Abstract
We aimed to evaluate 10 biomarkers related to inflammation and the kynurenine pathway, including neopterin, kynurenine:tryptophan ratio, C-reactive protein, tryptophan, and 6 kynurenines, as potential predictors of all-cause and cause-specific mortality in a general population sample. The study cohort was participants involved in a community-based Norwegian study, the Hordaland Health Study (HUSK). We used Cox proportional hazards models to assess associations of the biomarkers with all-cause mortality and competing-risk models for cause-specific mortality. Of the 7,015 participants, 1,496 deaths were recorded after a median follow-up time of 14 years (1998–2012). Plasma levels of inflammatory markers (neopterin, kynurenine:tryptophan ratio, and C-reactive protein), anthranilic acid, and 3-hydroxykynurenine were positively associated with all-cause mortality, and tryptophan and xanthurenic acid were inversely associated. Multivariate-adjusted hazard ratios for the highest (versus lowest) quartiles of the biomarkers were 1.19–1.60 for positive associations and 0.73–0.87 for negative associations. All of the inflammatory markers and most kynurenines, except kynurenic acid and 3-hydroxyanthranilic acid, were associated with cardiovascular disease (CVD) mortality. In this general population, plasma biomarkers of inflammation and kynurenines were associated with risk of all-cause, cancer, and CVD mortality. Associations were stronger for CVD mortality than for mortality due to cancer or other causes.
Keywords: cancer, cardiovascular disease, inflammation, mortality, tryptophan
Persistent systemic inflammation predisposes people to chronic disease and death (1). The proinflammatory cytokine interferon-γ induces the production of neopterin, a macrophage-derived metabolite. It also activates indoleamine 2,3-dioxygenase, which catalyzes the first and rate-limiting step in the kynurenine pathway of tryptophan catabolism, where tryptophan is converted to a variety of immunomodulating and neuroactive compounds, collectively termed kynurenines (2, 3).
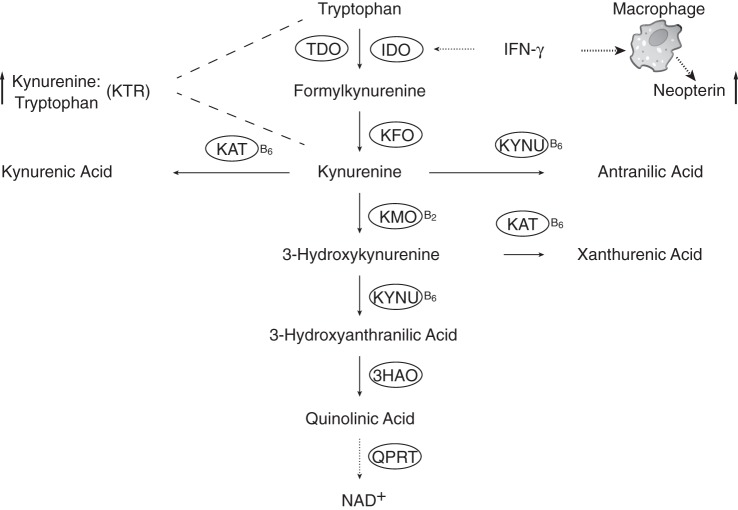
The kynurenine pathway ultimately results in the biosynthesis of nicotinamide adenine dinucleotide. The pathway involves vitamin B6- or B2-dependent enzymes catalyzing the formation of kynurenines, including anthranilic acid (AA), kynurenic acid (KA), 3-hydroxykynurenine (HK), 3-hydroxyanthranilic acid (HAA), and xanthurenic acid (XA) (4, 5) (Figure 1). The kynurenine pathway is well balanced under physiological conditions but up-regulated as part of the activated immune response (5), and it has been linked to several diseases, including neurodegenerative disorders, depression, and cancer (5).
Figure 1.
The kynurenine pathway of tryptophan catabolism and markers of interferon-γ-mediated immune activation. 3HAO, 3-hydroxyanthranilate dioxygenase; IDO, indoleamine 2,3-dioxygenase; IFN-γ, interferon-γ; KAT, kynurenine transaminase; KFO, kynurenine formylase; KMO, kynurenine monooxygenase; KTR, kynurenine:tryptophan ratio; KYNU, kynureninase; NAD+, nicotinamide adenine dinucleotide; QPRT, quinolinate phosphoribosyl transferase; TDO, tryptophan 2,3-dioxygenase.
Plasma neopterin level and kynurenine:tryptophan ratio (KTR) are correlated and are often considered systemic inflammatory markers reflecting interferon-γ activity (5, 6). Plasma neopterin has been found to predict all-cause mortality in patients with stable angina pectoris (6) and in renal transplant recipients (7). KTR in plasma or urine predicts all-cause and cardiovascular disease (CVD) mortality in patients with stable angina pectoris (6, 8) and mortality in human immunodeficiency virus–infected adults (9). Elevated neopterin level and KTR have been associated with increased risk of future coronary events (10) and cancer (11) and with more advanced stages of cancer (12).
C-reactive protein (CRP) reflects the activity of interleukin-6, a mediator of inflammatory cascades (13). It is a nonspecific systemic marker of inflammation. Elevated CRP level is a well-documented risk factor for CVD outcomes (14) and is a possible risk factor for cancer (11, 15), diabetes (16, 17), and fractures (18, 19), conditions that contribute to increased morbidity and mortality. Previous studies have demonstrated associations of CRP with all-cause mortality (14, 20) and death from vascular and nonvascular diseases, including cancer and respiratory diseases (13, 21, 22).
To date, there have been no published studies on prospective associations of biomarkers related to interferon-γ-mediated inflammation and the kynurenine pathway with mortality risk in the general population. Our aim in this study was to evaluate levels of interferon-γ-mediated inflammatory markers (neopterin and KTR), tryptophan, and 6 kynurenines, in addition to CRP, as potential predictors of all-cause and cause-specific mortality in a large community-based cohort.
METHODS
Study population
Data were drawn from the Hordaland Health Study (HUSK) (23), in which baseline examinations were conducted in Hordaland County, western Norway, in 1998–1999 (http://husk.b.uib.no). The study population consisted of 7,051 men and women born during 1925–1927 and 1950–1951 who had previously (1992–1993) participated in the Hordaland Homocysteine Study (24). We excluded participants with missing blood measurements at baseline (n = 35) and with missing information on vital status (n = 1). The cohort for all-cause mortality comprised 7,015 participants (3,117 men and 3,898 women). For the analysis of cause-specific mortality, we further excluded participants who died during the follow-up period but had no information on cause of death (n = 5); this yielded 7,010 participants. The Regional Committee for Medical and Health Research Ethics in Western Norway approved the study protocol, and all participants gave signed informed consent at baseline.
Laboratory measurements
Nonfasting blood samples were collected at baseline. Aliquots of ethylenediaminetetraacetic acid plasma were frozen at −80°C until analyses. Plasma levels of neopterin, tryptophan, and 6 kynurenines (kynurenine, AA, KA, HK, HAA, and XA) were measured by means of liquid chromatography–tandem mass spectrometry (25). KTR was calculated as the ratio of the concentrations of kynurenine (in nmol) to the concentrations of tryptophan (in µmol). Plasma high-sensitivity CRP level was determined using an immuno-MALDI [matrix-assisted laser desorption/ionization] mass spectrometry method (26). For neopterin and the 6 kynurenines, limits of detection ranged from 0.5 nmol/L to 7 nmol/L, while the limit was 0.4 µmol/L for tryptophan. Within-day and between-day coefficients of variation were 3.0%–9.5% and 5.7%–16.9%, respectively. For CRP, the limit of detection was 0.1 mg/L, and within-day and between-day coefficients of variation were 3.3%–5.5% and 2.4%–7.0%, respectively. Serum creatinine was measured colorimetrically using the alkaline picrate method with reagents from Roche (Basel, Switzerland) (23). All biochemical analyses were performed at Bevital A/S (Bergen, Norway; www.bevital.no), except for serum creatinine, which was analyzed at Ullevål University Hospital (Oslo, Norway).
Follow-up and ascertainment of deaths
Follow-up began at baseline and ended at the date of death, emigration, or the end of follow-up period, whichever came first. Deaths were included in the analysis if they occurred between baseline and the end of the follow-up period. Information on all-cause mortality was collected by linkage with Statistics Norway (through the year 2012) and information on cause-specific mortality by linkage with the Cause of Death Registry (through the year 2011). Primary cause of death was coded according to the 2010 version of the International Classification of Diseases, Tenth Revision (ICD-10). Deaths due to cancer (ICD-10 codes C00–C97), CVD (ICD-10 codes I00–I99), all other diseases, and external causes (ICD-10 codes V01–Y98) were combined.
Other baseline variables
Sociodemographic covariates included sex and age. Data on lifestyle measures, including smoking (never, former, or current smoker) and physical activity (none/light or moderate/vigorous), were collected by self-administered questionnaires. Body mass index was calculated as weight in kilograms divided by height in meters squared and was used as a continuous covariate. Calculation of estimated glomerular filtration rate (eGFR), which reflects renal function, was based on serum creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration equation (27).
Information on history of cancer (diagnosed with cancer before baseline, other than nonmelanoma skin cancer) was obtained through linkage with the Cancer Registry of Norway. Information on CVD (myocardial infarction, angina, and stroke) at baseline was self-reported. Participants were considered to have diabetes at baseline if they reported use of hypoglycemic medication or had a blood glucose level ≥126 mg/dL (time since last meal ≥8 hours) or ≥200 mg/dL (time since last meal <8 hours) (28). The numbers of participants with histories of cancer, CVD, and diabetes at baseline were 422, 719, and 192, respectively. A total of 1,189 participants had a history of morbidity, including cancer, CVD, or diabetes.
Statistical analysis
Analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, North Carolina) and R, version 3.0.3 for Windows (cmprsk and survival package; R Foundation for Statistical Computing, Vienna, Austria (www.r-project.org)). Log transformation was applied to all plasma biomarkers to normalize their distributions. Summary statistics for continuous variables are reported as median values (with interquartile ranges) and categorical variables as percentages. Differences between decedents and those remaining alive during follow-up were compared using χ2 tests for categorical variables and Wilcoxon-Mann-Whitney tests for continuous variables. Pearson correlation coefficients were calculated to examine the relationships of plasma CRP with tryptophan and kynurenines. Other relationships between the biomarkers have been previously reported (11, 29).
For the analysis of all-cause mortality, we used Cox proportional hazards models to calculate hazard ratios and 95% confidence intervals. The models were tested and plotted based on scaled Schoenfeld residuals (30) to ensure that assumptions of proportional hazards were not violated. Plasma measures were analyzed both as sex-specific quartiles and on continuous scales (per standard-deviation increment after log transformation). Associations between exposures and risk of all-cause mortality were visualized using generalized additive model regression plots. For the analysis of cause-specific mortality, we used competing-risk models to account for competing events (31, 32). Multivariate models included adjustment for sex, age group (46–49 years vs. 70–74 years), body mass index (continuous), smoking (never, former, or current smoker), and eGFR (continuous). Addition of interaction terms for interaction between sex and all exposures in models of mortality risk suggested no differences in predictor-outcome associations between the sexes (P for interaction > 0.05). Therefore, we combined men and women in the analyses.
Sensitivity analyses were conducted to assess the robustness of our results. We restricted the analyses to those participants who had no history of cancer, CVD, or diabetes at baseline. Moreover, we replicated the analyses by excluding the first 5 years of follow-up to eliminate the possible contribution of patients with preexisting disease that was fatal within the first 5 years of follow-up. All tests were 2-sided, and a P value less than 0.05 was considered statistically significant.
RESULTS
Population characteristics at baseline
Among the 7,015 participants, 1,496 deaths (817 men and 679 women) were recorded after a median follow-up time of 13.9 years (range, 0.1–14.7 years). Baseline characteristics of participants by vital status are shown in Table 1. A higher proportion of those who died during follow-up were male, and they were older at baseline (P < 0.001). In addition, decedents had a higher baseline body mass index and decreased renal function as estimated by eGFR. A significantly higher percentage of decedents were former smokers and engaged in less physical activity. Moreover, they were more likely to report a baseline history of cancer, CVD, or diabetes. Decedents had higher levels of inflammatory markers and kynurenines (other than XA, which was lower) and lower levels of tryptophan at baseline than their counterparts (P < 0.001).
Table 1.
Baseline Characteristics of Participants After a Median of 14 Years of Follow-up, by Vital Status, Hordaland Health Study, 1998–2012
| Characteristic | Vital Status |
P Valuea | |||||
|---|---|---|---|---|---|---|---|
| Overall (n = 7,015) |
Alive (n = 5,519) |
Deceased (n = 1,496) |
|||||
| % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | ||
| Age group, years | |||||||
| 46–49 | 52.8 | 64.5 | 9.5 | <0.001 | |||
| 70–74 | 47.2 | 35.5 | 90.5 | ||||
| Male sex | 44.4 | 41.7 | 54.6 | <0.001 | |||
| Duration of follow-up, years | 13.9 (13.6, 14.2) | 14.1 (13.8, 14.3) | 9.3 (5.7, 11.8) | <0.001 | |||
| Body mass indexb | 25.4 (23.1, 27.9) | 25.3 (23.1, 27.8) | 25.7 (23.2, 28.4) | 0.004 | |||
| Smoking status | |||||||
| Never smoker | 40.7 | 42.6 | 33.4 | <0.001 | |||
| Former smoker | 33.7 | 31.5 | 41.7 | ||||
| Current smoker | 25.6 | 25.9 | 24.9 | ||||
| Physical activity level | |||||||
| None/light | 43.7 | 39.2 | 61.1 | <0.001 | |||
| Moderate/vigorous | 56.3 | 60.8 | 38.9 | ||||
| eGFR, mL/minute/1.73 m2 | 79.9 (68.9, 89.7) | 82.2 (71.6, 92.0) | 70.9 (62.9, 81.0) | <0.001 | |||
| Prevalent disease at baseline | |||||||
| Cancerc | 6.0 | 4.1 | 13.0 | <0.001 | |||
| Cardiovascular disease | 10.3 | 6.4 | 24.5 | <0.001 | |||
| Diabetes | 2.7 | 1.4 | 7.6 | <0.001 | |||
| Inflammatory markers | |||||||
| Neopterin, nmol/L | 7.63 (6.37, 9.32) | 7.37 (6.21, 8.90) | 8.77 (7.26, 11.1) | <0.001 | |||
| Kynurenine:tryptophan ratio, nmol/µmol | 22.6 (18.5, 28.0) | 21.6 (18.0, 26.5) | 26.6 (21.8, 32.5) | <0.001 | |||
| C-reactive protein, mg/L | 1.59 (0.69, 3.62) | 1.40 (0.60, 3.24) | 2.28 (1.13, 5.27) | <0.001 | |||
| Kynurenine pathway metabolites | |||||||
| Tryptophan, µmol/L | 67.0 (58.3, 76.6) | 67.8 (58.9, 77.3) | 64.3 (55.8, 74.2) | <0.001 | |||
| Kynurenine, µmol/L | 1.52 (1.27, 1.83) | 1.48 (1.24, 1.76) | 1.72 (1.44, 2.05) | <0.001 | |||
| Anthranilic acid, nmol/L | 14.2 (11.4, 18.0) | 13.8 (11.1, 17.2) | 16.2 (12.7, 20.4) | <0.001 | |||
| Kynurenic acid, nmol/L | 45.7 (35.9, 58.5) | 44.4 (35.4, 56.3) | 50.9 (38.5, 67.6) | <0.001 | |||
| 3-Hydroxykynurenine, nmol/L | 32.2 (25.7, 40.3) | 31.4 (25.0, 38.7) | 36.2 (29.2, 45.7) | <0.001 | |||
| 3-Hydroxyanthranilic acid, nmol/L | 33.4 (26.1, 42.8) | 33.2 (26.1, 42.3) | 34.4 (26.3, 45.0) | 0.002 | |||
| Xanthurenic acid, nmol/L | 15.6 (11.3, 21.3) | 15.8 (11.5, 21.3) | 15.1 (10.5, 21.2) | <0.001 | |||
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range.
a Difference between the alive and deceased groups.
b Weight (kg)/height (m)2.
c Diagnosed cancers other than nonmelanoma skin cancer.
Correlations of CRP with other biomarkers
The inflammatory markers CRP, KTR, and neopterin were positively intercorrelated, as reported previously (11). Plasma CRP was positively correlated with kynurenine (r =0.17, P < 0.01), HK (r = 0.15, P < 0.01), and HAA (r = 0.11, P < 0.01) and negatively correlated with XA and tryptophan (r = −0.04 and P < 0.01 for both catabolites) in age- and sex-adjusted analyses.
Mortality risk
As shown in Table 2, the 3 inflammatory markers (neopterin, KTR, and CRP), kynurenine, AA, and HK were positively associated with risk of all-cause mortality both in models adjusted for sex and age only and in fully adjusted models (with further adjustment for body mass index, smoking, and eGFR). Tryptophan and XA were inversely associated with mortality risk. There were no linear associations of KA and HAA with risk of all-cause mortality. Results derived using quartiles and continuous scales were consistent for all of the exposures. After additional adjustment for physical activity (none/light and moderate/vigorous) or plasma CRP level, these associations remained essentially unchanged. Associations between kynurenines and all-cause mortality were attenuated by additional adjustment for neopterin (Appendix Table 1). There were no significant interactions with age for the majority of these markers, except KTR (P for interaction =0.05), kynurenine (P for interaction = 0.03), and HAA (P for interaction = 0.01). Age-stratified analyses showed that the risk estimates for the 3 markers were somewhat higher in the younger age group than in the older one.
Table 2.
Hazard Ratios for All-Cause Mortality According to Levels of Inflammatory Markers and Kynurenine Pathway Metabolites (n = 7,015), Hordaland Health Study, 1998–2012
| Biomarker and Model | Quartile of Biomarker Level |
P for Trend | Continuousa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Neopterin, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 1.07 | 0.88, 1.29 | 1.14 | 0.95, 1.37 | 1.54 | 1.29, 1.83 | <0.001 | 1.27 | 1.20, 1.33 |
| Multivariate-adjustedb | 1.00 | Referent | 1.09 | 0.90, 1.32 | 1.14 | 0.95, 1.38 | 1.55 | 1.29, 1.86 | <0.001 | 1.28 | 1.21, 1.36 |
| Kynurenine:tryptophan ratio, nmol/µmol | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 1.16 | 0.95, 1.42 | 1.34 | 1.11, 1.61 | 1.60 | 1.34, 1.92 | <0.001 | 1.22 | 1.16, 1.29 |
| Multivariate-adjusted | 1.00 | Referent | 1.14 | 0.93, 1.39 | 1.34 | 1.11, 1.63 | 1.60 | 1.32, 1.94 | <0.001 | 1.23 | 1.16, 1.31 |
| C-reactive protein, mg/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 1.27 | 1.05, 1.52 | 1.30 | 1.09, 1.56 | 1.60 | 1.34, 1.90 | <0.001 | 1.22 | 1.15, 1.30 |
| Multivariate-adjusted | 1.00 | Referent | 1.25 | 1.04, 1.50 | 1.22 | 1.02, 1.47 | 1.49 | 1.24, 1.78 | <0.001 | 1.18 | 1.11, 1.25 |
| Tryptophan, µmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.90 | 0.79, 1.03 | 0.80 | 0.70, 0.92 | 0.70 | 0.60, 0.81 | <0.001 | 0.85 | 0.81, 0.90 |
| Multivariate-adjusted | 1.00 | Referent | 0.93 | 0.81, 1.06 | 0.85 | 0.74, 0.99 | 0.73 | 0.63, 0.85 | <0.001 | 0.87 | 0.83, 0.92 |
| Kynurenine, µmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.86 | 0.72, 1.03 | 0.87 | 0.73, 1.02 | 1.10 | 0.94, 1.29 | 0.027 | 1.09 | 1.03, 1.16 |
| Multivariate-adjusted | 1.00 | Referent | 0.92 | 0.76, 1.10 | 0.93 | 0.78, 1.10 | 1.18 | 0.99, 1.41 | 0.011 | 1.11 | 1.04, 1.18 |
| Anthranilic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.90 | 0.75, 1.07 | 0.91 | 0.77, 1.07 | 1.05 | 0.90, 1.23 | 0.180 | 1.05 | 1.00, 1.11 |
| Multivariate-adjusted | 1.00 | Referent | 0.95 | 0.80, 1.14 | 1.02 | 0.86, 1.21 | 1.19 | 1.01, 1.41 | 0.006 | 1.10 | 1.04, 1.17 |
| Kynurenic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.74 | 0.63, 0.87 | 0.72 | 0.62, 0.84 | 0.90 | 0.78, 1.03 | 0.460 | 1.00 | 0.95, 1.05 |
| Multivariate-adjusted | 1.00 | Referent | 0.80 | 0.68, 0.94 | 0.76 | 0.64, 0.89 | 0.89 | 0.76, 1.05 | 0.318 | 0.99 | 0.93, 1.06 |
| 3-Hydroxykynurenine, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.97 | 0.81, 1.15 | 0.98 | 0.83, 1.15 | 1.28 | 1.10, 1.49 | <0.001 | 1.18 | 1.12, 1.24 |
| Multivariate-adjusted | 1.00 | Referent | 1.01 | 0.85, 1.20 | 1.01 | 0.85, 1.19 | 1.28 | 1.08, 1.51 | 0.001 | 1.18 | 1.11, 1.25 |
| 3-Hydroxyanthranilic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.91 | 0.78, 1.05 | 0.85 | 0.73, 0.98 | 0.98 | 0.86, 1.13 | 0.684 | 0.98 | 0.93, 1.03 |
| Multivariate-adjusted | 1.00 | Referent | 0.96 | 0.83, 1.12 | 0.88 | 0.76, 1.03 | 1.07 | 0.93, 1.24 | 0.539 | 1.00 | 0.95, 1.06 |
| Xanthurenic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.83 | 0.72, 0.95 | 0.81 | 0.70, 0.93 | 0.86 | 0.74, 0.98 | 0.021 | 0.89 | 0.85, 0.94 |
| Multivariate-adjusted | 1.00 | Referent | 0.86 | 0.75, 0.99 | 0.83 | 0.72, 0.96 | 0.87 | 0.75, 1.01 | 0.041 | 0.89 | 0.85, 0.94 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a HR (and 95% CI) per standard-deviation increment of log-transformed exposure.
b The multivariate-adjusted model adjusted for sex, age group (46–49 years vs. 70–74 years), body mass index (weight (kg)/height (m)2; continuous), smoking (never, former, or current smoker), and estimated glomerular filtration rate (continuous).
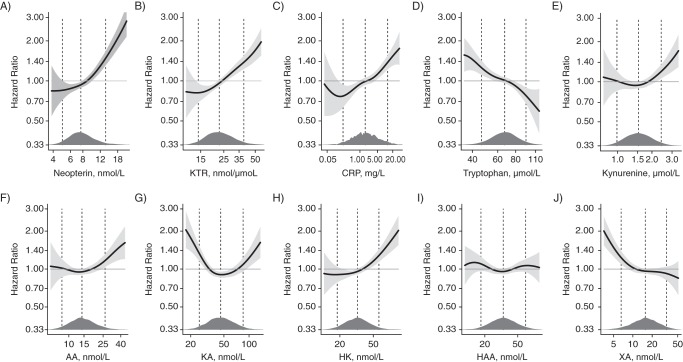
Nonlinear associations were explored by means of a generalized additive model, which showed positive associations with mortality in the upper ranges of neopterin, KTR, CRP, kynurenine, AA, and HK, inverse associations for tryptophan and in the lower range of XA, and a U-shaped association for KA (Figure 2).
Figure 2.
Associations of 10 biomarkers (neopterin, kynurenine:tryptophan ratio (KTR), C-reactive protein (CRP), tryptophan, kynurenine, anthranilic acid (AA), kynurenic acid (KA), 3-hydroxykynurenine (HK), 3-hydroxyanthranilic acid (HAA), and xanthurenic acid (XA)) with all-cause mortality in the Hordaland Health Study, 1998–2012. Models were constructed by using generalized additive model regression with adjustment for sex, age, body mass index (weight (kg)/height (m)2), smoking, physical activity, and renal function (estimated glomerular filtration rate). The solid lines show hazard ratios and the shaded areas 95% confidence intervals. Density plots indicate the distributions of the biomarkers, and dashed lines denote the 5th, 50th, and 95th percentiles. All exposure data were log-transformed before being entered into the models.
In sensitivity analyses, restricting the study population to 5,826 participants with no history of cancer, CVD, or diabetes at baseline gave essentially the same associations as for the entire cohort, except for kynurenine, which had no significant association with risk of all-cause mortality (data not shown). In addition, repeating the analyses after excluding those who died during the first 5 years of follow-up (n = 334) yielded results similar to those reported in Table 2 (data not shown).
Cause-specific mortality analyses (Table 3) showed that all markers except KA and HAA were related to CVD death. Neopterin, KTR, CRP, tryptophan, and HK were also related to death from other diseases, whereas only KTR, tryptophan, and XA were related to death from cancer. Thus, it appears that plasma inflammatory markers and kynurenines were more associated with CVD mortality than with cancer mortality or mortality due to other diseases.
Table 3.
Multivariate-Adjusteda Hazard Ratiosb for Cause-Specific Mortality According to Levels of Inflammatory Markers and Kynurenine Pathway Metabolites (n = 7,010), Hordaland Health Study, 1998–2011
| Biomarker | Cause of Death |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cancer (n = 465) |
CVD (n = 385) |
Other Diseases (n = 410) |
External Causes (n = 50)c |
|||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Neopterin, nmol/L | 1.10 | 0.98, 1.24 | 1.32 | 1.19, 1.47 | 1.23 | 1.10, 1.36 | 1.16 | 0.90, 1.49 |
| Kynurenine:tryptophan ratio, nmol/µmol | 1.20 | 1.07, 1.34 | 1.22 | 1.09, 1.37 | 1.19 | 1.07, 1.33 | 1.00 | 0.70, 1.42 |
| C-reactive protein, mg/L | 1.01 | 0.90, 1.13 | 1.38 | 1.21, 1.57 | 1.16 | 1.01, 1.33 | 0.92 | 0.69, 1.21 |
| Tryptophan, µmol/L | 0.90 | 0.82, 0.98 | 0.90 | 0.81, 0.99 | 0.85 | 0.77, 0.93 | 1.11 | 0.80, 1.52 |
| Kynurenine, µmol/L | 1.10 | 0.98, 1.24 | 1.14 | 1.00, 1.30 | 1.03 | 0.91, 1.17 | 1.15 | 0.76, 1.73 |
| Anthranilic acid, nmol/L | 1.07 | 0.96, 1.19 | 1.14 | 1.02, 1.28 | 1.05 | 0.93, 1.18 | 1.14 | 0.84, 1.55 |
| Kynurenic acid, nmol/L | 0.90 | 0.80, 1.01 | 1.08 | 0.96, 1.23 | 1.02 | 0.89, 1.17 | 0.95 | 0.66, 1.35 |
| 3-Hydroxykynurenine, nmol/L | 1.02 | 0.92, 1.12 | 1.20 | 1.05, 1.32 | 1.24 | 1.10, 1.40 | 0.98 | 0.71, 1.35 |
| 3-Hydroxyanthranilic acid, nmol/L | 1.06 | 0.96, 1.16 | 0.96 | 0.87, 1.06 | 1.00 | 0.90, 1.11 | 0.96 | 0.69, 1.33 |
| Xanthurenic acid, nmol/L | 0.90 | 0.82, 0.98 | 0.88 | 0.79, 0.98 | 0.97 | 0.87, 1.08 | 0.89 | 0.60, 1.33 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
a Adjusted for sex, age group (46–49 years vs. 70–74 years), body mass index (weight (kg)/height (m)2; continuous), smoking (never, former, or current smoker), and estimated glomerular filtration rate (continuous).
b HR (and 95% CI) per standard-deviation increment of log-transformed exposure, obtained by means of competing-risk models.
c Deaths due to accidents, violence, poisoning, suicide, etc.
When participants with a history of cancer, CVD, or diabetes at baseline were excluded from the analysis, risk estimates were essentially unchanged for cancer mortality and mortality due to other diseases. For CVD mortality, however, the associations remained significant for neopterin and CRP, whereas the associations were no longer significant for all other metabolites (data not shown).
DISCUSSION
Principal findings
In the present study, we identified plasma inflammatory markers (neopterin, KTR, and CRP), kynurenine, AA, HK, tryptophan (inversely), and XA (inversely) as independent predictors of all-cause mortality in a general population during a median follow-up period of 14 years. These associations persisted in analyses in which participants with baseline cancer, CVD, or diabetes mellitus were excluded or the first 5 years of follow-up were excluded. KTR and tryptophan were significantly associated with death due to cancer, CVD, or other diseases. CVD mortality correlated with the highest number of biomarkers (all except KA and HAA).
Inflammatory markers, the kynurenine pathway, and mortality
In this large-scale cohort of community-dwelling middle-aged and older adults, we found that neopterin and KTR were associated with all-cause mortality, with risk estimates similar to those for patients with specific conditions (6–9). Such associations have not been previously reported in the general population. Our findings on CRP also resemble results previously reported (13, 14, 20). The 2 interferon-γ-mediated inflammatory markers appeared to be better than the nonspecific inflammatory marker CRP for the prediction of non-CVD mortality, whereas CRP seemed slightly better for the prediction of CVD mortality. However, no strong conclusion can be made on the basis of the presented data. Other than for KTR, associations of tryptophan and individual kynurenines with mortality risk have not previously been reported.
Possible mechanisms
Systemic inflammation is involved in the pathophysiology of a large number of chronic conditions, including CVD (33), cancer (34, 35), and diabetes mellitus (16). Notably, all of the 10 predictors except HAA were significantly associated with CVD mortality as compared with non-CVD mortality. This indicates that inflammation may be more important for CVD-related pathogenesis and death than for that related to other conditions. This is in line with the current knowledge of atherosclerosis, the major underlying cause of CVD, as a chronic inflammatory process in which immune mechanisms interact with metabolic risk factors to initiate, propagate, and activate arterial lesions (6, 36, 37).
Positive associations of neopterin and KTR with mortality risk indicate a role of interferon-γ activity and T-helper 1-type cellular immune activation (12). Evidence suggests a role of interferon-γ in CVD pathogenesis (6). We previously reported that neopterin is associated with cancer risk (11), and we observed a similar magnitude for the association with cancer mortality in this study, although the finding was nonsignificant, probably because of fewer events.
Activation of the kynurenine pathway has been involved in the pathophysiology of chronic disorders (5, 38). We observed associations of both tryptophan (inverse) and kynurenine (positive) with all types of deaths due to diseases, albeit weaker than the associations for KTR. Tryptophan depletion via indoleamine 2,3-dioxygenase is part of the cytostatic and antiproliferative activity mediated by interferon-γ in monocyte-derived macrophages and dendritic cells (2, 12). In states of persistent immune activation, however, a low tryptophan concentration is likely to decrease the response of stimulated T-cells and contribute to immunosuppression and immunodeficiency (5, 12).
XA was inversely associated with mortality risk, especially deaths due to cancer or CVD. The biological function of XA remains obscure. However, XA has been shown to be a powerful antioxidant due to its metal-chelating activity, and it also serves as a peroxyl radical scavenger (39).
Although AA is generally accepted to be biologically inert (39), we observed a weak positive association with CVD mortality. HK, which has been reported to be positively related to several inflammatory markers (29, 40), had the strongest association with CVD mortality and noncancer mortality among all of the individual kynurenines in the present study. HK has also been shown to be inversely related to plasma pyridoxal 5′-phosphate (vitamin B6), levels of which are decreased in persons with CVD (41). In contrast, we observed no association of HAA with mortality risk, although HAA has been shown to have beneficial effects on lipid metabolism, inflammation, and atherosclerosis in recent experimental studies (42, 43). Controversial findings have been reported on pro- and antioxidant properties of HK and HAA (38). Similarly, the U-shaped association of KA with mortality in our study may be due to its combined pro- and antioxidant properties (39, 44, 45).
Strengths and limitations
To our knowledge, this is the first study to have prospectively assessed the risks of all-cause and cause-specific mortality associated with interferon-γ-mediated inflammatory markers and metabolites of the kynurenine pathway in a general population sample. The main strengths of this study include the large sample size and the long and nearly complete follow-up. In addition, all main biomarker analyses were performed at the same laboratory. The study included 10 different markers, which facilitated an extensive exploration of exposure-outcome associations. Most biomarkers are stable in stored plasma samples, and all have fair-to-good within-person reproducibility (46) that allows single-time-point measurement of biomarker status and suggests moderate regression dilution bias. Since all-cause mortality is a nonspecific endpoint of disease risk and prognosis, we additionally included cause-specific mortality to aid the discussion of mechanisms. However, our study had several limitations. Information on CVD and diabetes at baseline may have been subject to misclassification due to the reliance on self-reporting. Another limitation was the adjustment for kidney function by eGFR, an imperfect measure. Thus, we cannot rule out the possibility of residual confounding. In addition, the study cohort was from a small geographical area representing 2 narrow age groups, which may limit the generalizability of the findings.
Conclusions
Our study showed that interferon-γ-mediated inflammatory markers (neopterin and KTR), tryptophan, and several kynurenines, in addition to CRP, were associated with all-cause and cause-specific mortality in a large community-based population. Notably, interferon-γ-mediated inflammation and activation of the kynurenine pathway seem to have a stronger relationship with CVD mortality than with mortality due to cancer or other causes, possibly highlighting the role of systemic inflammation and immune system activation in the development of CVD.
ACKNOWLEDGMENTS
Author affiliations: Department of Global Public Health and Primary Care, Faculty of Medicine and Dentistry, University of Bergen, Bergen, Norway (Hui Zuo, Simone J. P. M. Eussen, Stein E. Vollset, Despoina Theofylaktopoulou, Grethe S. Tell); Department of Clinical Science, Faculty of Medicine and Dentistry, University of Bergen, Bergen, Norway (Hui Zuo, Per M. Ueland, Simone J. P. M. Eussen, Ottar Nygård, Despoina Theofylaktopoulou); Laboratory of Clinical Biochemistry, Haukeland University Hospital, Bergen, Norway (Per M. Ueland); Department of Heart Disease, Haukeland University Hospital, Bergen, Norway (Ottar Nygård); Bevital A/S, Bergen, Norway (Arve Ulvik, Øivind Midttun, Klaus Meyer); Department of Epidemiology, School for Public Health and Primary Care, Maastricht University, Maastricht, the Netherlands (Simone J. P. M. Eussen); and Division of Epidemiology, Norwegian Institute of Public Health, Bergen, Norway (Stein E. Vollset).
This work was supported by the Research Council of Norway (project 204650).
We thank the staff of Statistics Norway and the Cause of Death Registry for providing the outcome information on the study participants.
Conflict of interest: none declared.
Appendix Table 1.
Hazard Ratios for All-Cause Mortality According to Quartile of Kynurenine Concentration (Additionally Adjusted for Neopterin Level) Among 7,015 Participants in the Hordaland Health Study, 1998–2012
| Kynurenine | Quartile of Kynurenine Concentration |
P for Trend | Continuousa |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Kynurenine, µmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.86 | 0.72, 1.03 | 0.87 | 0.73, 1.02 | 1.10 | 0.94, 1.29 | 0.027 | 1.09 | 1.03, 1.16 |
| Multivariate-adjustedb | 1.00 | Referent | 0.88 | 0.73, 1.06 | 0.86 | 0.72, 1.02 | 0.98 | 0.82, 1.18 | 0.813 | 1.01 | 0.95, 1.08 |
| Anthranilic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.90 | 0.75, 1.07 | 0.91 | 0.77, 1.07 | 1.05 | 0.90, 1.23 | 0.180 | 1.05 | 1.00, 1.11 |
| Multivariate-adjusted | 1.00 | Referent | 0.92 | 0.77, 1.10 | 0.98 | 0.82, 1.16 | 1.07 | 0.90, 1.27 | 0.197 | 1.05 | 0.99, 1.11 |
| Kynurenic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.74 | 0.63, 0.87 | 0.72 | 0.62, 0.84 | 0.90 | 0.78, 1.03 | 0.460 | 1.00 | 0.95, 1.05 |
| Multivariate-adjusted | 1.00 | Referent | 0.79 | 0.67, 0.94 | 0.74 | 0.63, 0.87 | 0.83 | 0.71, 0.98 | 0.048 | 0.96 | 0.90, 1.02 |
| 3-Hydroxykynurenine, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.97 | 0.81, 1.15 | 0.98 | 0.83, 1.15 | 1.28 | 1.10, 1.49 | <0.001 | 1.18 | 1.12, 1.24 |
| Multivariate-adjusted | 1.00 | Referent | 0.98 | 0.82, 1.16 | 0.95 | 0.80, 1.12 | 1.12 | 0.94, 1.32 | 0.157 | 1.10 | 1.04, 1.17 |
| 3-Hydroxyanthranilic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.91 | 0.78, 1.05 | 0.85 | 0.73, 0.98 | 0.98 | 0.86, 1.13 | 0.684 | 0.98 | 0.93, 1.03 |
| Multivariate-adjusted | 1.00 | Referent | 0.94 | 0.81, 1.10 | 0.85 | 0.73, 0.99 | 1.02 | 0.88, 1.18 | 0.918 | 0.99 | 0.94, 1.04 |
| Xanthurenic acid, nmol/L | |||||||||||
| Sex- and age-adjusted | 1.00 | Referent | 0.83 | 0.72, 0.95 | 0.81 | 0.70, 0.93 | 0.86 | 0.74, 0.98 | 0.021 | 0.89 | 0.85, 0.94 |
| Multivariate-adjusted | 1.00 | Referent | 0.90 | 0.78, 1.04 | 0.87 | 0.75, 1.00 | 0.90 | 0.77, 1.04 | 0.120 | 0.91 | 0.86, 0.96 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a HR (and 95% CI) per standard-deviation increment of log-transformed exposure.
b The multivariate-adjusted model adjusted for sex, age group (46–49 years vs. 70–74 years), body mass index (weight (kg)/height (m)2; continuous), smoking (never, former, or current smoker), estimated glomerular filtration rate (continuous), and neopterin (log-transformed).
REFERENCES
- 1.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;3396116:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson CM, Hale PT, Carlin JM. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J Interferon Cytokine Res. 2005;251:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vécsei L, Szalárdy L, Fülöp F et al. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;121:64–82. [DOI] [PubMed] [Google Scholar]
- 4.Zinger A, Barcia C, Herrero MT et al. The involvement of neuroinflammation and kynurenine pathway in Parkinson's disease. Parkinsons Dis. 2011;2011:716859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen ER, Midttun Ø, Ueland PM et al. Systemic markers of interferon-γ-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;313:698–704. [DOI] [PubMed] [Google Scholar]
- 7.Pihlstrøm H, Mjøen G, März W et al. Neopterin is associated with cardiovascular events and all-cause mortality in renal transplant patients. Clin Transplant. 2014;281:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen ER, Svingen GF, Schartum-Hansen H et al. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J. 2013;3434:2689–2696. [DOI] [PubMed] [Google Scholar]
- 9.Byakwaga H, Boum Y 2nd, Huang Y et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;2103:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulo G, Vollset SE, Nygård O et al. Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int J Cardiol. 2013;1682:1435–1440. [DOI] [PubMed] [Google Scholar]
- 11.Zuo H, Tell GS, Vollset SE et al. Interferon-γ-induced inflammatory markers and the risk of cancer: the Hordaland Health Study. Cancer. 2014;12021:3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schröcksnadel K, Wirleitner B, Winkler C et al. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364(1-2):82–90. [DOI] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors Collaboration; Kaptoge S, Di Angelantonio E et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;3759709:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kengne AP, Batty GD, Hamer M et al. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care. 2012;352:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo YZ, Pan L, Du CJ et al. Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev. 2013;141:243–248. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Bao W, Liu J et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;361:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CC, Adler AI, Sandhu MS et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia. 2009;526:1040–1047. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson AL, Movérare-Skrtic S, Ljunggren Ö et al. High-sensitivity CRP is an independent risk factor for all fractures and vertebral fractures in elderly men: the MrOS Sweden study. J Bone Miner Res. 2014;292:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii S, Cauley JA, Greendale GA et al. C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res. 2013;287:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and all-cause mortality—the Copenhagen City Heart Study. Eur Heart J. 2010;3113:1624–1632. [DOI] [PubMed] [Google Scholar]
- 21.Ko YJ, Kwon YM, Kim KH et al. High-sensitivity C-reactive protein levels and cancer mortality. Cancer Epidemiol Biomarkers Prev. 2012;2111:2076–2086. [DOI] [PubMed] [Google Scholar]
- 22.Man SF, Connett JE, Anthonisen NR et al. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;6110:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vikse BE, Vollset SE, Tell GS et al. Distribution and determinants of serum creatinine in the general population: the Hordaland Health Study. Scand J Clin Lab Invest. 2004;648:709–722. [DOI] [PubMed] [Google Scholar]
- 24.Refsum H, Nurk E, Smith AD et al. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136(6 suppl):1731S–1740S. [DOI] [PubMed] [Google Scholar]
- 25.Midttun Ø, Hustad S, Ueland PM. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;239:1371–1379. [DOI] [PubMed] [Google Scholar]
- 26.Meyer K, Ueland PM. Targeted quantification of C-reactive protein and cystatin c and its variants by immuno-MALDI-MS. Anal Chem. 2014;8612:5807–5814. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;1509:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 29.Theofylaktopoulou D, Midttun Ø, Ulvik A et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;1731:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;1415:1707–1723. [DOI] [PubMed] [Google Scholar]
- 31.Andersen PK, Geskus RB, de Witte T et al. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;413:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satagopan JM, Ben-Porat L, Berwick M et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;917:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;832:456S–460S. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;1406:883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elinav E, Nowarski R, Thaiss CA et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;1311:759–771. [DOI] [PubMed] [Google Scholar]
- 36.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;35216:1685–1695. [DOI] [PubMed] [Google Scholar]
- 37.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leipnitz G, Schumacher C, Dalcin KB et al. In vitro evidence for an antioxidant role of 3-hydroxykynurenine and 3-hydroxyanthranilic acid in the brain. Neurochem Int. 2007;501:83–94. [DOI] [PubMed] [Google Scholar]
- 39.Reyes Ocampo J, Lugo Huitrón R, González-Esquivel D et al. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid Med Cell Longev. 2014;2014:646909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midttun O, Ulvik A, Ringdal Pedersen E et al. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr. 2011;1414:611–617. [DOI] [PubMed] [Google Scholar]
- 41.Paul L, Ueland PM, Selhub J. Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr Rev. 2013;714:239–244. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Ovchinnikova O, Jönsson A et al. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J. 2012;3316:2025–2034. [DOI] [PubMed] [Google Scholar]
- 43.Krause D, Suh HS, Tarassishin L et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: role of hemeoxygenase-1. Am J Pathol. 2011;1793:1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone TW, Forrest CM, Darlington LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;2798:1386–1397. [DOI] [PubMed] [Google Scholar]
- 45.Sas K, Robotka H, Toldi J et al. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257(1-2):221–239. [DOI] [PubMed] [Google Scholar]
- 46.Midttun O, Townsend MK, Nygård O et al. Most blood biomarkers related to vitamin status, one-carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within-person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J Nutr. 2014;1445:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]