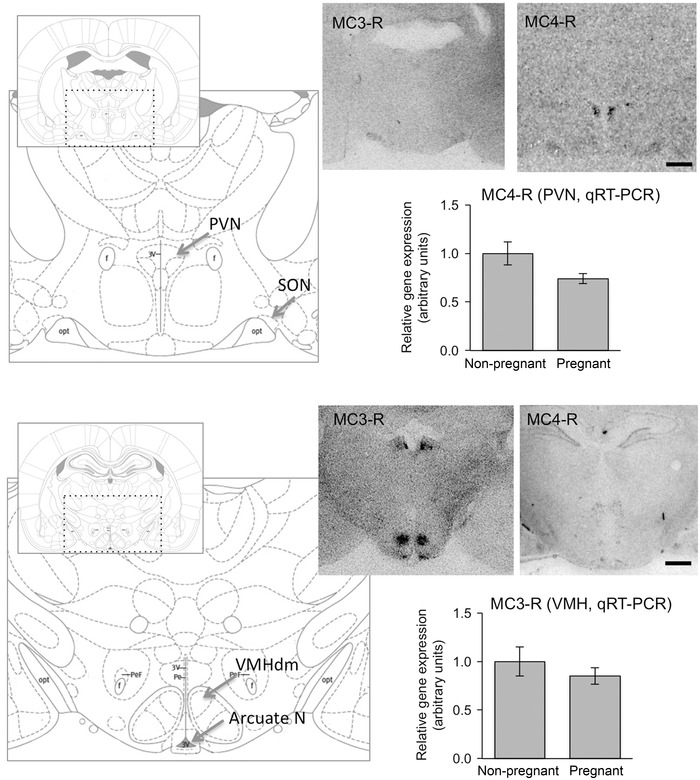
Representative autoradiographic images of coronal brain sections from a female rat showing expression of MC3‐R (left) and MC4‐R (right) in the paraventricular nucleus (top) and the arcuate nucleus and ventromedial nucleus of the hypothalamus (VMH) (bottom). Scale bars = 1000 μm. Schematics represent coronal rat brain sections through regions of the hypothalamus −1.56 and −2.40 mm relative to Bregma (Paxinos & Watson, 2005) that correspond to areas shown in the autoradiographic images. Graphs show no significant difference in VMH MC3‐R mRNA levels and PVN MC4‐R mRNA levels in laser capture microdissected samples from non‐pregnant rats (n = 6) and day 14 pregnant rats (n = 6). Data are presented as relative gene expression with comparison to the control group ± SEM.
AgRP immunoreactivity in the PVN during pregnancy
AgRP fibre immunoreactivity was observed within the PVN (Fig. 6), as described previously (Broberger et al. 1998; Bagnol et al. 1999). There was no significant difference in the extent of fibre coverage in the PVN of pregnant and non‐pregnant rats (P = 0.4).
Figure 6. AgRP immunoreactivity .
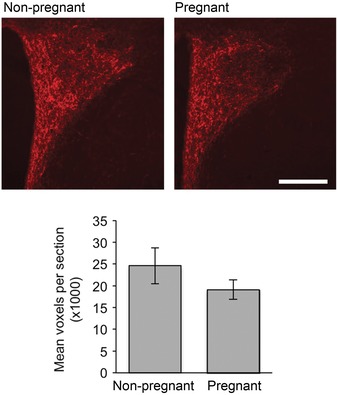
AgRP‐positive immunoreactivity in the paraventricular nucleus in representative images from non‐pregnant (top) and pregnant (bottom) rats. Scale bar = 200 μm. Graph shows no significant difference in AgRP fibre density in the PVN of non‐pregnant and pregnant rats (n = 3 per group).
Discussion
This study has documented a profound change in the hypothalamic response to α‐MSH during pregnancy. In all hypothalamic areas investigated, there was a marked decrease in the ability of α‐MSH to induce c‐fos expression in cells. As a representative example of a known population of α‐MSH‐responsive neurons, we examined the electrophysiological response to α‐MSH in supraoptic oxytocin neurons in vivo. In non‐pregnant female rats, α‐MSH decreased oxytocin neuron firing rate, as described previously (Sabatier et al. 2003). In contrast, there was an overall loss of α‐MSH‐induced inhibition of firing rate of SON oxytocin neurons in mid‐pregnancy, consistent with the loss of α‐MSH‐induced c‐fos expression, with some neurons even showing an opposite response. These data, coupled with our prior demonstration that the satiety response to α‐MSH is lost during pregnancy, indicate a widespread insensitivity to α‐MSH in the hypothalamus during pregnancy.
Several studies have previously documented that α‐MSH induces c‐fos expression in the PVN and SON (Thiele et al. 1998; McMinn et al. 2000; Olszewski et al. 2001; Sabatier et al. 2003; Kawasaki et al. 2009; Rowland et al. 2010). As elevated c‐fos expression is widely thought to reflect neuronal activity, many of these studies were interpreted to suggest that α‐MSH excited neurons within these nuclei. As discussed in several reviews (Hoffman & Lyo 2002; Kovacs 2008), however, it is important to recognise that c‐fos is activated by several signal transduction pathways, and it is possible to dissociate changes in these 2nd messenger systems from depolarisation of neurons. The most direct demonstration of this was provided by Luckman et al. (1994), who showed that cholinergic receptor activation increased both action potential firing and c‐fos expression in oxytocin neurons, but that electrical stimulation of action potential firing using the pattern recorded during cholinergic receptor activation did not induce c‐fos expression. Hence, c‐fos expression was mediated by receptor‐mediated signal transduction, rather than by neuronal firing, per se. There are many examples where strong activation of neuronal firing is not accompanied by c‐fos expression (Hoffman & Lyo 2002; Kovacs 2008). For example, oxytocin neurons do not show elevated c‐fos during lactation, despite robust increases in neuronal activity (Fenelon et al. 1993). In particular, it is known that c‐fos does not provide a marker of activity in neurons experiencing a net synaptic inhibition (Chan et al. 1993; Kovacs 2008). While less common, there are also examples where expression of c‐fos can be induced in the absence of an increase in neuronal firing rate or, indeed, in the presence of inhibition of firing. For example, in the striatum, dopamine induces c‐fos expression through an increase in cAMP, and at the same time inhibits neuronal firing via D1 receptors (Berreta et al. 1992). α‐MSH action on oxytocin neurons provides another similar example, with α‐MSH administration directly into the SON shown to stimulate c‐fos expression in oxytocin neurons, associated with an α‐MSH‐induced decrease in firing rate of these cells (Sabatier et al. 2003). Our data in non‐pregnant animals replicate these observations, and confirm that the normal response of oxytocin neurons to α‐MSH is an increase in c‐fos expression together with an inhibition of neuronal firing rate. The physiological consequences of this complex response have been nicely reviewed (Sabatier, 2006), and it potentially provides a mechanism to allow differential secretion of oxytocin from different compartments within the neurons. The induction of c‐fos probably involves activation of cAMP in the oxytocin neurons, as the MC4‐R predominantly acts via Gs to activate adenylate cyclase (Tao, 2010), and cAMP represents one of the major pathways involved in activating c‐fos expression (Hoffman & Lyo 2002). Alternatively, some recent evidence from amygdala neurons suggests that activation of presynaptic MC4‐Rs can induce an inhibition of N‐type calcium channels, potentially reducing inhibitory inputs to the postsynaptic neurons to induce an increase in c‐fos expression (Agosti et al. 2014). Whether a similar mechanism is involved in oxytocin neurons is not known. In contrast, the inhibition of firing appears to involve α‐MSH‐induced increases in intracellular calcium in oxytocin neurons, which triggers a release of endocannabinoids to act in a retrograde manner to inhibit presynaptic inputs to these cells (Sabatier & Leng, 2006). Remarkably, both the increase in c‐fos expression and the inhibition of firing of oxytocin neurons in response to α‐MSH are lost during pregnancy.
The melanocortin system is a vital neuronal network involved in the regulation of energy balance. Leptin acts to increase α‐MSH release from POMC neurons in the arcuate nucleus. α‐MSH binds to MCR‐4, predominantly located on neurons within the PVN, to suppress food intake (Balthasar et al. 2005). Leptin also suppresses production of the melanocortin antagonist AgRP from another population of neurons in the arcuate nucleus, thereby facilitating activation of the melanocortin‐sensitive pathways. In diet‐induced obesity, these arcuate nucleus neurons become less responsive to leptin, with the resulting reduction in signalling through the melanocortin pathway contributing to increased food intake, despite rising levels of leptin. Bypassing this leptin resistance by administering α‐MSH or melanocortin receptor agonists induces a profound suppression of food intake (Pierroz et al. 2002; Hansen et al. 2005), demonstrating that the melanocortin system remains responsive. In contrast, pregnant animals are leptin resistant, and also do not show a satiety response to α‐MSH (Ladyman et al. 2009). It therefore seems likely that the observed suppression of responses to α‐MSH during pregnancy is a key mechanism underlying the development of leptin resistance during pregnancy. The loss of response in these second‐order neurons provides an adaptive change in energy homeostasis, facilitating increased appetite in pregnant females and promoting a positive energy balance in preparation for the energy demands of lactation.
The central melanocortin system is also involved in glucose homeostasis, and α‐MSH action, particularly within the PVN and VMH, plays a role in stimulating glucose uptake by peripheral tissues (Toda et al. 2009). It is possible that suppressed activation of cells by α‐MSH in these areas during pregnancy may contribute to the decrease in glucose uptake in maternal peripheral tissues, a pregnancy‐specific adaption of glucose homeostasis to reduce insulin sensitivity in the mother and preferentially provide glucose to the fetus (Leturque et al. 1980, 1984; Rossi et al. 1993).
While our observation of a loss of response to α‐MSH during pregnancy seems quite clear, these data are not supported by a previous study using chronic third‐cerebral ventricular infusion of SHU9119, a melanocortin‐3,4 receptor blocker. That study found that SHU9119 administered from day 4 of pregnancy significantly increased food intake and weight gain during pregnancy, suggesting that there is ongoing melanocortin tone (Heinsbroek & van Dijk, 2009). It seems likely that this apparent contradiction is explained by the different timing in the two experiments. We have not examined responses to α‐MSH earlier than day 14 of pregnancy, but our previous work examining leptin responses has demonstrated that leptin resistance does not develop until between day 7 and day 14 of pregnancy (Ladyman & Grattan, 2004), and it is likely that it is driven by changes in hormone secretion from the placenta around day 11 (Augustine & Grattan, 2008). Given the role of the melanocortin system in mediating the effects of leptin, a similar time frame could be predicted for changes in the melanocortin system during pregnancy. Hence, administration of the antagonist from day 4 may have been inducing an effect prior to the pregnancy‐induced change in sensitivity of the melanocortin system.
With reference to the representative example of oxytocin neurons specifically investigated in this study, recent evidence suggests that oxytocin neurons represent the primary output neurons in the PVN mediating action of melanocortins to regulate appetite (Atasoy et al. 2012). Both peripheral (Morton et al. 2012; Ho et al. 2014) and central (Arletti et al. 1990; Olson et al. 1991; Richard et al. 1991) oxytocin is anorectic, involved in both reducing both meal duration and overall food intake. While central administration of an oxytocin antagonist induces a significant increase in food intake in non‐pregnant rats, it has no effect in pregnant females (Douglas et al. 2007), suggesting that central oxytocin secretion is reduced. This is consistent with our observation of a loss of α‐MSH action in the hypothalamus. While we have not specifically looked at PVN oxytocin neurons, we have observed that pregnancy was associated with a loss of α‐MSH‐induced inhibition of firing rate of SON oxytocin neurons. Oxytocin neurons in the SON are activated during feeding (Johnstone et al. 2006), although the exact nature of their role in regulating appetite (as opposed to PVN neurons) is unclear (Sabatier et al. 2013). They would certainly contribute to peripheral oxytocin levels, but there is also some evidence that axons from these magnocellular neurons in the SON branch and provide innervation of pre‐autonomic neurons (Knobloch et al. 2012), thereby potentially contributing to the oxytocinergic regulation of appetite. In non‐pregnant rats, central α‐MSH inhibits the firing rate of oxytocin neurons in the SON (Sabatier et al. 2003), via endocannabinoids (Sabatier & Leng, 2006), along with decreasing oxytocin secretion from the pituitary gland, but increases dendritic release of oxytocin within the hypothalamus (Sabatier et al. 2003). Hence, it is also possible that oxytocin released from the dendritic parts of these neurons could influence preautonomic neurons elsewhere in the hypothalamus, as has been shown for dendritically released vasopressin (Son et al. 2013). Whether the loss of effect of α‐MSH on these SON oxytocin neurons during pregnancy contributes to the overall lack of α‐MSH‐induced satiety or indeed the hyperphagia of pregnancy is unknown, but it is certainly consistent with this hypothesis.
In addition to a possible role in appetite regulation, oxytocin has numerous other functions, including critical roles both in parturition and in milk ejection during lactation. To enable these functions, the magnocellular oxytocin neurons undergo significant adaptive modifications during pregnancy and lactation resulting in the novel capacity to fire intermittent, co‐ordinated, high‐frequency bursts of action potentials that result in pulses of oxytocin secreted into the blood at regular intervals (Russell et al. 2003; Brown et al. 2013). The adaptive changes that prepare these neurons for this new pattern of secretion are initiated during pregnancy, when oxytocin neurons are strongly inhibited and their responses to many stimuli are attenuated (Douglas et al. 2007). The loss of an inhibitory effect of α‐MSH during pregnancy might be anticipated to induce elevated oxytocin secretion, which would be potentially counter‐productive to pregnancy. There are multiple pregnancy‐specific factors contributing to the inhibition of peripheral oxytocin secretion at this time, however, including the progesterone metabolite allopregnenalone (Brunton et al. 2014), nitric oxide (Srisawat et al. 2000), endogenous opioids (Douglas et al. 1995) and prolactin (Kokay et al. 2006). Together these factors are thought to restrain activity of oxytocin neurons to prevent premature birth. Hence, the change in response of these neurons to α‐MSH during pregnancy may be part of the broader adaptive changes in this neuronal system that enable successful parturition and lactation.
Our attempts to elucidate the mechanism underlying the loss of response to α‐MSH during pregnancy have not been completely successful. Within the hypothalamus, α‐MSH acts through the MC3‐R and MC4‐R subtypes. Similar to previous studies, MC4‐R mRNA was detected in the PVN and more weakly in the SON and arcuate nucleus, while MC3‐R mRNA was detected in the arcuate nucleus and dorsomedial region of the VMH (Roselli‐Rehfuss et al. 1993; Mountjoy et al. 1994; Xia & Wikberg, 1997; Kishi et al. 2003; Mountjoy, 2010). While we observed slightly lower levels of MC3‐R and MC4‐R mRNA during pregnancy in the VMH and PVN, respectively, these differences were not statistically significant. Hence, these data suggest that it is unlikely that a down‐regulation of melanocortin receptor may underlie the decrease in α‐MSH‐induced expression of c‐fos in hypothalamic cells that was observed during pregnancy. This finding is supported by previous work using RT‐PCR techniques, showing that MC4‐R mRNA levels in the whole hypothalamus were not significantly different between late pregnant and non‐pregnant rats (Rocha et al. 2003). We also investigated whether the endogenous melanocortin receptor antagonist AgRP might be upregulated during pregnancy to oppose the actions of α‐MSH. There is some inconsistency in prior reports regarding AgRP mRNA during pregnancy. Our previous work, examining AgRP mRNA by in situ hybridization, suggested that levels remain stable in the arcuate nucleus during pregnancy, although dramatically increasing during lactation (Ladyman et al. 2009). In contrast, others, using RT‐PCR on RNA extracted from the whole hypothalamus, have suggested that levels of AgRP mRNA may be increased during pregnancy (Rocha et al. 2003; Szczepankiewicz et al. 2009). In the present study, we observed that AgRP fibre density in the PVN was similar in pregnant and non‐pregnant animals. Taken together with our previous in situ hybridization study (Ladyman et al. 2009), these data suggest that it is unlikely that increased AgRP could provide a mechanism to explain the pregnancy‐induced loss of α‐MSH responses, or the hyperphagia of pregnancy. It is possible that some post‐receptor mechanism may impair responses to α‐MSH during pregnancy. For example, melanocortin receptor accessory proteins (MRAPs) are expressed in the brain, including the PVN (Chan et al. 2009), and at least in vitro, these are required for normal melanocortin signalling (Chan et al. 2009). Hence, pregnancy‐induced changes in the expression or function of MRAPs might contribute to the lack of response to α‐MSH.
Overall, the results from the present study show a marked suppression of α‐MSH‐induced c‐fos expression in multiple regions of the hypothalamus during pregnancy compared with non‐pregnant controls. In addition, we observed that the α‐MSH‐induced inhibition of firing rate in SON oxytocin neurons seen in non‐pregnant animals was lost during pregnancy in the rat. These data suggest a widespread insensitivity to the actions of α‐MSH in the hypothalamus during pregnancy. These studies provide further insight into the complex adaptive changes occurring in the maternal brain during pregnancy, altering the neural networks regulating appetite to favour positive energy balance.
Additional information
Competing interests
None declared.
Author contributions
Experiments were conceived and designed by S.R.L., R.A.A., H.R.P., C.H.B. and D.R.G. Collection, analysis and interpretation of immunohistochemistry experiments was performed by S.R.L., E.S. and D.R.G.; collection, analysis and interpretation of mRNA experiments was performed by H.R.P., R.A.A. and D.R.G.; collection, analysis and interpretation of electrophysiological experiments was performed by C.H.B. and R.A.A. The manuscript was written by S.R.L., with critical revisions by D.R.G., C.H.B. and R.A.A.
Funding
This research was supported by a grant from the Health Research Council of New Zealand, and by the Marsden Fund of the Royal Society of New Zealand (Contract UOO0905).
Acknowledgements
We gratefully acknowledge the technical assistance of Elliot Wilks and Dr Victoria Scott.
References
- Agosti F, Lopez Soto EJ, Cabral A, Castrogiovanni D, Schioth HB, Perello M & Raingo J (2014). Melanocortin 4 receptor activation inhibits presynaptic N‐type calcium channels in amygdaloid complex neurons. Eur J Neurosci 40, 2755–2765. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A & Bertolini A (1990). Oxytocin inhibits food and fluid intake in rats. Physiol Behav 48, 825–830. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH & Sternson SM (2012). Deconstruction of a neural circuit for hunger. Nature 488, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine RA & Grattan DR (2008). Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology 149, 1049–1055. [DOI] [PubMed] [Google Scholar]
- Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS & Watson SJ (1999). Anatomy of an endogenous antagonist: relationship between Agouti‐related protein and proopiomelanocortin in brain. J Neurosci 19, RC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK & Lowell BB (2005). Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505. [DOI] [PubMed] [Google Scholar]
- Begriche K, Girardet C, McDonald P, Butler AA (2013). Melanocortin‐3 receptors and metabolic homeostasis. Prog Mol Biol Transl Sci 114, 109–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Robertson HA & Graybiel AM (1992). Dopamine and glutamate agonists stimulate neuron‐specific expression of Fos‐like protein in the striatum. J Neurophysiol 68, 767–777. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW & Baskin DG (2009). Forebrain melanocortin signaling enhances the hindbrain satiety response to CCK‐8. Am J Physiol Regul Integr Comp Physiol 296, R476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M & Hökfelt T (1998). The neuropeptide Y/agouti gene‐related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate‐treated mice. Proc Natl Acad Sci USA 95, 15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M & Stern JE (2013). Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol 25, 678–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Munro G, Murphy NP, Leng G & Russell JA (1996). Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol 496, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA & Hirst JJ (2014). Allopregnanolone in the brain: protecting pregnancy and birth outcomes. Prog Neurobiol 113, 106–136. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD (2000) A unique metabolic syndrome causes obesity in the melanocortin‐3 receptor‐deficient mouse. Endocrinology 141, 3518–3521. [DOI] [PubMed] [Google Scholar]
- Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertova M, Elphick MR, Cheetham ME, Metherell LA & Clark AJ (2009). MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA 106, 6146–6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RK, Brown ER, Ericsson A, Kovacs KJ & Sawchenko PE (1993). A comparison of two immediate‐early genes, c‐fos and NGFI‐B, as markers for functional activation in stress‐related neuroendocrine circuitry. J Neurosci 13, 5126–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF & Cone RD (1999). Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163. [DOI] [PubMed] [Google Scholar]
- Douglas AJ, Neumann I, Meeren HK, Leng G, Johnstone LE, Munro G & Russell JA (1995). Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci 15, 5049–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AJ, Johnstone LE & Leng G (2007). Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav 91, 352–365. [DOI] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P & Cone RD (2004). Cholecystokinin‐mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7, 335–336. [DOI] [PubMed] [Google Scholar]
- Fenelon VS, Poulain DA, Theodosis DT (1993). Oxytocin neuron activation and Fos expression: a quantitative immunocytochemical analysis of the effect of lactation, parturition, osmotic and cardiovascular stimulation. Neuroscience 53, 77–89. [DOI] [PubMed] [Google Scholar]
- Garza JC, Kim CS, Liu J, Zhang W & Lu XY (2008). Adeno‐associated virus‐mediated knockdown of melanocortin‐4 receptor in the paraventricular nucleus of the hypothalamus promotes high‐fat diet‐induced hyperphagia and obesity. J Endocrinol 197, 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MJ, Schioth HB & Morris MJ (2005). Feeding responses to a melanocortin agonist and antagonist in obesity induced by a palatable high‐fat diet. Brain Res 1039, 137–145. [DOI] [PubMed] [Google Scholar]
- Heinsbroek AC & van Dijk G (2009). Gestational weight gain by reduced brain melanocortin activity affects offspring energy balance in rats. Int J Obesity 33, 104–114. [DOI] [PubMed] [Google Scholar]
- Ho JM, Anekonda VT, Thompson BW, Zhu M, Curry RW, Hwang BH, Morton GJ, Schwartz MW, Baskin DG, Appleyard SM, Blevins JE (2014) Hindbrain oxytocin receptors contribute to the effects of circulating oxytocin on food intake in male rats. Endocrinology 155, 2845–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE & Lyo D (2002). Anatomical markers of activity in neuroendocrine systems: are we all ‘fos‐ed out’? J Neuroendocrinol 14, 259–268. [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM & Leng G (2006). Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab 4, 313–321. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Ponzio TA, Yue C, Fields RL & Gainer H (2009). Neurotransmitter regulation of c‐fos and vasopressin gene expression in the rat supraoptic nucleus. Exp Neurol 219, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB & Elmquist JK (2003). Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457, 213–235. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R & Grinevich V (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566. [DOI] [PubMed] [Google Scholar]
- Kokay IC, Bull PM, Davis RL, Ludwig M & Grattan DR (2006). Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol 290, R1216–1225. [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua SC, Jr , Leibel RL & Wardlaw SL (2001). Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13, 959–966. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ (2008). Measurement of immediate‐early gene activation – c‐fos and beyond. J Neuroendocrinol 20, 665–672. [DOI] [PubMed] [Google Scholar]
- Ladyman SR & Grattan DR (2004). Region‐specific reduction in leptin‐induced phosphorylation of signal transducer and activator of transcription‐3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145, 3704–3711. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Sapsford TJ & Grattan DR (2011). Loss of acute satiety response to cholecystokinin in pregnant rats. J Neuroendocrinol 23, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Tups A, Augustine RA, Swahn‐Azavedo A, Kokay IC & Grattan DR (2009). Loss of hypothalamic response to leptin during pregnancy associated with development of melanocortin resistance. J Neuroendocrinol 21, 449–456. [DOI] [PubMed] [Google Scholar]
- Leturque A, Burnol AF, Ferre P & Girard J (1984). Pregnancy‐induced insulin resistance in the rat: assessment by glucose clamp technique. Am J Physiol 246, E25–31. [DOI] [PubMed] [Google Scholar]
- Leturque A, Ferre P, Satabin P, Kervran A & Girard J (1980). In vivo insulin resistance during pregnancy in the rat. Diabetologia 19, 521–528. [DOI] [PubMed] [Google Scholar]
- Luckman SM, Dyball RE, Leng G (1994) Induction of c‐fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci 14, 4825–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P & Palmiter RD (1999). Response of melanocortin‐4 receptor‐deficient mice to anorectic and orexigenic peptides. Nat Genet 21, 119–122. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Wilkinson CW, Havel PJ, Woods SC & Schwartz MW (2000). Effect of intracerebroventricular α‐MSH on food intake, adiposity, c‐Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol 279, R695–703. [DOI] [PubMed] [Google Scholar]
- Mizuno TM & Mobbs CV (1999). Hypothalamic agouti‐related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140, 814–817. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden‐Hanson T, Baskin DG, Schwartz MW, Blevins JE (2012) Peripheral oxytocin suppresses food intake and causes weight loss in diet‐induced obese rats. Am J Physiol Endocrinol Metab 302, E134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG (2010). Distribution and function of melanocortin receptors within the brain. Adv Expt Med Biol 681, 29–48. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB & Cone RD (1994). Localization of the melanocortin‐4 receptor (MC4‐R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8, 1298–1308. [DOI] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM & Verbalis JG (1991). Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12, 113–118. [DOI] [PubMed] [Google Scholar]
- Olszewski PK, Wirth MM, Shaw TJ, Grace MK, Billington CJ, Giraudo SQ & Levine AS (2001). Role of α‐MSH in the regulation of consummatory behavior: immunohistochemical evidence. Am J Physiol Regul Integr Comp Physiol 281, R673–680. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (2005). The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press, New York. [Google Scholar]
- Phillipps HR, Ladyman SR & Grattan DR (2013). Maintained expression of genes associated with metabolism in the ventromedial hypothalamic nucleus despite development of leptin resistance during pregnancy in the rat. Physiol Rep 1, e00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS & Mantzoros CS (2002). Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet‐induced obesity. Diabetes 51, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Richard P, Moos F & Freund‐Mercier MJ (1991). Central effects of oxytocin. Physiol Rev 71, 331–370. [DOI] [PubMed] [Google Scholar]
- Rocha M, Bing C, Williams G & Puerta M (2003). Pregnancy‐induced hyperphagia is associated with increased gene expression of hypothalamic agouti‐related peptide in rats. Regul Pept 114, 159–165. [DOI] [PubMed] [Google Scholar]
- Roselli‐Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB & Cone RD (1993). Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90, 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Sherwin RS, Penzias AS, Lapaczewski P, Jacob RJ, Shulman GI & Diamond MP (1993). Temporal changes in insulin resistance and secretion in 24‐h‐fasted conscious pregnant rats. Am J Physiol 265, E845–851. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Schaub JW, Robertson KL, Andreasen A & Haskell‐Luevano C (2010). Effect of MTII on food intake and brain c‐Fos in melanocortin‐3, melanocortin‐4, and double MC3 and MC4 receptor knockout mice. Peptides 31, 2314–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Leng G & Douglas AJ (2003). The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol 24, 27–61. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L & Leng G (2003). α‐Melanocyte‐stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci 23, 10351–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N (2006). α‐Melanocyte‐stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol 18, 703–710. [DOI] [PubMed] [Google Scholar]
- Sabatier N & Leng G (2006). Presynaptic actions of endocannabinoids mediate α‐MSH‐induced inhibition of oxytocin cells. Am J Physiol Regul Integr Comp Physiol 290, R577‐584. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Leng G & Menzies J (2013). Oxytocin, feeding, and satiety. Front Endocrinol 4, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M & Stern JE (2013). Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron 78, 1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepankiewicz D, Pruszynska‐Oszmalek E, Kaczmarek P, Skrzypski M, Andralojc K, Wojciechowicz T, Sassek M & Nowak KW (2009). Changes of agouti‐related protein in hypothalamus, placenta, and serum during pregnancy in the rat. J Endocrinol 202, 35–41. [DOI] [PubMed] [Google Scholar]
- Srisawat R, Ludwig M, Bull PM, Douglas AJ, Russell JA & Leng G (2000). Nitric oxide and the oxytocin system in pregnancy. J Neurosci 20, 6721–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX (2010). The melanocortin‐4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev 31, 506–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, van Dijk G, Yagaloff KA, Fisher SL, Schwartz M, Burn P & Seeley RJ (1998). Central infusion of melanocortin agonist MTII in rats: assessment of c‐Fos expression and taste aversion. Am J Physiol 274, R248–254. [DOI] [PubMed] [Google Scholar]
- Toda C, Shiuchi T, Lee S, Yamato‐Esaki M, Fujino Y, Suzuki A, Okamoto S & Minokoshi Y (2009). Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58, 2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y & Wikberg JE (1997). Postnatal expression of melanocortin‐3 receptor in rat diencephalon and mesencephalon. Neuropharmacology 36, 217–224. [DOI] [PubMed] [Google Scholar]


