Abstract
The E. coli flagellar types H1 and H12 show a high serological cross-reactivity and molecular serotyping appears an advantageous method to establish a clear discrimination between these flagellar types. Analysis of fliCH1 and fliCH12 gene sequences showed that they were 97.5% identical at the nucleotide level. Because of this high degree of homology we developed a two-step real-time PCR detection procedure for reliable discrimination of H1 and H12 flagellar types in E. coli. In the first step, a real-time PCR assay for common detection of both fliCH1 and fliCH12 genes is used, followed in a second step by real-time PCR assays for specific detection of fliCH1 and fliCH12, respectively. The real-time PCR for common detection of fliCH1 and fliCH12 demonstrated 100% sensitivity and specificity as it reacted with all tested E. coli H1 and H12 strains and not with any of the reference strains encoding all the other 51 flagellar antigens. The fliCH1 and fliCH12 gene specific assays detected all E. coli H1 and all E. coli H12 strains, respectively (100% sensitivity). However, both assays showed cross-reactions with some flagellar type reference strains different from H1 and H12. The real-time PCR assays developed in this study can be used in combination for the detection and identification of E. coli H1 and H12 strains isolated from different sources.
Keywords: E. coli, molecular serotyping, fliC type H1 gene, fliC type H12 gene, STEC, ExPEC
Introduction
Strains belonging to the species of Escherichia coli are ubiquitous as commensals in the gut of humans and warm-blooded animals. Apart from their role as beneficial microbes, some E. coli strains are known to behave as human and animal pathogens, causing a wide spectrum of extraintestinal and enteric diseases, with urinary tract infection and diarrhea as most frequent (Kaper et al., 2004; Stenutz et al., 2006). Pathogenic and apathogenic E. coli cannot be discerned from each other by their morphology, cultural properties or fermentation reactions. As a consequence, serotyping is used since the 1940s as a diagnostic tool for identification of animal and human pathogenic E. coli strains (Orskov and Orskov, 1984).
E. coli serogroups are commonly defined by the antigenic properties of the lipopolysaccharide which is part of the outer membrane (O-antigen) (Stenutz et al., 2006). Motile E. coli strains can be additionally typed for their flagellar filaments (H-antigen) (Orskov and Orskov, 1984). E. coli O- and H-antisera are usually produced by immunization of rabbits with respective reference strains (Orskov and Orskov, 1984; Edwards and Ewing, 1986). At present, 182 O-antigens and 53 H-antigens have been described (Scheutz et al., 2004; Scheutz and Strockbine, 2005). The resulting O:H serotype (for example O157:H7) is commonly used for describing E. coli isolates (Bettelheim, 1978; Orskov and Orskov, 1984).
Complete serotyping of E. coli is laborious and time-consuming and performed only in a few specialized reference laboratories worldwide. Moreover, cross-reactivity which is observed between some E. coli O-groups and H-types can complicate the interpretation of serotyping results. Last but not least, serotyping fails if autoagglutinating (O-antigen or H-antigen rough) and non-motile (NM) E. coli strains have to be examined (Orskov and Orskov, 1984; Edwards and Ewing, 1986). For these reasons, attempts were made to substitute serotyping by molecular typing of O-antigen and H-antigen encoding genes.
In the recent years, the nucleotide sequences of all known O and H-antigen genes in E. coli have been elucidated (Wang et al., 2003; Iguchi et al., 2015a). Molecular methods such as PCR and nucleotide sequencing have been successfully employed for typing of O- and H-antigen genes in E. coli (Beutin and Fach, 2014; Joensen et al., 2015; Iguchi et al., 2015b). Molecular serotyping was shown to be specific and sensitive and can substitute conventional serological detection of E. coli surface antigens (Bugarel et al., 2010; Fratamico et al., 2011; Clotilde et al., 2015; Iguchi et al., 2015b; Joensen et al., 2015). In contrast to serotyping, molecular detection of O- and H-antigen genes is easier and faster to perform and O-rough and non-motile strains can be typed on the basis of their O- and H-antigen genes (Beutin and Fach, 2014; Joensen et al., 2015).
We have previously investigated the genetic variability of flagellar types H19, H25 and H28 in E. coli (Beutin et al., 2015a,b). These flagellar types are widespread in strains belonging to numerous O-serogroups but are also associated with enterohemorrhagic E. coli O145:H25, O145:H28, and O121:H19 strains. By nucleotide sequence analysis of fliC (flagellin) genes encoding H19, H25, and H28 flagella we have observed a high genetic variability among fliCH19, flicH25, and fliCH28 alleles, respectively. To some part, this sequence alterations were associated with some O-groups of strains which allowed the development of real-time PCR protocols for specific typing of flagellar variants encoded by enterohemorrhagic E. coli O145:H25, O145:H28, and O121:H19 strains (Beutin et al., 2015a,b). Such real-time PCR protocols were found useful for improvement of horizontal real-time PCR detection methods for EHEC from food samples (Beutin et al., 2015a,b).
In this work, we compared E. coli fliC genes that encode flagellar types H1 and H12. These flagellar types show a high serological cross-reactivity and cross-absorbed H1 and H12 antisera are used for definite H-typing (Orskov and Orskov, 1984; Edwards and Ewing, 1986). Moreover, three subtypes of H1 were detected by serological typing using factor specific antisera (Ratiner et al., 1995). Serological cross reactions may cause confounding results in diagnostic laboratories where absorbed antisera are not available. The development of molecular typing procedures for reliable detection of H1 and H12 flagellar types could overcome this specific problem.
A clear discrimination between E. coli flagellar types H1 and H12 has a value for clinical diagnostics and for epidemiological investigations. Some human isolates of Shiga Toxin-producing E. coli (STEC) express H1 or H12 flagella (Scheutz and Strockbine, 2005). Moreover, flagellar type H1 is clinically significant as it is associated with worldwide occurring extraintestinal pathogenic E. coli (ExPEC) strains carrying capsular polysaccharides (O2:K2:H1, O4:K12:H1, O6:K2:H1, O6:K5:H1, O7:K1:H1, O15:K52:H1) that cause cystitis, pyelonephritis and urosepsis (Orskov and Orskov, 1985; Johnson et al., 1994, 2005, 2006; Olesen et al., 2009). Adherent-invasive E. coli (AIEC) O83:H1 strains were associated with Crohn's disease in human patients (Allen et al., 2008; Nash et al., 2010) and flagellar type H1 is associated with biofilm formation and invasive properties of AIEC strains (Eaves-Pyles et al., 2008; Martinez-Medina et al., 2009) as well as with intestinal colonization (Martinez-Medina and Garcia-Gil, 2014). Moreover, H1-type flagellum is a characteristic trait of Shiga toxin 2e-producing E. coli O139:H1 strains which are a major cause of edema disease in pigs (Tschape et al., 1992; Frydendahl, 2002; Fairbrother et al., 2005; Beutin et al., 2008). Conversely, the flagellar type H12 has not been associated with pathogenic E. coli, except from human enterotoxigenic O78:H12 and O128:H12 strains (Orskov and Orskov, 1977; Echeverria et al., 1982; Shaheen et al., 2004).
In this work we have analyzed the nucleotide sequences of E. coli H1 and H12 strains in order to detect characteristic fliC sequence alterations corresponding with these closely related H-types. Subsequently, we have developed a real-time PCR procedure for reliable discrimination of H1 and H12 flagellar types in E. coli. The protocol should be useful for diagnostic and epidemiological investigations of human and animal pathogenic strains of E. coli.
Materials and methods
Bacteria
E. coli strains used in this study were derived from the collections of the National Reference Laboratory for E. coli (NRL E. coli) at the Federal Institute for Risk Assessment (BfR) in Berlin, Germany and from the French Agency for Food, Environmental and Occupational Health and Safety (Anses) in Maisons-Alfort, France. E. coli strains used for specificity study included in particular the E. coli reference strains belonging to serogroups O1-O181 and H-types H1-H56 (Orskov and Orskov, 1984; Edwards and Ewing, 1986). All strains have been previously described for their serotypes and for virulence genes associated with STEC (Beutin et al., 2015a,b). All strains were grown overnight at 37°C in Luria broth, and DNA was extracted according to manufacturers instructions using InstaGene matrix (BioRad laboratories, Marnes-La-Coquette, France).
Real-time PCR assays were performed with an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA) in 25- μl reaction volumes, a LightCycler Nano (Roche Diagnostics, Meylan, France) in 10 μl reaction volumes or with a LightCycler 1536 (Roche Diagnostics, Meylan, France) in 1.5-μl reaction volumes according to the recommendations of the suppliers. Primers and TaqMan probes were used at 300 nM final concentrations. The following thermal profile was applied to all instruments: enzyme activation at 95°C for 1–10 min as recommended followed by 40 cycles of denaturation at 95°C and annealing at 60°C.
PCR detection and mapping of E. coli O-antigen and H-antigen genes
Mapping of fliC gene variants to their respective H-types was performed as previously described (Beutin et al., 2015a,b). Nucleotide sequence data obtained from thirteen fliCH1 and eight fliCH12 genes were used for designing TaqMan® real-time PCR probes and XS probes (minor groove binder replacement, Biolegio, Nijmegen, The Netherlands) and primers for specific detection of all genetic variants of thirteen fliCH1 and eight fliCH12 genes (this work). Real-time PCR probes and primers used in this work were designed with the software Primer Express V3.0 (Applied Biosystems) and are described in Table 1.
Table 1.
Primers and probes for real-time PCR detection of E. coli flagellar types H1 and H12.
| Target gene | Forward primer, reverse primer and probe sequences (5′–3 ′)a | Length and location within 21 sequences listed in Table 2 (5′ –3′) |
|---|---|---|
| fliCH1 | AGGACGAAATCAAATCCCGTCT | 338–359b |
| ACGGTTCGATGAAAATTCAGGTT | 422–444c | |
| [6FAM]- GACCGCGTATCC GGTCA-[BHQ1]a |
370–386d | |
| fliCH12 | TCCATTCAGGACGAAATCAAATC | 331–353b |
| CGTGAACGTACTGGCGAAAG | 402–421e | |
| [6FAM] -GTATCTGGCC AGACCCA-[BHQ1]a |
376–392f | |
| fliCH1∕H12 | TGATGGTGAAATGACTACAATTGGT | 1329–1353g |
| GGTAACTGTTGATTCTGGAACTGGT | 1395–1419g | |
| [6FAM]–CGAAGTATTCAATCG ATGCTAACAACGGCA–[BHQ1] |
1363–1393g |
XS probes (MGB-replacement) were used for fliCH1 and fliCH12 specific real-time PCRs.
Forward primer conserved in all analyzed fliCH1 and fliCH12 sequences.
FliCH1 reverse primer: one mismatch at position 429: fliCH1 = G, fliCH12 = A (underlined).
FliCH1 probe: one mismatch at position 381: fliCH1 = C, fliCH12 = T; and position 384: fliCH1 = T, fliCH12 = C (underlined).
FliCH12 reverse primer: one mismatch at position 402 with fliCH1 = T (4/13 strains), fliCH12 = C (underlined).
FliCH12probe: one mismatch at position 381: fliCH12 = T, fliCH1 = C; and position 384: fliCH12 = C, fliCH1 = T (underlined).
Conserved in all 21 fliCH1 and fliCH12 sequences from Table 2.
Nucleotide sequencing
The nucleotide sequence of the PCR products were determined as described (Beutin et al., 2015b) and analyzed with the Accelrys DS Gene software package (Accelrys Inc., USA). The nucleotide sequences of the respective products for fliC homologs were determined and have been submitted to European Nucleotide Archive (ENA). The GenBank Accession numbers are listed in Table 2.
Table 2.
Escherichia coli strains used for nucleotide sequencing of fliCH1 and fliCH12 genes and corresponding sequences obtained from GenBank.
| Strain | Serotype | fliC gene GenBank accession no | Pathotype | Source and References |
|---|---|---|---|---|
| CB11962 | O20:H12 | LN877748e | STEC | Calves feces, Germany, 2009, this work |
| CB13385 | O9:K9:H12 | LN877749e | No data | Chicken meat, Germany 2011, this work |
| Bi316-42 | O9:K9:H12 | AY249997 | ExPEC | Orskov and Orskov, 1984; Wang et al., 2003 |
| NX9861 | O157:H12 | AY337474 | No data | China, 2003, unpublished |
| 90 | O157:H12 | AY337471 | No data | China, 2003, unpublished |
| CB11070 | O1:H12 | LN877750e | No data | Pig feces, Germany, 2007, this work |
| CB12026 (07QMA185.1) | O153:H12 | LN877751e | STEC | Beef, France, 2009, this work |
| CB12530 | O55:H12 | LN877752e | STEC | Martin and Beutin, 2011 |
| Su1242 | O2:K2:H1 | AB028471 | ExPEC | Orskov and Orskov, 1984 |
| CFT073 | O6:H1 | AE014075 | ExPEC | Welch et al., 2002 |
| ABU83972 | O25:H1 | CP001671a | ExPEC | Zdziarski et al., 2010 |
| CB13658 | O6:H1 | LN877753e | No data | Pig intestine, Germany, 2011, this work |
| ATCC25922 | O6:H1 | CP009072 | Human, clinical | Minogue et al., 2014 |
| NRG 857C | O83:H1 | CP001855 | AIEC | Allen et al., 2008 |
| LF82 | O83:H1 | CU651637b | AIEC | Martinez-Medina et al., 2009 |
| Ec614 | O157:H1 | JF308285c | Beef, no data | Goulter et al., 2010 |
| CB13179 | O15:H1 | LN877754e | Human, ESBL-producerd | Geser et al., 2012 |
| CB295 | O139:H1 | LN877755e | STEC | Hampson et al., 1988 |
| CB13050 (D3648) | O139:H1 | LN877756e | STEC | Scheutz et al., 2012 |
| CB13107 | O139:H1 | LN877757e | STEC | Switzerland, pig intestine, 2011, this work |
| CB15303 | O139:H1 | LN877758e | STEC | France, edema disease pig, 2014, this work |
The whole genome sequence of the E. coli strain ABU 83972 (GenBank: CP001671.1) is available (Zdziarski et al., 2010). The O-serogroup of this strain was not reported but its wzx gene (position 2372093–2373353) is >99% similar to wzx of E. coli O25 strains E47a (GenBank GU014554.1) (Wang et al., 2010). Therefore, we classified ABU 83972 here as an O25:H1 strain.
The whole genome sequence of E. coli strain LF82 is available (GenBank: CU651637.1). The O-serogroup of this strain is not reported but its wzx gene (position 2127428–212804) is identical to the wzx gene of E. coli O83:H31 strain H17a GenBank: KJ778808.1 (unpublished) and of E. coli O83:H1 strain NRG857c (GenBank: CP001855.1) (Allen et al., 2008). Therefore, we suggest that LF82 is an O83:H1 strain.
The fliC sequence deposited under GenBank JF308285 is derived from strain EC614 reported as O157:H1 (Goulter et al., 2010). By Blast search, it is 100% identical to the fliC sequence of the H1 reference strain Su1242 (GenBank accession AY249997). Therefore, the flagellar type of EC614 was classified as H1.
Multiresistant, extended-spectrum-lactamase (ESBL)-producing E. coli from healthy human carrier.
The fliC sequence was determined in this study.
Results
Sources and properties of E. coli H1 and H12 strains
The E. coli H1 and H12 strains investigated in this study were from human, animal, food, and environmental sources (Table 3). The thirty-one flagellar type H1 strains were associated with 10 different E. coli O-serogroups, O-rough and O-untypable strains and originated from healthy and diseased humans and animals and from food. The thirty-eight H12 strains divided into thirteen different O-groups of E. coli, and in O-untypable and O-rough strains. The H12 strains were from healthy and diseased humans and animals, from food and the environment. Production of Shiga-toxins (Stx) was found in 16 (42.1%) of the H12 strains and associated with five different O-groups. Fourteen (45.2%) of the E. coli H1 strains produced Stx, however most of these were from pigs with edema disease (O139:H1, Or:H1) and harbored the stx2e gene. O:H types known to be associated with E. coli causing extraintestinal infections of humans (O2:H1, O4:H1, O6:H1, O25:H1) were detected among the investigated H1 strains. Interestingly, strains belonging to these serotypes originated not only from humans but also from animals and food. Certain strains belonged to serotypes which have not been previously associated with clinical disease and their role of pathogens for humans and animals is not yet known.
Table 3.
Source and origin of E. coli H1 and H12 strains.
| Serotypea | Nos. of strains | Source | Origin/References |
|---|---|---|---|
| O2:H1 | 1 | Calves feces, diarrhea | Germany, 2010 |
| O2:K2:H1 | 1 | Human blood | Orskov and Orskov, 1984 |
| O4:H1 | 1 | Raw milk cheese | Germany, 2010 |
| O6:H1 | 4 | Pig feces, diarrhea (1), Human (3) | Germany, 2011 Germany, 2009 |
| O6:K5:H1 | 1 | Human feces | Reister et al., 2014 |
| O15:H1 | 3 | Rabbit (2) Human (1) | Switzerland, 2007 Geser et al., 2012 |
| O22:H1 | 2 | Goat cheese (1) Human peritoneum (1) | Germany, 2013 Orskov and Orskov, 1984 |
| O25:H1 | 1 | Dog feces | Germany, 2011 |
| O77:H1 | 1 | salad | Germany, 2009 |
| O79:H1 | 1 | hare | Germany, 2007 |
| O139:H1 | 11b | Pig feces/organs, edema disease Wild boar feces/organs, edema disease | Orskov and Orskov, 1984: Beutin et al., 2008 France, 2013 |
| O149:H1 | 2c | Beef | Germany, 2011 |
| ONT:H1 | 1 | human | Germany, 2011 |
| Or:H1 | 1b | Pig feces, edema disease | Germany, 2014 |
| O1:H12 | 1 | Pig feces | Germany 2007 |
| O9:K9:H12 | 5 | Human peritoneum (1) Surface water (3) Chicken meat (1) | Orskov and Orskov, 1984 Germany, 2013 Germany, 2013 |
| O9:H12 | 4 | Pork | Martin and Beutin, 2011 |
| O11:H12 | 2 | Human (1) Pig feces (1) | Geser et al., 2012 Germany, 2009 |
| O20:H12 | 2d | Calves feces/organs, diarrhea | Germany, 2009 |
| O49:H12 | 1 | Human urine | Orskov and Orskov, 1984 |
| O55:H12 | 2e | Milk, beef | Martin and Beutin, 2011 |
| O79:H12 | 1 | Surface water | Germany, 2011 |
| O98:H12 | 1 | Pork | Germany, 2013 |
| O104:H12 | 2 | Human, diarrhea Surface water | Miko et al., 2013 Germany, 2013 |
| O118:H12 | 3d | Human, diarrhea | Pierard et al., 1998; Beutin et al., 2004 |
| O136:H12 | 4e | Milk Cattle feces | Martin and Beutin, 2011 France, 1998 Canada, 2012 |
| O153:H12 | 3e | beef | Martin and Beutin, 2011 |
| O157:H12 | 3 | Human Pig | Germany, 2007 Kaufmann et al., 2006 |
| ONT:H12 | 2 | Milk Surface water | Germany, 2014 Germany, 2013 |
| Or:H12 | 2e | Cattle feces | Germany, 2010 |
This list includes serotype reference strains: Nissle 1917 (O6:K5:H1) (Reister et al., 2014), EH250 (O118:H12), (Scheutz et al., 2012), Su 1242 (O2:K2:H1), E14a (O22:H1), CDC 63-57 (O139:H1), Bi316-42 (O9:K9:H12), U12-41 (O49:H1) (Orskov and Orskov, 1984).
Positive for stx2e.
Positive for stx1d.
Positive for stx2.
Positive for stx1.
Nucleotide analysis of E. coli fliCH1 and fliCH12 genes
The nucleotide sequences of the reference strains (Orskov and Orskov, 1984) for E. coli flagellar antigens H1 (strain Su1242, GenBank accession AB028471.1) and H12 (Bi 316-42, GenBank accession AY249997) (Wang et al., 2003) have been published previously. The length of coding sequence of each fliCH1 and fliCH12 gene is 1788 nucleotides and both sequences have 97.5% identity (44 nucleotide exchanges) at the nucleotide level and 98.98% identity and 99.16% similarity at the amino acid level (7 amino acids (aa) exchanges). Additional fliC nucleotide sequences from six E. coli H1 and five E. coli H12 strains were obtained in this work (Table 2). These sequences were compared with seven fliCH1 sequences and three fliCH12 sequences already available in GenBank (Table 2). All 21 H1 or H12 flagellin genes have a 1788 nucleotides length that codes for 595 amino acid residues.
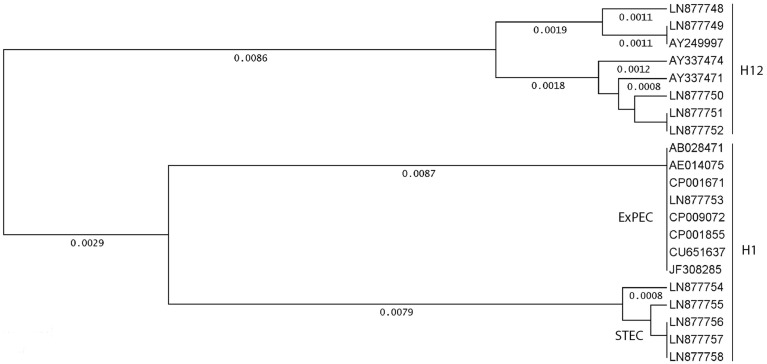
A cluster analysis performed with thirteen fliCH1 and eight fliCH12 sequences is shown in Figure 1. Four different genotypes were detected among the thirteen fliCH1 strains. Uropathogenic E. coli O2:H1, O6:H1, O25:H1, and AIEC O83:H1 strains were identical for their fliCH1 sequences and assigned to a large cluster composed by eight strains. A smaller cluster was formed by five fliCH1 strains; four of these were Stx2e producing O139:H1 causing edema disease in pigs.
Figure 1.
Genetic relationships between fliCH1 and fliCH12 genes in different strains and serotypes of E. coli. Cluster analysis was performed using eight fliCH12 and thirteen fliCH1 genes listed in Table 2. GenBank accession numbers are indicated for orientation. The unweighted-pair group method using average linkages was used as a tree-building mode, and the distances were calculated according to Tajima and Nei (1984) using the Accelrys DS Gene software package.
Six different genotypes were found among the eight fliCH12 strains. Identical fliCH12 sequences were only found between two O9:K9:H12 strains and each one O55:H12 and O153:H12 strain, respectively.
Amino acid alterations between flagellar antigens H1 and H12 in E. coli strains
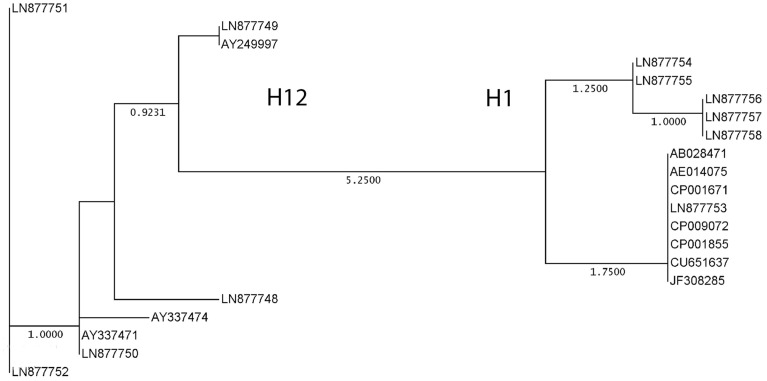
An alignment of the amino acid sequences of thirteen fliCH1 and eight fliCH12 strains is shown in Table S1. All translation products had a length of 595 amino acids (aa). The eight fliCH12 strains were showing only few alterations with one or more of the strains at aa positions 249, 258, 339, and 472 (99.2% similarity) (Table S1), generating six different protein sequences (Figure 2). The thirteen fliCH1 strains split into three protein sequences (Figure 2) differing at positions 258, 431, and 481 (99.5% similarity) (Table S1). The aa changes were all located in the variable region of fliC encoding flagellar antigen specificity (Wang et al., 2003). Differences in the aa sequence which could distinguish between all investigated fliCH1 and fliCH12 strains, respectively, were found at positions 302 (Glu/Lys), 340 (Asn/Lys), 361 (Gly/Asp), 391 (Thr/Lys), 396 (Asn/Asp), and 430 (Asn/Lys). The six flagellar type specific aa sequence differences were all located in the variable region of the fliCH1 and fliCH12 genes.
Figure 2.
Genetic relationships between translation products of thirteen fliCH1 and eight fliCH12 genes in different strains and serotypes of E. coli listed in Table 2. GenBank accession numbers are indicated for orientation. The neighbor joining method with absolute differences (best tree) was used as a tree-building mode (Nei, 1996) using the Accelrys DS Gene software package.
Development and evaluation of real-time PCR assays for identification of E. coli fliCH1 and fliCH12 strains
The close similarity between E. coli fliCH1 and fliCH12 translation products explains the serological cross-reactivity which was previously described for H1 and H12 antigens (Orskov and Orskov, 1984; Edwards and Ewing, 1986). As specific differences were found that distinguish between fliCH1 and fliCH12 sequences, molecular detection of the respective fliC genes could be more suitable than serotyping for clear identification of H1 and H12 strains of E. coli.
Based on the sequence data obtained for E. coli fliCH1 and fliCH12 genes we developed a TaqMan real-time PCR assay for common detection of fliCH1 and fliCH12 genes as well as real-time PCR assays for specific detection of fliCH1 and fliCH12, respectively (Table 1). Short-length XS-probes (minor groove binder replacement) had to be employed to develop real-time PCR assays specific for fliCH1 and fliCH12 sequences (Table 1). We used two nucleotide substitutions between the sequences of fliCH1 and fliCH12 to design specific probes (Table 1).
The assays were first tested for sensitivity and specificity on 31 E. coli H1 and 38 E. coli H12 strains (Table 4) as well as on the E. coli H-type reference strains (H1-H56) (Orskov and Orskov, 1984; Edwards and Ewing, 1986). The real-time PCR for common detection of fliCH1 and fliCH12 reacted with all tested E. coli H1 and H12 strains (Table 4) and not with any of the reference strains encoding all other flagellar antigens than H1 and H12.
Table 4.
Detection of different E. coli H1 and H12 strains belonging to different O-serogroups by fliCH1, fliCH12 and fliCH1/H12 Real Time PCR assays.
| Serotypeb | Nos. of strains | CT-valuesafliCH1 | CT-valuesafliCH12 | CT-valuesafliCH1/H12 |
|---|---|---|---|---|
| O2:H1 | 1 | 21.1 | – | 22.2 |
| O2:K2:H1b | 1 | 21.1–24.3 | – | 22.2–22.9 |
| O4:H1 | 1 | 24.7 | – | 27.1 |
| O6:H1 | 5 | 21.1–24.3 | – | 21.9–25.3 |
| O15:H1 | 3 | 19.6–25.5 | – | 21.2–24.0 |
| O22:H1 | 2 | 19.6–22.0 | – | 22.6–24.2 |
| O25:H1 | 1 | 22.5 | – | 24.5 |
| O77:H1 | 1 | 21.8 | – | 23.1 |
| O79:H1 | 1 | 24.5 | – | 24.7 |
| O139:H1 | 11 | 16.5–23.7 | – | 15.0–24.6 |
| O149:H1 | 2 | 213–21.4 | – | 23.5–24.8 |
| ONT:H1 | 1 | 22.6 | – | 22.7 |
| Or:H1 | 1 | 20.1 | – | 21.9 |
| O1:H12 | 1 | – | 20.9 | 24.2 |
| O9:K9:H12b | 5 | – | 18.6–23.2 | 21.6–23.1 |
| O9:H12 | 4 | – | 17.0–17.9 | 16.0–22.6 |
| O11:H12 | 2 | – | 18.2–22.4 | 21.7–22.6 |
| O20:H12 | 2 | – | 15.2–16.9 | 20.4–21.1 |
| O49:H12 | 1 | – | 19.6 | 24.5 |
| O55:H12 | 2 | – | 20.1–22.8 | 22.5–22.7 |
| O79:H12 | 1 | – | 22.8 | 23.0 |
| O98:H12 | 1 | – | 22.6 | 24.3 |
| O104:H12 | 2 | – | 20.9–23.6 | 22.1–23.4 |
| O118:H12 | 3 | – | 18.0–23.1 | 17.1–23.4 |
| O136:H12 | 4 | – | 16.8–23.6 | 15.9–23.2 |
| O153:H12 | 3 | – | 18.3–20.9 | 22.2–23.2 |
| O157:H12 | 3 | – | 18.7–23.4 | 21.7–23.7 |
| ONT:H12 | 2 | – | 21.7–22.8 | 22.5–23.4 |
| Or:H12 | 2 | – | 19.2–20.7 | 23.0–23.3 |
Range of real time PCR cycle thresholds. Negative reactions are indicated with the “–” sign.
Reference strain Orskov non-motile and the fliC-genotype was detected by nucleotide sequencing of fliC PCR products.
The fliCH1 and fliCH12 gene specific assays detected all E. coli H1 and all E. coli H12 strains, respectively (Table 4). However, both assays showed cross-reactions with some flagellar type reference strains different from H1 and H12. With the fliCH1 real-time PCR, cross-reactions were observed with H6, H7, H15, H20, H34, H37, H41, H45, H46, H49, and H52 strains. The fliCH12 specific PCR reacted also with H7, H28, H31, and H41 strains (Table 5). Although the overall sequences of the fliC genes of H-types cross-reacting with the fliCH1 and fliCH12 real-time PCR are widely different from those of fliCH1 and fliCH12, they show high local similarities with the primers and probes sequences. In cases of cross reactivity, no or only minor differences (0–3 mismatches) were found between target-sequences and fliCH1 and fliCH12, primers and probes (Table 5). Three and more mismatches were found in cases of negative real-time PCR results.
Table 5.
Cross-reactions of fliCH1 and fliCH12 real time PCR assays with other flagellar types of E. coli.
| Reference straina | H-type | GenBank Accession No. | Detector testedb | Ct-valuec | Mismatch FP/P/RPd |
|---|---|---|---|---|---|
| A20 | H6 | AY249991.1 | fliCH1 | 26.8 | 0/0/0 |
| fliCH12 | – | 0/2/1 | |||
| U5-41 | H7 | AB028474.1 | fliCH1 | 26.0 | 0/1/0 |
| fliCH12 | 24.5 | 0/1/1 | |||
| E39a | H15 | AY249999.1 | fliCH1 | 28.2 | 1/1/1 |
| fliCH12 | – | 3/2/2 | |||
| H3306 | H20 | AY250003.1 | fliCH1 | 27.0 | 0/0/1 |
| fliCH12 | – | 2/2/2 | |||
| HW30 | H28 | AY337469.1 | fliCH1 | – | 0/2/1 |
| fliCH12 | 21.9 | 0/0/0 | |||
| HW33 | H31 | AF345849.1 | fliCH1 | – | 0/2/4 |
| fliCH12 | 22.9 | 0/0/0 | |||
| BP 12665 | H34 | AY250016.1 | fliCH1 | 20.9 | 0/0/0 |
| fliCH12 | – | 0/2/1 | |||
| P11a | H37 | AY250017.1 | fliCH1 | 26.4 | 1/0/1 |
| fliCH12 | – | 3/2/1 | |||
| RVC1787 | H41 | AY250020.1 | fliCH1 | 27.1 | 0/1/1 |
| fliCH12 | 24.0 | 0/1/1 | |||
| 4106-54 | H45 | AY250023.1 | fliCH1 | 25.5 | 0/0/0 |
| fliCH12 | – | 0/3/1 | |||
| 5306-56 | H46 | AY250024.1 | fliCH1 | 27.6 | 0/0/1 |
| fliCH12 | – | 0/1/2 | |||
| 2147-59 | H49 | AY250026.1 | fliCH1 | 24.6 | 0/0/0 |
| fliCH12 | – | 0/3/1 | |||
| C2187-69 | H52 | AY250028.1 | fliCH1 | 26.8 | 0/1/1 |
| fliCH12 | – | 0/3/1 | |||
| Su1242 | H1 | AB028471.1 | fliCH1 | 21.1–24.3 | 0/0/0 |
| fliCH12 | – | 0/2/1 | |||
| Bi316/42 | H12 | AY249997.1 | fliCH1 | – | 0/2/1 |
| fliCH12 | 18.6–23.6 | 0/0/0 |
H-type reference strains (Orskov and Orskov, 1984).
As listed in Table 1.
Mean of real-time cycle threshold (CT-values) calculated from duplicate PCRs. Negative reactions are indicated with the “–” sign.
Number of mismatches found between real-time detector sequence and target gene sequence. FP, forward primer; P, gene probe; RP, reverse primer.
In respect to these findings, the assays were then tested on a second panel of 78 strains comprising strains with H-types previously found to cross-react with FlicH1 or FlicH12 PCR assays as well as strains from O-groups that can be found associated with H1 and H12, but with H-types different from H1 and H12 (Table 6).
Table 6.
Reaction of the fliCH1/H12, fliCH1 and fliCH12 real-time PCR assays with non-H1 and non-H12 strains.
| Serotype | Number of strains | Ct-valuesafliCH1/H12 | Ct-valuesafliCH1 | Ct-valueafliCH12 |
|---|---|---|---|---|
| O33:H6 | 1 | – | 20.6 | – |
| O40:H6 | 1 | – | 19.35 | – |
| O55:H6 | 1 | – | 17.2 | – |
| O63:H6 | 1 | – | 18.98 | – |
| O113:H6 | 2 | – | 19.38–19.46 | – |
| O125:H6 | 1 | – | 24.29 | – |
| O126:H6 | 1 | – | 20.17 | – |
| O127:H6 | 1 | – | 21.78 | – |
| O41:H7 | 1 | – | – | 21.11 |
| O55:H7 | 3 | – | – | 22.35–26.16 |
| O153:H7 | 1 | – | – | 18.21 |
| O157:H7 | 4 | – | – | 18.46–24.7 |
| O23:H15 | 1 | – | – | – |
| O157:H15 | 1 | – | – | – |
| O28:H28 | 1 | – | – | 18.73 |
| O91:H28 | 1 | – | – | 16.34 |
| O110:H28 | 1 | – | – | 15.53 |
| O116:H28 | 1 | – | – | 30.55 |
| O145:H28 | 1 | – | – | 14.99 |
| OX185:H28 | 1 | – | – | 17.18 |
| O51:H49 | 1 | – | 21.1 | – |
| O114:H49 | 1 | – | 20.65 | – |
| O181:H49 | 1 | – | 20.6 | – |
| O45:H31 | 1 | – | 19.44 | – |
| O179:H31 | 1 | – | – | 19.56 |
| O6:H34 | 2 | – | – | – |
| O86:H34 | 1 | – | 21.4 | – |
| O142:H34 | 1 | – | 21.99 | – |
| O145:H34 | 1 | – | – | 21.67 |
| O132:H34 | 1 | – | – | – |
| O132:H34 | 1 | – | – | 20.84 |
| O76:H41 | 1 | – | – | 20.75 |
| O17/77:H41 | 1 | – | 21.93 | – |
| O8:H45 | 1 | – | – | – |
| O121:H45 | 1 | – | 22.32 | – |
| O157:H45 | 1 | – | 24.34 | – |
| O186:[H45] | 1 | – | 20.92 | – |
| O119:[H52] | 1 | – | – | – |
| O2:H8 | 1 | – | – | – |
| O2:H25 | 1 | – | 27.15 | – |
| O2:H27 | 1 | – | – | – |
| O2:H40 | 1 | – | – | – |
| O4:H5 | 1 | – | – | – |
| O4:H16 | 1 | – | – | – |
| O6 | 2 | – | – | – |
| O6:H4 | 1 | – | 24.17 | – |
| O6:H10 | 1 | – | – | – |
| O7:H4 | 1 | – | – | – |
| O15:H2 | 1 | – | – | – |
| O15:H11 | 1 | – | – | – |
| O15:H16 | 1 | – | – | – |
| O15:H21 | 1 | – | – | – |
| O139:H4 | 1 | – | – | – |
| O139:H19 | 1 | – | – | – |
| O128:H2 | 1 | – | – | – |
| O128:H8 | 1 | – | – | – |
| O20:H9 | 1 | – | – | 23.23 |
| O20:H30 | 1 | – | – | – |
| O20:H33 | 1 | – | – | – |
| O20:NM | 1 | – | – | – |
| O55:H19 | 1 | – | – | 23.43 |
| O55:H21 | 1 | – | – | – |
| O55:H51 | 1 | – | – | – |
| O118:H2 | 1 | – | – | – |
| O118:H5 | 1 | – | – | – |
| O118:H8 | 1 | – | – | – |
| O118:H16 | 1 | – | – | – |
| O153:H14 | 1 | – | – | 20.86 |
| O153:H21 | 1 | – | – | – |
| O153:H25 | 1 | – | 28.37 | – |
Range of real time PCR cycle thresholds. Negative reactions are indicated with the “–” sign.
None of the 78 strains with H–types different from H1 and H12 reacted with the common fliCH1 / fliCH12 real-time PCR assay. Cross reactions with the fliCH1 real-time PCR-assay were observed with H6 (9/9), H49 (3/3), H31 (1/2), H34 (2/7), H41 (1/2), H45 (3/4) as well as with one O6:H4 strain. Weak cross-reactions were also observed with one O2:H25 strain and one O153:H25 strain. Cross-reactions with the fliCH12 real-time PCR-assay were observed with H7 (9/9), H28 (6/6), H31 (1/2), H34 (2/7), H41 (1/2), as well as one O20:H9, one O55:H19 and one O153:H14 strains. In contrast to the respective reference strains, cross-reactions were not observed with either real-time PCR-assay with two other H15 and one H52 strain tested (Tables 5, 6). We do not know if these three strains show further differences in the PCR-target region which could explain these findings.
Overall, molecular typing of E. coli H1 and H12 strains requires first identification of H1/H12 strains with the common fliCH1/fliCH12 real-time PCR assay, followed by specific identification of fliCH1 and fliCH12, by their respective real-time PCR-assays. The real-time PCR for common detection of fliCH1 and fliCH12 was found 100% sensitive and 100% specific. The fliCH1 and fliCH12 gene specific assays were found 100% sensitive as they detected all E. coli H1 and all E. coli H12 strains, respectively. When used exclusively on H1 and H12 strains (as identified by the common primers/probe set in a first step), the fliCH1 and fliCH12 gene specific assays were found 100% specific. Thus, 100% of H1 and H12 strains would be accurately typed with this system.
Discussion
The genetically and serologically closely related flagellar antigens H1 and H12 were found in heterogeneous types of E. coli strains belonging to 26 different O-serogroups, O-untypable and O-rough strains. With one exception (O79:H1 and O79:H12), H1 and H12 strains did not share common O-serogroups which would indicate that flagellar types H1 and H12 have separated from each other not very recently in evolution. They may have evolved independently following rearrangements in the O-group loci of ancestor strains carrying the closely related H1/H12 flagellar types and do not directly derive from a common O-group ancestor.
By comparing nucleotide sequences of fliC genes from thirteen H1 and eight H12 strains we identified six H-type specific aa changes at positions 302, 340, 361, 391, 396, and 430. All these are located in the variable part of flagellin determining antigen specificity (Wang et al., 2003). As these changes are characteristic for the respective flagellar antigen, we suppose them to determine the antigen specificities of H1 and H12. The few other aa changes detected in some H1 and H12 strains might thus not be significant as specific characteristics of H1 or H12 types. However, such aa-changes could explain the finding of serological subtypes of H1 which were detected using factor specific H-antisera (Ratiner et al., 1995).
Interestingly, the genetic distance between fliCH1 (Su1242, GenBank accession AB028471.1) and fliCH12 sequences (Bi 316-42, GenBank accession AY249997) (97.5% similarity) is less than that found between different alleles of fliCH28 (92.0% similarity) (Beutin et al., 2015b). It is slightly bigger than the distance found among different alleles of fliCH19 (98.5% similarity) (Beutin et al., 2015a). Multiple allelelic types of fliC were also detected in E. coli H6, H7, H8, H25, and H40 strains, respectively (Reid et al., 1999; Wang et al., 2000; Beutin and Strauch, 2007; Beutin et al., 2015b). Already, a considerable number of serological cross-reactions were observed when flagellar types H1–H56 were compared (Orskov and Orskov, 1984; Edwards and Ewing, 1986). Some of these flagellar antigens (H1/H12, H8/H40, H11/H21, and H37/H41) are so closely related that the use of cross-absorbed antisera is needed to obtain unambiguous serotyping results (Edwards and Ewing, 1986).
The presence of allelic subtypes within fliC genes encoding different H-types of E. coli and the finding that different flagellar types are serologically cross-reacting may complicate E. coli strain typing using H-antisera. The use of molecular typing procedures, such as real-time PCR can solve the typing problem caused by serologically closely related H-antigens, as we have shown for H1 and H12 in this work. Using primer express V3.0 software, it was not possible to design real-time PCRs specific exclusively for fliCH1 and fliCH12, respectively. For this reason, we employed a two-step real-time detection procedure. The first step uses a real-time PCR highly specific for both H1 and H12 strains, followed by subtyping of H1/H12-positive strains with the respective fliCH1 and fliCH12 specific real-time PCRs. Short probe sequence lengths as obtained with minor groove binder (MGB) or MGB-replacements (XS-probe) are needed to ensure specificity between closely similar DNA-targets as previously shown for fliCH19 allelic discrimination (Beutin et al., 2015a). The PCRs could be used in parallel for examination of large number of isolates using high throughput PCR platforms as described previously for analysis of large numbers of Clostridia and E. coli strains (Delannoy et al., 2013; Woudstra et al., 2013).
Unambiguous typing of fliCH1 and fliCH12 sequences is of interest for clinical and epidemiological investigations since some H1 and H12 strains were shown to play a role as pathogens in humans and animals.
More than one third of investigated H1 and H12 strains produced Shiga toxins. Strains showing O:H types characteristic for ExPEC associated with human diseases (O2:H1, O4:H1, O6:H1, O15:H1) were detected in this work. Interestingly, these were not only from humans but also found in animals and food. It was previously described that animals, food and water can be a source of pandemic ExPEC strains (Jakobsen et al., 2010; Riley, 2014; Gomi et al., 2015; Singer, 2015). Flagellar type H12 strains encompass mainly STEC (O20:H12, O55:H12, O118:H12, O136:H12, O153:H12, and Or:H12) and were isolated from diseased animals and humans, food and the environment (Scheutz and Strockbine, 2005).
The specific molecular detection of H1 and H12 flagellins as described in this study will be useful for diagnosis and for source attribution of human and animal pathogenic ExPEC and STEC strains in outbreaks and sporadic cases of infection.
Author contributions
Conceived and designed the experiments: LB, SD, PF. Performed the experiments: LB, SD. Analyzed the data: LB, SD, PF. Contributed reagents/materials/analysis tools: LB, SD, PF. Wrote the paper: LB, SD, PF. Critical revision of the paper for important intellectual content: LB, SD, PF.
Funding
The project was partially financed by the French “joint ministerial program of R&D against CBRNE risks” (Grant number C17609- 2).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00135
References
- Allen C. A., Niesel D. W., Torres A. G. (2008). The effects of low-shear stress on Adherent-invasive Escherichia coli. Environ. Microbiol. 10, 1512–1525. 10.1111/j.1462-2920.2008.01567.x [DOI] [PubMed] [Google Scholar]
- Bettelheim K. A. (1978). The sources of “OH” serotypes of Escherichia coli. J. Hyg. 80, 83–113. 10.1017/S0022172400053420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Delannoy S., Fach P. (2015a). Genetic diversity of the fliCH19 genes encoding the flagellar antigen H19 of Escherichia coli and application to the specific identification of enterohemorrhagic E. coli (EHEC) O121:H19. Appl. Environ. Microbiol. 81, 4224–4230. 10.1128/AEM.00591-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Delannoy S., Fach P. (2015b). Sequence variations in the flagellar antigen genes fliCH25 and fliCH28 of Escherichia coli and their use in identification and characterization of enterohemorrhagic E. coli (EHEC) O145:H25 and O145:H28. PLoS ONE 10:e0126749. 10.1371/journal.pone.0126749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Fach P. (2014). Detection of Shiga toxin-producing Escherichia coli from nonhuman sources and strain typing, in Microbiology Spectrum, eds Sperandio V., Hovde C. J. (Washington, DC: American Society for Microbiology; ), 1–23. [DOI] [PubMed] [Google Scholar]
- Beutin L., Krause G., Zimmermman S., Kaulfuss S., Gleier K. (2004). Characterization of Shiga toxin-producing Escherichia coli strains isolated from human patients in Germany over a 3-year period. J. Clin. Microbiol. 42, 1099–1108. 10.1128/JCM.42.3.1099-1108.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Kruger U., Krause G., Miko A., Martin A., Strauch E. (2008). Evaluation of major types of Shiga toxin 2e producing Escherichia coli present in food, pigs and in the environment as potential pathogens for humans. Appl. Environ. Microbiol. 74, 4806–4816. 10.1128/AEM.00623-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutin L., Strauch E. (2007). Identification of sequence diversity in the Escherichia coli fliC genes encoding flagellar types H8 and H40 and its use in typing of Shiga toxin-producing E. coli O8, O22, O111, O174, and O179 strains. J. Clin. Microbiol. 45, 333–339. 10.1128/JCM.01627-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugarel M., Beutin L., Martin A., Gill A., Fach P. (2010). Micro-array for the identification of Shiga toxin-producing Escherichia coli (STEC) seropathotypes associated with Hemorrhagic Colitis and Hemolytic Uremic Syndrome in humans. Int. J. Food Microbiol. 142, 318–329. 10.1016/j.ijfoodmicro.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Clotilde L. M., Salvador A., Bernard C., Lin A., Lauzon C., Muldoon M., et al. (2015). Comparison of multiplex immunochemical and molecular serotyping methods for shiga toxin-producing Escherichia coli. Foodborne Pathog. Dis. 12, 118–121. 10.1089/fpd.2014.1827 [DOI] [PubMed] [Google Scholar]
- Delannoy S., Beutin L., Fach P. (2013). Discrimination of enterohemorrhagic Escherichia coli (EHEC) from non-EHEC strains based on detection of various combinations of type III effector genes. J. Clin. Microbiol. 51, 3257–3262. 10.1128/JCM.01471-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves-Pyles T., Allen C. A., Taormina J., Swidsinski A., Tutt C. B., Jezek G. E., et al. (2008). Escherichia coli isolated from a Crohn's disease patient adheres, invades, and induces inflammatory responses in polarized intestinal epithelial cells. Int. J. Med. Microbiol. 298, 397–409. 10.1016/j.ijmm.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Echeverria P., Orskov F., Orskov I., Plianbangchang D. (1982). Serotypes of enterotoxigenic Escherichia coli in Thailand and the Philippines. Infect. Immun. 36, 851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. R., Ewing W. H. (1986). The genus Escherichia, in Identification of Enterobacteriaceae, 4th Edn., ed Ewing W. H. (New York, NY: Elsevier Science Publishing Co.), 93–136. [Google Scholar]
- Fairbrother J. M., Nadeau E., Gyles C. L. (2005). Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6, 17–39. 10.1079/AHR2005105 [DOI] [PubMed] [Google Scholar]
- Fratamico P. M., Bagi L. K., Cray W. C., Jr., Narang N., Yan X., Medina M., et al. (2011). Detection by multiplex real-time polymerase chain reaction assays and isolation of Shiga toxin-producing Escherichia coli serogroups O26, O45, O103, O111, O121, and O145 in ground beef. Foodborne Pathog. Dis. 8, 601–607. 10.1089/fpd.2010.0773 [DOI] [PubMed] [Google Scholar]
- Frydendahl K. (2002). Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 85, 169–182. 10.1016/S0378-1135(01)00504-1 [DOI] [PubMed] [Google Scholar]
- Geser N., Stephan R., Korczak B. M., Beutin L., Hachler H. (2012). Molecular identification of extended-spectrum-beta-lactamase genes from enterobacteriaceae isolated from healthy human carriers in Switzerland. Antimicrob. Agents Chemother. 56, 1609–1612. 10.1128/AAC.05539-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi R., Matsuda T., Fujimori Y., Harada H., Matsui Y., Yoneda M. (2015). Characterization of pathogenic Escherichia coli in river water by simultaneous detection and sequencing of 14 virulence genes. Environ. Sci. Technol. 49, 6800–6807. 10.1021/acs.est.5b00953 [DOI] [PubMed] [Google Scholar]
- Goulter R. M., Gentle I. R., Dykes G. A. (2010). Characterisation of curli production, cell surface hydrophobicity, autoaggregation and attachment behaviour of Escherichia coli O157. Curr. Microbiol. 61, 157–162. 10.1007/s00284-010-9589-2 [DOI] [PubMed] [Google Scholar]
- Hampson D. J., Fu Z. F., Bettleheim K. A., Wilson M. W. (1988). Managemental influences on the selective proliferation of two strains of haemolytic Escherichia coli in weaned pigs. Epidemiol. Infect. 100, 213–220. 10.1017/S0950268800067340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi A., Iyoda S., Kikuchi T., Ogura Y., Katsura K., Ohnishi M., et al. (2015a). A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Res. 22, 101–107. 10.1093/dnares/dsu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi A., Iyoda S., Seto K., Morita-Ishihara T., Scheutz F., Ohnishi M. (2015b). Escherichia coli O-Genotyping PCR: a Comprehensive and practical platform for molecular O Serogrouping. J. Clin. Microbiol. 53, 2427–2432. 10.1128/JCM.00321-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen L., Spangholm D. J., Pedersen K., Jensen L. B., Emborg H. D., Agerso Y., et al. (2010). Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int. J. Food Microbiol. 142, 264–272. 10.1016/j.ijfoodmicro.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Joensen K. G., Tetzschner A. M., Iguchi A., Aarestrup F. M., Scheutz F. (2015). Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 53, 2410–2426. 10.1128/JCM.00008-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Orskov I., Orskov F., Goullet P., Picard B., Moseley S. L., et al. (1994). O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J. Infect. Dis. 169, 119–126. 10.1093/infdis/169.1.119 [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Owens K., Gajewski A., Kuskowski M. A. (2005). Bacterial characteristics in relation to clinical source of Escherichia coli isolates from women with acute cystitis or pyelonephritis and uninfected women. J. Clin. Microbiol. 43, 6064–6072. 10.1128/JCM.43.12.6064-6072.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Owens K. L., Clabots C. R., Weissman S. J., Cannon S. B. (2006). Phylogenetic relationships among clonal groups of extraintestinal pathogenic Escherichia coli as assessed by multi-locus sequence analysis. Microbes Infect. 8, 1702–1713. 10.1016/j.micinf.2006.02.007 [DOI] [PubMed] [Google Scholar]
- Kaper J. B., Nataro J. P., Mobley H. L. (2004). Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- Kaufmann M., Zweifel C., Blanco M., Blanco J. E., Blanco J., Beutin L., et al. (2006). Escherichia coli O157 and non-O157 Shiga toxin-producing Escherichia coli in fecal samples of finished pigs at slaughter in Switzerland. J. Food Prot. 69, 260–266. [DOI] [PubMed] [Google Scholar]
- Martin A., Beutin L. (2011). Characteristics of Shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources. Int. J. Food Microbiol. 146, 99–104. 10.1016/j.ijfoodmicro.2011.01.041 [DOI] [PubMed] [Google Scholar]
- Martinez-Medina M., Garcia-Gil L. J. (2014). Escherichia coli in chronic inflammatory bowel diseases: an update on adherent invasive Escherichia coli pathogenicity. World J. Gastrointest. Pathophysiol. 5, 213–227. 10.4291/wjgp.v5.i3.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Medina M., Naves P., Blanco J., Aldeguer X., Blanco J. E., Blanco M., et al. (2009). Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC). BMC Microbiol. 9:202. 10.1186/1471-2180-9-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miko A., Delannoy S., Fach P., Strockbine N. A., Lindstedt B. A., Mariani-Kurkdjian P., et al. (2013). Genotypes and virulence characteristics of Shiga toxin-producing Escherichia coli O104 strains from different origins and sources. Int. J. Med. Microbiol. 303, 410–21. 10.1016/j.ijmm.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Minogue T. D., Daligault H. A., Davenport K. W., Bishop-Lilly K. A., Broomall S. M., Bruce D. C., et al. (2014). Complete genome assembly of Escherichia coli ATCC 25922, a serotype O6 reference strain. Genome Announc. 2:e00969–14. 10.1128/genomeA.00969-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash J. H., Villegas A., Kropinski A. M., Aguilar-Valenzuela R., Konczy P., Mascarenhas M., et al. (2010). Genome sequence of adherent-invasive Escherichia coli and comparative genomic analysis with other E. coli pathotypes. BMC Genomics. 11:667. 10.1186/1471-2164-11-667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. (1996). Phylogenetic analysis in molecular evolutionary genetics. Annu. Rev. Genet. 30, 371–403. 10.1146/annurev.genet.30.1.371 [DOI] [PubMed] [Google Scholar]
- Olesen B., Scheutz F., Menard M., Skov M. N., Kolmos H. J., Kuskowski M. A., et al. (2009). Three-decade epidemiological analysis of Escherichia coli O15:K52:H1. J. Clin. Microbiol. 47, 1857–1862. 10.1128/JCM.00230-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov F., Orskov I. (1984). Serotyping of Escherichia coli, in Methods in Microbiology, ed Bergan T. (London, UK: Academic Press; ), 43–112. [Google Scholar]
- Orskov I., Orskov F. (1977). Special O:K:H serotypes among enterotoxigenic E. coli strains from diarrhea in adults and children. Occurrence of the CF (colonization factor) antigen and of hemagglutinating abilities. Med. Microbiol. Immunol. 163, 99–110. 10.1007/BF02121825 [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. (1985). Escherichia coli in extra-intestinal infections. J. Hyg. 95, 551–575. 10.1017/S0022172400060678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierard D., Muyldermans G., Moriau L., Stevens D., Lauwers S. (1998). Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36, 3317–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratiner Y., Latto I., Ulisko I., Alekseeva M., Siitonen A. (1995). Serological variety of flagellar antigen H1 in natural Escherichia coli population. FEMS Immunol. Med. Microbiol. 10, 125–131. 10.1111/j.1574-695X.1995.tb00021.x [DOI] [PubMed] [Google Scholar]
- Reid S. D., Selander R. K., Whittam T. S. (1999). Sequence Diversity of Flagellin (fliC) Alleles in Pathogenic Escherichia coli. J. Bacteriol. 181, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reister M., Hoffmeier K., Krezdorn N., Rotter B., Liang C., Rund S., et al. (2014). Complete genome sequence of the gram-negative probiotic Escherichia coli strain Nissle 1917. J. Biotechnol. 187, 106–107. 10.1016/j.jbiotec.2014.07.442 [DOI] [PubMed] [Google Scholar]
- Riley L. W. (2014). Pandemic lineages of extraintestinal pathogenic Escherichia coli. Clin. Microbiol. Infect. 20, 380–390. 10.1111/1469-0691.12646 [DOI] [PubMed] [Google Scholar]
- Scheutz F., Cheasty T., Woodward D., Smith H. R. (2004). Designation of O174 and O175 to temporary O groups OX3 and OX7, and six new E. coli O groups that include Verocytotoxin-producing E. coli (VTEC): O176, O177, O178, O179, O180 and O181. APMIS 112, 569–584. 10.1111/j.1600-0463.2004.apm1120903.x [DOI] [PubMed] [Google Scholar]
- Scheutz F., Strockbine N. A. (2005). Genus I. Escherichia, in Bergey's Manual of Systematic Bacteriology, 2nd Edn., eds Garrity G. M., Brenner D. J., Krieg N. R., Staley J. T. (New York, NY: Springer; ), 607–624. [Google Scholar]
- Scheutz F., Teel L. D., Beutin L., Pierard D., Buvens G., Karch H., et al. (2012). Multicenter evaluation of a sequence-based protocol for subtyping shiga toxins and standardizing stx nomenclature. J. Clin. Microbiol. 50, 2951–2963. 10.1128/JCM.00860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen H. I., Khalil S. B., Rao M. R., Abu E. R., Wierzba T. F., Peruski L. F., Jr., et al. (2004). Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J. Clin. Microbiol. 42, 5588–5595. 10.1128/JCM.42.12.5588-5595.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. S. (2015). Urinary tract infections attributed to diverse ExPEC strains in food animals: evidence and data gaps. Front. Microbiol. 6:28. 10.3389/fmicb.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenutz R., Weintraub A., Widmalm G. (2006). The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol. Rev. 30, 382–403. 10.1111/j.1574-6976.2006.00016.x [DOI] [PubMed] [Google Scholar]
- Tajima F., Nei M. (1984). Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1, 269–285. [DOI] [PubMed] [Google Scholar]
- Tschape H., Bender L., Ott M., Wittig W., Hacker J. (1992). Restriction fragment length polymorphism and virulence pattern of the veterinary pathogen Escherichia coli O139:K82:H1. Zentralbl. Bakteriol. 276, 264–272. 10.1016/S0934-8840(11)80013-7 [DOI] [PubMed] [Google Scholar]
- Wang L., Rothemund D., Curd H., Reeves P. R. (2000). Sequence diversity of the Escherichia coli H7 fliC genes: implication for a DNA-based typing scheme for E. coli O157:H7. J. Clin. Microbiol. 38, 1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Rothemund D., Curd H., Reeves P. R. (2003). Species-wide variation in the Escherichia coli flagellin (H-Antigen) gene. J. Bacteriol. 185, 2936–2943. 10.1128/JB.185.9.2936-2943.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Wang S., Beutin L., Cao B., Feng L., Wang L. (2010). Development of a DNA microarray for detection and serotyping of enterotoxigenic Escherichia coli. J. Clin. Microbiol. 48, 2066–2074. 10.1128/JCM.02014-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Burland V., Plunkett G., III, Redford P., Roesch P., Rasko D., et al. (2002). Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 99, 17020–17024. 10.1073/pnas.252529799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudstra C., Lambert D., Anniballi F., De Medici D., Austin J., Fach P. (2013). Genetic diversity of the flagellin genes of Clostridium botulinum groups I and II. Appl. Environ. Microbiol. 79, 3926–3932. 10.1128/AEM.00686-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdziarski J., Brzuszkiewicz E., Wullt B., Liesegang H., Biran D., Voigt B., et al. (2010). Host imprints on bacterial genomes–rapid, divergent evolution in individual patients. PLoS Pathog. 6:e1001078. 10.1371/journal.ppat.1001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.