Abstract
Objective:
South Asians are a high-risk group for type 2 diabetes and coronary heart disease. We sought to determine ethnic differences in newborn adiposity comparing South Asians (SA) to White Caucasians (Whites).
Methods:
Seven hundred ninety pregnant women (401 SA, 389 Whites) and their full-term offspring from two birth cohorts in Canada were analyzed. Pregnant women completed a health assessment including a 75-g oral glucose tolerance test to assess for dysglycemia. Birthweight, length, waist and hip circumference, and triceps and subscapular skinfold thickness (a surrogate measure of body adiposity) were measured in all newborns. Multivariate regression was used to identify maternal factors associated with newborn skinfold measurements.
Results:
South Asian women were younger (30.1 vs 31.8 years, P<0.001), their prepregnancy body mass index was lower (23.7 vs 26.2, P<0.0001) and gestational diabetes was substantially higher (21% vs 13%, P=0.005) compared with Whites. Among full-term newborns, South Asians had lower birthweight (3283 vs 3517 g, P=0.0001), had greater skinfold thickness (11.7 vs 10.6 mm; P=0.0001) and higher waist circumference (31.1 vs 29.9 cm, P=0.0001) compared with Whites. Risk factors for newborn skinfold thickness included South Asian ethnicity (standardized estimate (s.e.): 0.24; P<0.0001), maternal glucose (s.e.: 0.079; P=0.04) and maternal body fat (s.e.: 0.14; P=0.0002).
Conclusions:
South Asian newborns are lower birthweight and have greater skinfold thickness, compared with White newborns, and this is influenced by maternal body fat and glucose. Interventions aimed at reducing body fat prior to pregnancy and gestational diabetes during pregnancy in South Asians may favorably alter newborn body composition and require evaluation.
Introduction
People who originate from the Indian subcontinent, known as South Asians, suffer a high prevalence of type 2 diabetes and cardiovascular disease.1, 2 Although both sociocultural and genetic factors have been implicated as potential explanations, more recently attention has turned to early-life influences as determinants of these adult diseases.3
Previous studies that compared South Asians living in India with those in the United Kingdom reported that South Asian newborns had lower birthweight and had relatively more adipose tissue compared with White Caucasian (White) newborns of the same gestational age.3, 4 This has been termed the ‘thin-fat' phenotype and may represent an early feature of increased cardiometabolic risk, as there is a growing body of evidence associating lower birthweight with abdominal obesity, hypertension, future type 2 diabetes and cardiovascular disease.5, 6 Interestingly, this phenotype is present among South Asian immigrants to the United Kingdom and persists across multiple generations.7
Health system databases in Canada indicate greater maternal gestational diabetes (GDM) in South Asian women,8, 9 and that South Asian newborns have lower birthweight compared with White newborns.10 These two observations are at odds with each other because elevated maternal glucose is generally associated with higher birthweight or macrosomia.11
To further understand these observations, among women with full-term pregnancies recruited in two contemporary birth cohorts in Ontario, Canada, we sought to investigate: (1) the ethnic differences in newborn skinfold thickness; and (2) the antenatal maternal factors which influence these measurements.
Patients and methods
Eligibility and recruitment
Participants from the FAMILY and START prospective birth cohorts were potentially eligible for this analysis. Both studies were Research Ethics Board approved and all participants provided written informed consent. Eligible participants for this analysis were required to have a full-term pregnancy that is, ⩾37 weeks, have delivered a single offspring, and have the maternal baseline assessment conducted between 22 and 30 weeks gestational age. White participants were selected from the FAMILY cohort and were recruited from the greater Hamilton area in Ontario, Canada between 8 October 2002 and 8 July 2009. South Asian participants were from the START cohort and recruited from the Peel region, Ontario, Canada between 11 July 2011 and 20 September 2013. Detailed recruitment and baseline measures are reported in detail for both cohorts in prior publications.12, 13
Maternal measurements
During the second trimester, participants who did not have pre-existing diabetes completed a 75-g oral glucose tolerance test (OGTT). Individuals with pre-existing diabetes did not have the OGTT, but provided a fasting blood sample. GDM was defined using International Association of Diabetes and Pregnancy Study Groups criteria14 as fasting glucose⩾5.1 mmol l−1 or 1 h post OGTT glucose⩾10.0 mmol l−1 or 2 h post OGTT glucose of⩾8.5 mmol l−1 and area under the curve (AUC) glucose was used as a continuous measure of glucose response to a 75-g OGTT.15 All participants completed detailed health questionnaires including self-reported personal medical and family history, social and cultural questions, and previously validated ethnic-specific food frequency questionnaires.16 Physical activity in pregnancy was defined as hours of active sport per week during pregnancy as reported by the mother at her initial visit.
Social disadvantage was determined using a previously validated index which includes employment, marital status and income.17 Physical measurements using a protocol standardized between both cohorts included resting blood pressure using an oscillometric device, body weight, height, hip circumference and skinfold thickness. Maternal body fat in pregnancy was estimated by adding the skinfold thickness from triceps and subscapular sites.18 Participants also self-reported their prepregnancy weight, and gestational weight gain was calculated by subtracting the prepregnancy weight from the end pregnancy weight prior to delivery.
Delivery information and newborn measurements
Type, duration and outcomes of labor were obtained from hospital charts by trained research assistants using a standardized protocol. Measurements of newborns were completed by trained research assistants using a standardized protocol and 90% were taken within 24 up to 96 h after delivery. Specifically, newborn length was collected using the O'LEARLY length board. Birthweight was obtained from the hospital chart in 98% of newborns, or if unavailable, the newborn was weighed using the hospital newborn scale at the time of the birth visit. Head circumference was measured using a nonstretchable measuring tape. Waist and hip circumferences were measured using an OHAUS nonstretchable tape with an attached spring balance and the time of last feed prior to the measurements was not recorded. Skinfold thickness were taken in triplicate and recorded to the nearest mm using the HOLTAIN calipers in START (0.2 mm) and the LANGE calipers in FAMILY, (0.5 mm). The intraclass correlation (reliability) of the skinfold measurements was calculated and was 0.98 for subscapular skin folds and 0.96 for triceps skinfolds overall.19 Gestational weight gain was reported as a percent weight gain relative to the participant's prepregnancy weight. Ponderal index—a measure of neonatal leanness was calculated as birthweight per length3.3
Statistical considerations
We compared ~400 subjects per group to have 90% power to detect a difference of 10% in skinfold thickness between the ethnic groups. Statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC, USA). Means, s.d. and proportions are presented for descriptive variables. Between group comparisons were made using ANOVA or χ2-tests. Comparisons between South Asian and White newborns were adjusted for newborn sex and gestational age. A multiple regression model was constructed to assess the independent predictors of newborn skinfold measurements adjusting for newborn age and sex; mothers taking insulin at baseline were excluded. Individual factors which were significant predictors of newborn skinfold measurements, adjusting for ethnicity, with a P<0.10 was tested in a multivariable model. Backward stepwise regression was used to determine the final model with all variables significant at P<0.05. Interactions between ethnicity and each significant main effect were tested.
Results
Maternal comparisons
The baseline characteristics of mothers with full-term singleton pregnancies from the FAMILY (n=389) and START (n=401) cohorts are shown in Table 1. Briefly, compared with Whites, South Asian mothers were younger (30.1 vs 31.8 years, P<0.001), and were mostly immigrants, having lived in Canada an average of 8 years. The majority of South Asian women were of Indian origin (77.3%) followed by Pakistani (16.3%), Bangladeshi (0.75%), Sri Lankan (2.7%) and other (2.5%). South Asian women were less likely to be smokers during pregnancy (0% vs 13%, P<0.0001), more likely to be vegetarian (40.5% vs 0.8%, P<0.0001), have a stronger family history of type 2 diabetes (38.7% vs 19.0%, P<0.0001) and performed fewer hours of active sport per week compared with White women (1.7 (2.6) vs 2.2 (3.0) h per week, P=0.01). Despite having similar high school completion rates, South Asian women had greater social disadvantage (18.9% vs 5.6%, P<0.0001) as reflected by their lower annual household income and employment.
Table 1. Maternal baseline characteristics.
| South Asian | White Caucasian | P-value | |
|---|---|---|---|
| N per group | 401 | 389 | |
| Maternal age (years) | 30.1 (4.0) | 31.8 (5.0) | <0.001 |
| Years in Canada | 8 (7) | 30 (7) | <0.0001 |
| Gestational age at study enrolment (years) | 26.7 (1.3) | 26.9 (1.7) | 0.03 |
| Primiparous (%) | 38.7 | 43.7 | 0.15 |
| Smoked in pregnancy (%) | 0 | 13.3 | <0.0001 |
| Vegetarian (%) | 40.5 | 0.8 | <0.0001 |
| Hours of active sport per week | 1.7 (2.6) | 2.2 (3.0) | 0.01 |
| Family history of diabetes (%) | 38.7 | 19.0 | <0.0001 |
| Personal history of prepregnancy DM (%) | 0.5 | 3.1 | 0.006 |
| Social disadvantage index | |||
| High (%) | 18.9 | 5.6 | <0.0001 |
| Moderate (%) | 36.4 | 14.7 | |
| Low (%) | 44.7 | 79.7 | |
| Currently employed (%) | 52.8 | 83.2 | <0.0001 |
| Annual household income⩾50 K (%) | 40.8 | 80.2 | <0.0001 |
| High school education (%) | 99.2 | 97.9 | 0.12 |
| Prepregnancy weight, kg | 62.2 (11.3) | 71.1 (17.7) | <0.0001 |
| Height, cm | 162.1 (6.5) | 164.9 (6.2) | <0.0001 |
| Prepregnancy BMI, kg m−2 | 23.7 (4.3) | 26.2 (6.3) | <0.0001 |
| Gestational weight gain, kg | 13.8 (6.3) | 15.0 (5.3) | 0.01 |
| GWG / prepregnancy weight | 24% | 22% | 0.17 |
| Tricep skinfold thickness, mm | 28.2 (6.8) | 31.1 (12.1) | <0.0001 |
| Subscapular skinfold thickness, mm | 23.1 (6.9) | 23.6 (10.2) | 0.46 |
| Tricep+subscapular skinfold thickness thickness, mm | 51.2 (12.5) | 54.3 (20.9) | 0.01 |
| Gestational DM–IADPSG and recorded in chart (%) | 20.8 | 13.3 | 0.005 |
| Received insulin during pregnancy (%) (Use of insulin at baseline visit/current use of insulin) | 0.8 | 2.3 | 0.07 |
| Area under curve glucose, mmol × h | 13.3 (2.8) | 12.3 (2.3) | <0.0001 |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; GWG, gestational weight gain; IADPSG, International Association of Diabetes and Pregnancy Study Groups. Social Disadvantage Index, includes employment status, marital status and annual household income.15
Compared with White women, South Asians had lower weight (prepregnancy weight difference of 9 kg, P<0.0001), were shorter (prepregnancy height difference: 3 cm, P<0.0001), had a lower prepregnancy body mass index (23.7 vs 26.6 kg m−2, P<0.0001), and had lower triceps and subscapular skinfold thickness (51.2 vs 54.3 mm, P=0.01). During pregnancy, the percent of weight gain relative to the prepregnancy weight comparing South Asian and White women was similar (24% vs 22%, P=0.17; Table 1).
South Asian women had significantly more gestational dysglycemia. Specifically using the IADPSG definition to diagnose GDM, South Asian mothers had almost a twofold higher rate of GDM than White mothers (20.8% vs 13.3% P=0.005) and higher AUC glucose (13.3 vs 12.3 mmol × h; P<0.0001; Table 1). Fourteen women had pre-existing diabetes (2 SA vs 12 White) and 12 women were treated with insulin during pregnancy (SA: 3 vs White: 9).
Newborn comparisons
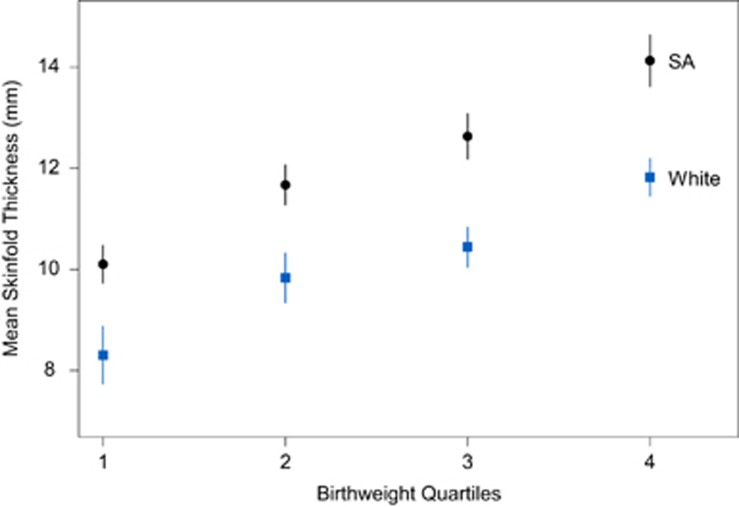
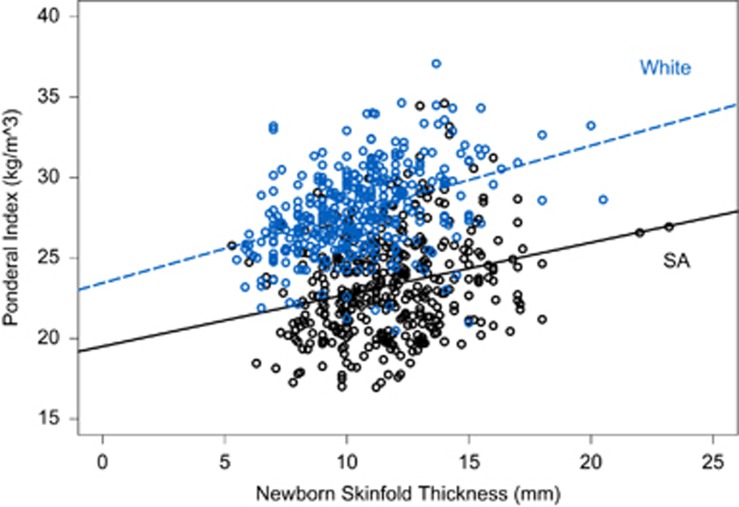
South Asian newborns had significantly lower birthweight (3283 vs 3517 g, P<0.0001), and had significantly greater skinfold thickness (11.7 (0.1) vs 10.6 (0.1); P<0.0001) compared with White newborns. Further, compared with White newborns, South Asian newborns had greater skinfold thickness across each quartile of birthweight (Figure 1). Interestingly South Asian newborns waist circumference, a measure of abdominal obesity in adults but not validated in newborns was significantly higher (31.1 vs 29.9 cm, P=0.0001), although no difference in the waist to height ratio was observed between the groups (0.598 in SA vs 0.595 in Whites; P=0.40; Table 2). Also, the ponderal index was substantially lower (23.3 vs 27.9, P<0.0001) in South Asian than in White newborns. Furthermore, at any level of skinfold thickness, South Asian newborns had a lower ponderal index (that is, are thinner) than White newborns (P<0.0001; Figure 2).
Figure 1.
Mean skinfold thickness by birthweight quartiles comparing South Asian and White newborns adjusting for newborn age, sex and age at birth visit. Using continuous birthweight, South Asian newborns have higher skinfolds over all birthweights (P<0.0001); the increasing trend in both ethnic groups is significant (P<0.0001). Note that adjustment for GDM makes no appreciable difference in the results.
Table 2. Newborn baseline characteristicsa.
| South Asian | White Caucasian | P-value | |
|---|---|---|---|
| N per Group | 400 | 389 | |
| Male (%) | 47.9 | 48.1 | 0.96 |
| Gestational age at birth, weeks | 39.3 | 39.6 | 0.002 |
| Birthweight, g | 3283 (22) | 3517 (22) | <0.0001 |
| Newborn head circumference, cm | 34.1 (0.1) | 34.9 (0.1) | <0.0001 |
| Newborn length, cm | 52.1 (0.1) | 50.2 (0.1) | <0.0001 |
| Newborn Waist circumference, cm | 31.1 (0.1) | 29.9 (0.1) | <0.0001 |
| Waist to height ratio | 0.598 (0.002) | 0.595 (0.002) | 0.40 |
| Triceps skinfolds, mm | 6.2 (0.1) | 5.4 (0.1) | <0.0001 |
| Subscap skinfolds, mm | 5.6 (0.1) | 5.2 (0.1) | <0.0001 |
| Tricep+subscapular skinfolds, mm | 11.7 (0.1) | 10.6 (0.1) | <0.0001 |
| Ponderal index, kg m−3 | 23.3 (0.2) | 27.9 (0.2) | <0.0001 |
Ponderal index defined as, Birthweight in kg per birthlength in m3.
Means (s.e.) Adjusted for gestational age and sex.
Figure 2.
Newborn skinfold thickness by ponderal index (kg m−3). White newborns have a higher ponderal index over all skinfolds (P<0.0001); the increasing trend in both ethnic groups is significant (P<0.0001). There is no significant interaction (P=0.19).
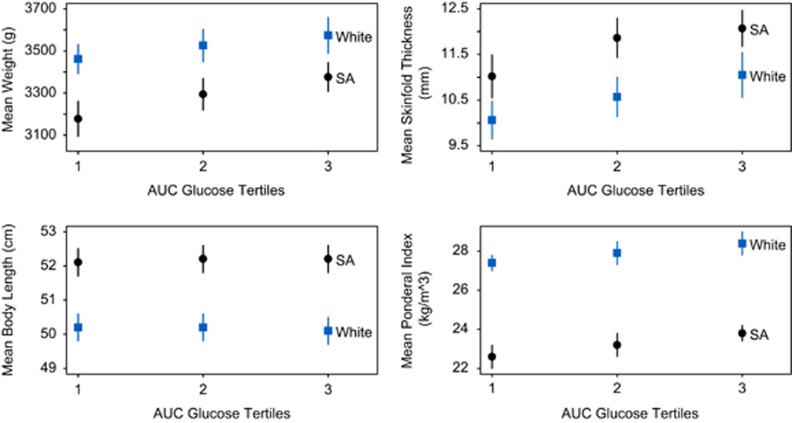
To determine the impact of increased maternal dysglycemia on newborn body composition, the AUC glucose was divided into tertiles, and the anthropometric characteristics of South Asian and White newborns were examined. Birthweight (P<0.0001), skinfold thickness (P=0.006) and ponderal index (P<0.0001) increased progressively with increasing maternal glucose in both ethnic groups, but neonatal length (P=0.57) did not (Figure 3).
Figure 3.
Newborn body composition by maternal AUC glucose tertiles (women receiving insulin during pregnancy excluded), adjusting for newborn sex and gestational age. Ethnic differences are significant for each newborn parameter (P<0.0001 for each). Birthweight, skinfold thickness, and ponderal index increase with increasing AUC glucose (continuous measure) adjusting for newborn sex and gestational age (P<0.0001, P=0.0006, P<0.0001 respectively); while there is no trend found with birthlength (P=0.57).
Predictors of newborn skinfold thickness
Individual maternal factors, adjusting for ethnicity were first tested in regression models. Factors with a P<0.10 (years in Canada, prepregnancy weight, tricep plus subscapular skinfolds thickness, AUC glucose, social disadvantage index and physical activity) as well as newborn male sex and gestational age were retained in a multiple regression analysis (Table 3). The final model showed that in addition to male sex and gestational age, South Asian ethnicity (standardized estimate (s.e.): 0.24; P<0.0001), maternal skinfold thickness—a surrogate measure of maternal body fat (s.e.: 0.14; P=0.0002)—and maternal AUC glucose (s.e.: 0.079; P=0.04) were significantly associated with newborn skinfold thickness (Table 3).
Table 3. Predictors of newborn skinfold thickness.
| Factor |
Single variable models adjusting for ethnicity |
Full multivariable modela |
Final multivariable modelb |
|||
|---|---|---|---|---|---|---|
| Standardized parameter estimate | P-value | Standardized parameter estimate | P-value | Standardized parameter estimate | P-value | |
| Maternal characteristics | ||||||
| South Asian | 0.234 | <0.0001 | 0.326 | <0.0001 | 0.237 | <0.0001 |
| Primiparous | −0.034 | 0.34 | NA | |||
| Years in Canada | 0.108 | 0.10 | 0.066 | 0.36 | ||
| Prepregnancy weight (kg) | 0.161 | <0.0001 | 0.054 | 0.34 | ||
| Maternal height (m) | 0.029 | 0.43 | NA | |||
| Tricep+subscapular skinfold (mother) | 0.145 | <0.0001 | 0.117 | 0.03 | 0.141 | 0.0002 |
| Gestational weight gain | 0.021 | 0.57 | NA | |||
| AUC glucose (mmol × h) | 0.119 | 0.001 | 0.054 | 0.19 | 0.079 | 0.04 |
| Smoking during pregnancy | −0.002 | 0.96 | NA | |||
| Vegetarian | −0.045 | 0.27 | NA | |||
| Social Disadvantage Index (relative to low) | ||||||
| Moderate | −0.076 | 0.06 | −0.059 | 0.14 | ||
| High | −0.046 | 0.24 | −0.025 | 0.54 | ||
| Physical activity | −0.063 | 0.08 | −0.047 | 0.22 | ||
| Newborn Characteristics | ||||||
| Male sex | −0.097 | 0.006 | −0.090 | 0.02 | −0.083 | 0.02 |
| Gestational age at birth | 0.059 | 0.10 | 0.084 | 0.03 | 0.082 | 0.02 |
Factors with a univariate P<0.10 were tested in a multiple regression model holding newborn age and sex in the model.
Backward stepwise regression methods were used to determine the final model with all variables significant at P<0.05 holding newborn age and sex in the model (R2=0.10).
Discussion
South Asian newborns have lower birthweight and greater skinfold thickness—considered to represent ‘fatness', when compared with White newborns. The skinfold thickness is independently influenced by maternal glucose and maternal body fat.
We identified intriguing differences in newborn body composition as South Asian offspring were lower birthweight, more adipose and had higher waist circumference compared to Whites. Lower birthweight and increased adipose tissue have been observed in some but not all prior comparisons of South Asian and White offspring.3, 4, 20 We observed that across a range of birth weights, South Asians have significantly higher adipose tissue compared with Whites. South Asian ethnicity, mother's body fat and increased maternal glucose were positively associated with skinfold thickness—a surrogate measure of adiposity in newborns. However, collectively these factors accounted for only 10% of the variance of the newborn skinfold thickness, which suggests that additional factors including genetic and epigenetic factors may also be important determinants of this phenotype.21, 22
Our observation that South Asian newborns have an increased waist circumference compared with White newborns may be important because increased waist circumference is associated with increased trunk fat mass in children aged 3 to 5 years,23 insulin resistance in children ages 9 to 11 years of age,24 cardiometabolic risk factors in children and adolescents25 and is strongly correlated with visceral adiposity and cardiometabolic factors in adults.1, 22 Our observation is supported by a prior report using whole-body magnetic resonance imaging, which found that full-term South Asian newborns in India compared with White newborns in London, UK (within 2 weeks of birth) had increased abdominal adiposity (including more visceral, deep subcutaneous, and superficial subcutaneous fat) and less nonabdominal superficial subcutaneous fat.26 However, the waist to height ratio was not different between South Asian and White newborns. Waist to height ratio has been proposed as a simple screen for visceral adiposity in adults, adolescents and children,27, 28 but to our knowledge has not been evaluated in full-term newborns. Therefore, although the waist circumference taken at birth may be a useful predictor of future cardiometabolic risk in the growing offspring, both waist circumference and waist to height ratio in newborns require validation in newborns and confirmation of their predictive abilities in longitudinal studies.
South Asian pregnant women were smaller stature yet had increased dysglycemia compared with White women. Thus, South Asian fetuses were exposed to greater hyperglycemia in utero compared with Whites. The expected physiologic response of the fetus to maternal dysglycemia is greater production of fetal insulin, and subsequently increased lean and adipose tissue growth, with the extreme hyperglycemic fetus becoming macrosomic.11 Birthweight, adipose tissue and lean body mass increased among South Asian and White newborns with increasing maternal glycemia. Despite having a significantly higher burden of dysglycemia, South Asians were lower birthweight, yet had higher skinfold thickness in each AUC glucose tertile (Figure 3). Future investigations of fetal/newborn insulin and adipokines, such as leptin and adiponectin, genetic and epigenetic factors, may further elucidate the reasons for differences in skinfold measurements relative to lower birthweight among South Asians.29, 30
Our study has a number of strengths. First, South Asians and White participants from the START and FAMILY birth cohorts both live in the same geographic regions of Southern Ontario, Canada. Second, standardized methods to assess hyperglycemia, maternal and infant anthropometry and health behaviors were used in both cohorts. These are advantages over previous transcontinental studies, which have indirectly compared South Asians with Whites. There are, however, some limitations of our interethnic comparisons. A potential for bias includes use of mother's self-reported prepregnancy weight, although this has been found to be reasonably reliable in previous birth cohorts.31 Additional limitations include: the use of two different instruments to measure skinfold thickness in the two cohorts with no assessment of interobserver variability, timing of measurement of skinfold thickness ranged between 0 to 96 h after birth although adjustment for time and measurement did not change our results, and birth weights that were extracted from hospital records. Another potential limitation is that the time period during which the cohorts were recruited is not identical, as the Whites were recruited between 2002 and 2009 and the South Asians between 2011 and 2013. However, we do not believe that there has been any considerable secular change in lifestyle factors, that is, in availability of food or walking environment, or in health care delivery especially as Canada has a public health care system within which there is equal access to prenatal and hospital care for all landed immigrants and citizens.
Conclusion
South Asian newborns are lower birthweight, and have greater skinfold thickness compared with White newborns, and this is influenced by increased maternal body fat and glucose. Interventions aimed at reducing body fat prior to pregnancy and GDM during pregnancy in South Asians may favorably alter newborn body composition and require evaluation.
Acknowledgments
We thank Dr David Mowat, Peel Public Health, Dr V Persad, Dr J Chanchlani. START Canada Investigators: G Pare, A Mente, J Beyene, R Anglin; START Research Staff: N Aga, S Hirjikaka, M Kooner, F Khan, M Nundy, Z Sohani, S Sothiratnam. This work was supported by Canadian Institutes of Health Research: Grant # 109205, Heart and Stroke Foundation of Canada: NA 7283. We also thank FAMILY Study Investigators: S Atkinson, J Bourgeois, M McQueen, S Yusuf; FAMILY Staff, (Population Health Research Institute, Hamilton Health Sciences and McMaster University, Hamilton, Canada), Principal Investigator: Dr K Teo, Project Manager: Sumathy Rangarajan, Study Statisticians: K Schulze, P RaoMelacini, Study Research Staff: N Abdalla, J Gross, L Helden, N Jakymyshyn, S Khurshid, C McLean-Price, L Rasnak, C Sim, S Steele, A Van Der Loo, V Vaughan Williams, C Wright. START-India Investigators: KS is the Principal Investigator, AV is the Co-Investigator and PD is the Study Coordinator for START India Collaborators St John Medical College Bangalore. We thank, Dr T S Sridhar (Molecular Medicine), Dr Jyothi S Prabhu, (Molecular Medicine), Dr Annamma Thomas, (Obstetrics and Gynecology), Dr Tinku Sarah Thomas (Biostatistics), Dr Sr Gladys Menezes, Director, Snehalaya Medical Relief Centre, Solur.
Author contributions
Researched data done by SA, MG, KM, KT, NA, DD, GW, CW, PS, KS; SA wrote the manuscript and researched the data; SA, MG, KS, AV, AK, PD Reviewed/edited the manuscript, and SA, MG, KT, KM, SM researched the data and contributed to discussion.
The authors declare no conflict of interest.
References
- 1Anand SS, Yusuf S, Vuksan V, Devanesen S, Montague P, Kelemen L et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic Groups (SHARE). Lancet 2000; 356: 279–284. [DOI] [PubMed] [Google Scholar]
- 2Abate N, Chandalia M. Ethnicity and type 2 diabetes: focus on Asian Indians. J Diabetes Complications 2001; 15: 320–327. [DOI] [PubMed] [Google Scholar]
- 3Yajnik C, Fall C, Coyaji K, Hirve SS, Rao S, Barker DJ et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Ind J Obes Relat Metabol Disord 2003; 27: 173–180. [DOI] [PubMed] [Google Scholar]
- 4West J, Lawlor DA, Fairley L, Bhopal R, Cameron N, McKinney PA et al. UK-born Pakistani-origin infants are relatively more adipose than white British infants: findings from 8704 mother-offspring pairs in the Born-in-Bradford prospective birth cohort. J Epidemiol Community Health. 2013; 67: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Barker D, Osmond C, Golding J, Kuh D, Wadsworth M. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989; 289: 564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Wahi G, Anand SS. Race/Ethnicity, Obesity, and Related Cardio-Metabolic Risk Factors: A Life-Course Perspective. Curr Cardiovasc Risk Rep 2013; 7: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Stanfield KM, Wells JC, Fewtrell MS, Frost C, Leon DA. Differences in body composition between infants of South Asian and European ancestry: the London Mother and Baby Study. Int J Epidemiol. 2012; 41: 1409–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Mukerji G, Chiu M, Shah BR. Gestational diabetes mellitus and pregnancy outcomes among Chinese and South Asian women in Canada. J Matern Fetal Neonatal Med 2013; 26: 279–284. [DOI] [PubMed] [Google Scholar]
- 9Urquia M, Glazier RH, Berger H, Ying I, De Souza L, Ray JG. Gestational diabetes among immigrant women. Epidemiology 2011; 22: 879–880. [DOI] [PubMed] [Google Scholar]
- 10Ray JG, Jiang D, Sgro M, Shah R, Singh G, Mamdani MM. Thresholds for small for gestational age among newborns of East Asian and South Asian ancestry. J Obstet Gynaecol Can 2009; 31: 322–330. [DOI] [PubMed] [Google Scholar]
- 11HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 12Morrison KM, Anand SS, Yusuf S, Atkinson SA, Schulze KM, Rao-Melacini P et al. Maternal and pregnancy related predictors of cardiometabolic traits in newborns. PLoS One 2013; 8: e55815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13Anand SS, Vasudevan A, Gupta M, Morrison K, Kurpad A, Teo KK et al. Rationale and design of South Asian Birth Cohort (START): a Canada-India collaborative study. BMC Public Health 2013; 13: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Agarwal MM, Dhatt GS, Shah SM. Gestational diabetes mellitus: simplifying the international association of diabetes and pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care 2010; 33: 2018–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuler L. Blood glucose area under the curve – methodological aspects. Diabetes Care 1990; Vol 13: 172–175. [DOI] [PubMed] [Google Scholar]
- 16Kelemen LE, Anand SS, Vuksan V, Yi Q, Teo KK, Devanesen S et al. Development and evaluation of cultural food frequency questionnaires for South Asians, Chinese, and Europeans in North America. J Am Diet Assoc. 2003; 103: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 17Anand S, Razak F, Davis A, Jacobs R, Vuksan V, Teo K et al. Social disadvantage and cardiovascular disease: development of an index and analysis of age, sex, and ethnicity effects. Int J Epidemiol 2006; 35: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 18Sommer C, Mørkrid K, Jenum AK, Sletner L, Mosdøl A, Birkeland KI. Weight gain, total fat gain and regional fat gain during pregnancy and the association with gestational diabetes: a population-based cohort study. Int J Obes (Lond) 2014; 38: 76–81. [DOI] [PubMed] [Google Scholar]
- 19Winer BJ. Statistical Principles in Experimental Design. 2nd edn. McGraw-Hill Kogakusha, Ltd.: London, UK, 1971. [Google Scholar]
- 20Muthayya S, Dwarkanath P, Thomas T, Vaz M, Mhaskar A, Mhaskar R et al. Anthropometry and body composition of south Indian babies at birth. Public Health Nutr 2006; 9: 896–903. [DOI] [PubMed] [Google Scholar]
- 21Krishnaveni G, Hill J, Veena S, Bhat DS, Wills AK, Karat CL et al. Low plasma vitamin B(12) in pregnancy is associated with gestational ‘diabesity' and later diabetes. Diabetologia 2009; 52: 2350–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Hattersley AT, Tooke JE. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 1999; 353: 1789–1792. [DOI] [PubMed] [Google Scholar]
- 23Taylor RW, Williams SM, Grant AM, Ferguson E, Taylor BJ, Goulding A. Waist circumference as a measure of trunk fat mass in children aged 3 to 5 years. Int J Pediatr Obes 2008; 3: 226–233. [DOI] [PubMed] [Google Scholar]
- 24Rodríguez G, Moreno LA, Blay MG, Blay VA, Garagorri JM, Sarria A et al. Body composition in adolescents: measurements and metabolic aspects. Int J Obes Relat Metab Disord 2004; 28: S54–S58. [DOI] [PubMed] [Google Scholar]
- 25Katzmarzyk PT, Srinivasan SR, Chen W, Malina RM, Bouchard C, Berenson GS. Body mass index, waist circumference, and clustering of cardiovascular disease risk factors in a biracial sample of children and adolescents. Pediatrics 2004; 114: e198–e205. [DOI] [PubMed] [Google Scholar]
- 26Modi N, Thomas EL, Uthaya SN, Umranikar S, Bell JD, Yajnik C. Whole body magnetic resonance imaging of healthy newborn infants demonstrates increased central adiposity in Asian Indians. Pediatr Res 2009; 65: 584–587. [DOI] [PubMed] [Google Scholar]
- 27Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev 2012; 13: 275–286. [DOI] [PubMed] [Google Scholar]
- 28Hara M, Saitou E, Iwata F, Okada T, Harada K. Waist-to-height ratio is the best predictor of cardiovascular disease risk factors in Japanese schoolchildren. Atheroscler Thromb 2002; 9: 127–132. [DOI] [PubMed] [Google Scholar]
- 29Lango Allen H, Estrada K. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 2010; 467: 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30West J, Wright J, Fairley L, Sattar N, Whincup P, Lawlor DA. Do ethnic differences in cord blood leptin levels differ by birthweight category? Findings from the Born in Bradford cohort study. Int J Epidemiol. 2014; 43: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: The Coronary Artery Risk Development in Young Adults Study. J Am Heart Assoc 2014; 3: e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]