Abstract
Aim:
To evaluate the SimCYP simulator ethnicity-specific population model for predicting the pharmacokinetics of midazolam, a typical CYP3A4/5 substrate, in Chinese after oral administration.
Methods:
The physiologically based pharmacokinetic (PBPK) model for midazolam was developed using a SimCYP population-based simulator incorporating Chinese population demographic, physiological and enzyme data. A clinical trial was conducted in 40 Chinese subjects (the half was females) receiving a single oral dose of 15 mg midazolam. The subjects were separated into 4 groups based on age (20–50, 51–65, 66–75, and above 76 years), and the pharmacokinetics profiles of each age- and gender-group were determined, and the results were used to verify the PBPK model.
Results:
Following oral administration, the simulated profiles of midazolam plasma concentrations over time in virtual Chinese were in good agreement with the observed profiles, as were AUC and Cmax. Moreover, for subjects of varying ages (20–80 years), the ratios of predicted to observed clearances were between 0.86 and 1.12.
Conclusion:
The SimCYP PBPK model accurately predicted the pharmacokinetics of midazolam in Chinese from youth to old age. This study may provide novel insight into the prediction of CYP3A4/5-mediated pharmacokinetics in the Chinese population relative to Caucasians and other ethnic groups, which can support the rational design of bridging clinical trials.
Keywords: midazolam, pharmacokinetics, PBPK model, CYP3A4/5, Chinese
Introduction
As of 2014, China is the second largest pharmaceutical market, behind the United States1. Many large pharmaceutical companies have increased their presence in China, resulting in a dramatic increase in the number of clinical trials in the last decade2,3. It is not surprising that an area of strategic focus for industry is faster product registration and entry into this emerging market; however, delays occur due to the critical concern that potential ethnic differences may result in substantial variability in the dose-response relationship, which ultimately affects safety and efficacy4,5.
Physiologically based pharmacokinetic modeling (PBPK) is a key component of model-based drug development and is increasingly embraced by industry6,7 and regulatory authorities8,9,10. It is well-recognized that PBPK modeling can assess ethnicity sensitivity in virtual populations, thereby informing the need for and design of real studies. Currently, PBPK models have been widely applied to the prediction of pharmacokinetics and drug-drug interactions in healthy subjects11,12,13,14,15 and in specific populations (eg, cancer patients) in China16,17,18; however, limited demographic and physiological knowledge (eg, liver weight, cardiac output, and enzyme abundance, etc) impede the development of the PBPK approach in the Chinese population. Presently, commercially available PBPK software (eg, SimCYP and Gastroplus) affords the opportunity to predict drug exposure and disposition in different ethnic groups. These population-based PBPK approaches have been widely used to address critical concerns such as pharmacokinetics in pediatric populations, organ impairment, and metabolic drug-drug interactions19,20; however, as these models use parameters gathered from primarily Western populations (ie, Caucasians) their capacities for predicting pharmacokinetics (PK) in Chinese are rather limited.
Midazolam is a short-acting hypnotic-sedative drug with anxiolytic and amnestic properties. Since the 1980s, it has become the most frequently used benzodiazepine for the induction of anesthesia and conscious sedation, as well as for long-term sedation in patients receiving mechanical ventilation. Midazolam is primarily metabolized by CYP3A4/5, and greater than 95% of the dose is metabolized by CYP3A4/521,22,23. Consequently, the FDA has recommended midazolam for use as a typical substrate to characterize the CYP3A metabolic pathway24. Recently, Barter et al developed an ethnicity population database for the prediction of differences in cytochrome P450 (CYP)-mediated pharmacokinetics between Chinese and Caucasians using alprazolam and midazolam as probe drugs for the CYP3A metabolic pathway11. This study was the first systematic attempt at investigating inter-ethnic differences in the Chinese population using mechanistic PBPK modeling. Unfortunately, the predicted oral clearance of midazolam in Chinese was 2-fold higher than previously reported, while the prediction for alprazolam was in good agreement with observed data. With the limitations to the gut physiology and enzymology of the Chinese population model11,12, the scarcity of quality of clinical data and appreciable inter-study variability may account for the overprediction11. Moreover, the PK data used for model verification were mostly acquired from young Chinese male subjects (19–31 years) with homogeneous demographics25,26,27 and special populations such as the elderly were not involved.
It is well-recognized that CYP3A is an important metabolic pathway that accounts for the metabolism of approximately 50%–60% of therapeutic drugs28; therefore, it is necessary to evaluate the feasibilityof the CYP3A PBPK models for the Chinese population. This study aimed to evaluate the SimCYP Chinese population model for midazolam (a CYP3A4/5 substrate) using a top-down approach and to investigate whether the pharmacokinetics of drugs that are predominantly metabolized by CYP3A4/5 were well captured.
Materials and methods
Clinical study of midazolam in Chinese after oral administration
To acquire reliable pharmacokinetic data for the model evaluation and investigate midazolam pharmacokinetics in Chinese, an open-label, parallel group single-dose study was performed. The study was performed in accordance with Good Clinical Practice regulations and the ethical principles stated in the Declaration of Helsinki and was approved by the Ethical Committee of the Peking Union Medical College Hospital (Beijing, China) prior to subject enrollment. After giving written informed consent, 40 Chinese subjects were enrolled based on the following inclusion and exclusion criteria: men and women of Chinese ethnicity (three generations living in mainland China), 20 to 85 years of age, and a body mass index of 19–32 kg/m2. For subjects ≤65 years, there was no evidence of clinically significant abnormalities in cardiac, hepatic, renal, pulmonary, neurological, gastrointestinal, hematological and psychiatric function as determined by medical history, physical examination and laboratory screens; no history of drug or substance abuse; and no allergic history to benzodiazepines. For elderly subjects (>65 years), minor stable diseases frequently encountered in persons (eg, mild hypertension or arthritis) were permitted. Female subjects were required to be surgically incapable of pregnancy, or practicing an acceptable double-barrier birth control method such as a condom or diaphragm with spermicide. For all the volunteers, we excluded those that used medications with a pronounced effect on the central nervous system within the last 3 months, or had impaired hearing or epilepsy. Intake of concomitant drugs was not permitted.
Forty Chinese subjects were enrolled and divided into 4 groups (20–50, 51–65, 66–75, and >76 years). Each age group included 10 subjects (half men and half women). On the study day, the subjects were given one tablet of midazolam (15 mg/tablet, Nhwa Pharmaceutical Co, Xuzhou, China) with 250 mL of water following overnight fasting. Standard meals were provided at 4 and 10 h post-dose. Serial blood samples (5 mL at each time point) were collected at the following time points: 0 (pre-dose), 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 12 h post-dose. A validated HPLC-MS/MS method was utilized to determine the plasma concentrations of midazolam. The assay was linear between 0.5 and 250 ng/mL, and the lower limit of quantification (LLOQ) was 0.5 ng/mL. Three quality control samples (1.5, 100 and 200 ng/mL) were also analyzed to ensure bioassay quality.
Pharmacokinetic parameters were calculated using non-compartmental analysis (NCA) with WinNonlin (Version 6.3; Pharsight, NC, USA). Cmax was the maximum plasma concentration in a profile, and Tmax was the time to Cmax. The elimination half-life (t1/2) was estimated by linear regression of log-transformed concentration-time data. The area under the plasma concentration-time curve from time zero to time t (AUC0-t), where t is the time of last measurable sample, was calculated according to the linear trapezoidal rule. The AUC from time zero to infinity (AUC0-∞) was estimated as AUC0-t +Ct/λz, where Ct was the plasma concentration of the last measurable sample. The apparent total clearance (CL/F) was calculated as Dose/AUC0-∞ and the apparent total volume of distribution (V/F) as calculated as CL/λz. Statistical analysis was performed using SPSS software (version 19, SPSS Inc, Chicago, IL, USA). Descriptive statistics were expressed as the mean±standard deviation (SD). Cmax and AUC data were transformed by natural logarithm prior to statistical analysis. Differences among the age groups were identified by analysis of variance (ANOVA) and were considered to be significant when P<0.05.
PBPK model development
The PBPK model was developed using the SimCYP population-based simulator® (version 13.0, SimCYP Ltd, Sheffield, UK).
Virtual population
There are two Chinese population databases in the SimCYP population-based simulator. One database is constructed based on demographic data of 8118 Han Chinese obtained from the China Health and Nutrition Survey 200629, and the other is a volunteer database (594 healthy Chinese) provided by Pfizer Ltd (Sandwich, UK). In the current study, the first database was selected for simulation, with ages ranging between 20 and 80 years and with a proportion of females of 0.5. The age distributions were fitted independently in males and females using a Weibull 2 parameter model30. The mean bodyweights for males and females were 62 and 60 kg, respectively, and the mean heights were 1.73 and 1.58 m, respectively. The mean BSA of the general Chinese population were 1.73 and 1.55 m2 for males and females, respectively. Moreover, the mean abundances of CYP3A4 and 3A5 in the liver were 120 and 82 pmol/mg protein, respectively. As no data were available regarding intestinal CYP abundances in Chinese, the Chinese CYP3A4/5 intestinal abundances (58 and 21.5 pmol/mg) in SimCYP were scaled from Caucasians11,31.
Midazolam PBPK model
The SimCYP compound file for midazolam was used for modeling and the input parameters are summarized in Table 1. The SimCYP segmental advanced dissolution absorption metabolism (ADAM) model32 was used for absorption. As the midazolam tablets used in this study were formulated for immediate release (IR), IR tablet dissolution profiles were acquired by in vitro dissolution assays and incorporated into the ADAM model. Given the high lipophilicity of midazolam, a full PBPK model was utilized to improve recovery of plasma concentration-time profiles. The volume of distribution was predicted using the Rodgers et al method33, and the default SimCYP enzyme kinetics of midazolam were used to model elimination. Given the lack of hepatic and intestinal CYP3A4/A5 abundance data in Chinese populations, the following assumptions were made, as previously mentioned in Barter et al11: (1) the abundances of hepatic CYP3A5 in Chinese and Japanese were assumed identical; (2) the abundance of Chinese intestinal CYP3A4/5 was scaled from that of Caucasians based upon the relationship between hepatic CYP abundances in Caucasians and Chinese31.
Table 1. Summary of input parameters for midazolam PBPK model.
| Parameter | Midazolam | Method/reference |
|---|---|---|
| Absorption | ||
| Absorption model | ADAM | |
| Caco-2 permeability (×10−6 cm/s) | 6.59 | Calculated using SimCYP |
| fuGut | 1 | Assumed |
| Formulation | Immediate release tablet | |
| Dissolution | ||
| Distribution | Dissolution profile (>95% was released within 0.5 h) | From an in vitro dissolution test |
| Distribution model | Full PBPK model | |
| Vss (L/kg) | 10.048 | Calculated using SimCYP |
| Enzymes kinetics | 1-OH/CYP 3A4: Vmax 5.23, Km 2.18* | Ref 11 |
| 1-OH/CYP 3A5: Vmax 19.7, Km 4.16 | Ref 11 | |
| 4-OH/CYP 3A4: Vmax 5.2, Km 31.8 | Ref 11 | |
| 4-OH/CYP 3A4: Vmax 4.03, Km 34.8 | Ref 11 | |
*Vmax, pmol·min−1·pmol−1 CYP enzyme; Km, μmol/L.
Simulation of midazolam pharmacokinetics in Chinese after oral administration
Simulations were performed for subjects in 4 age groups (20–50, 51–65, 66–75 and >76 years) using the SimCYP Chinese general population profile. Each simulation was performed for 10 trials with 100 subjects (total n=1000, with the proportion of females being 0.5). The trial design was identical to the real clinical study: a single dose of 15 mg of midazolam was administered to fasted subjects with 250 mL of water at 8:00 AM, and the study was 12 h in duration.
Results
Demographics
Forty Chinese subjects completed the clinical study and demographics are shown in Table 2. The distributions of mean demographics (age, height, weight and body mass index) of these subjects were comparable to the virtual Chinese population simulated by SimCYP.
Table 2. Subject demographics (mean±SD).
| Cohort | Age (year) | Weight (kg) | Height (m) | BMI (m/kg2) | |
|---|---|---|---|---|---|
| Observed subjects | 20–50 years (n=10) | 32 (23–43)* | 61.5±8.6 | 1.64±0.07 | 22.3±2.1 |
| 51–65 years (n=10) | 57 (55–62) | 73.9±13.4 | 1.66±0.07 | 26.9±4.7 | |
| 66–75 years (n=10) | 72 (67–74) | 67.4±9.4 | 1.57±0.08 | 27.3±2.5 | |
| >76 years (n=10) | 77 (76–80) | 70.8±9.8 | 1.63±0.09 | 26.7±2.7 | |
| Virtual subjects | 20–50 years (n=1000) | 40 (20–50)* | 59.0±10.5 | 1.63±6.83 | 22.0±3.4 |
| 51–65 years (n=1000) | 57 (51–65) | 57.0±10.3 | 1.61±6.7 | 23.2±6.6 | |
| 66–75 years (n=1000) | 69 (66–75) | 56.0±10.4 | 1.59±6.5 | 22.1±3.6 | |
| >76 years (n=1000) | 77 (76–80) | 54.0±9.6 | 1.57±6.73 | 22.6±3.7 |
*, range; BMI, body mass index.
Pharmacokinetics of midazolam in Chinese at 20–80 years
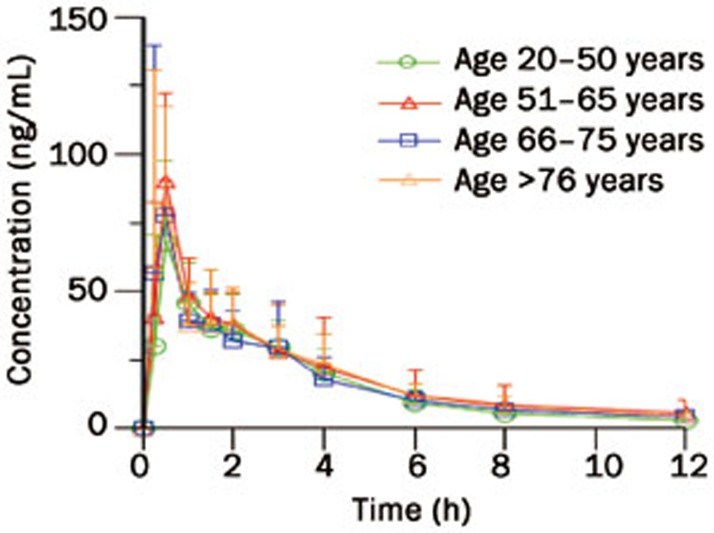
To obtain reliable PK data for evaluation of the model, a clinical study was designed and performed in which forty Chinese subjects (age 23–80 years) were treated with 15 mg of midazolam. The mean pharmacokinetic parameters and mean concentration-time profiles of oral midazolam are shown in Table 3 and Figure 1. Midazolam was absorbed rapidly, with median tmax of 0.7 h, and an elimination t1/2 of 3–5 h following oral administration. Mean oral clearance (CL/F) was 65–75 L/h and the mean volume of distribution (V/F) was 350–420 L. Statistical analysis indicated that there were no significant differences in system plasma exposure (AUC and Cmax), CL/F and V/F for midazolam among the 4 age groups (P>0.05). Moreover, there were no significant differences between female and male subjects with regards to these parameters (P>0.05). These results suggest that the pharmacokinetics of midazolam following oral administration were not affected by either subject age or gender.
Table 3. Pharmacokinetic parameters of the Chinese subjects after oral administration of 15 mg midazolam (mean±SD).
| PK parameter | Group 1 (Age 20–50 years) |
Group 2 (Age 51–65 years) |
Group 3 (Age 66–75 years) |
Group 4 (Age >76 years) |
||||
|---|---|---|---|---|---|---|---|---|
| Male (n=5) | Female (n=5) | Male (n=5) | Female (n=5) | Male (n=5) | Female (n=5) | Male (n=5) | Female (n=5) | |
| Cmax (ng/mL)a | 68.6±17.5 | 88.4±25.3 | 100.3±29.5 | 95.7±25.1 | 80.5±18.5 | 115.4±52.6 | 100.7±69.3 | 92.9±51.0 |
| Tmax (median, range) | 1 (0.5–1.5) | 0.5 (0.25–0.5) | 0.5 (0.5–0.5) | 0.5 (0.25–3) | 0.5 (0.5–1.5) | 0.5 (0.25–0.5) | 0.5 (0.25–0.5) | 0.5 (0.25–4) |
| T1/2 (h) | 2.9±0.9 | 3.6±1.0 | 3.7±0.6 | 6.1±0.9 | 4.7±1.3 | 4.8±1.9 | 3.8±0.9 | 4.4±1.3 |
| AUC0-∞(h·ng/mL) | 223.8±52.5 | 199.3±55.6 | 274.3±123.4 | 278.0±167.0 | 195.7±74.8 | 262.2±42.9 | 233.8±77.8 | 274.2±100.3 |
| CL/F (L/h) | 70.0±16.0 | 80.7±24.8 | 63.1±24.7 | 66.7±28.9 | 84.8±28.4 | 64.3±20.4 | 70.1±22.9 | 61.9±25.4 |
| V/F (L) | 306.4±149.6 | 398.7±86.6 | 326.2±107.7 | 568.9±194.3 | 559.7±238.6 | 387.2±102.5 | 382.6±172.5 | 387.6±172.6 |
aP>0.05.
Figure 1.
Mean plasma concentration-time profiles of midazolam in Chinese subjects after oral administration (n=40).
Simulation of midazolam plasma concentration-time profiles
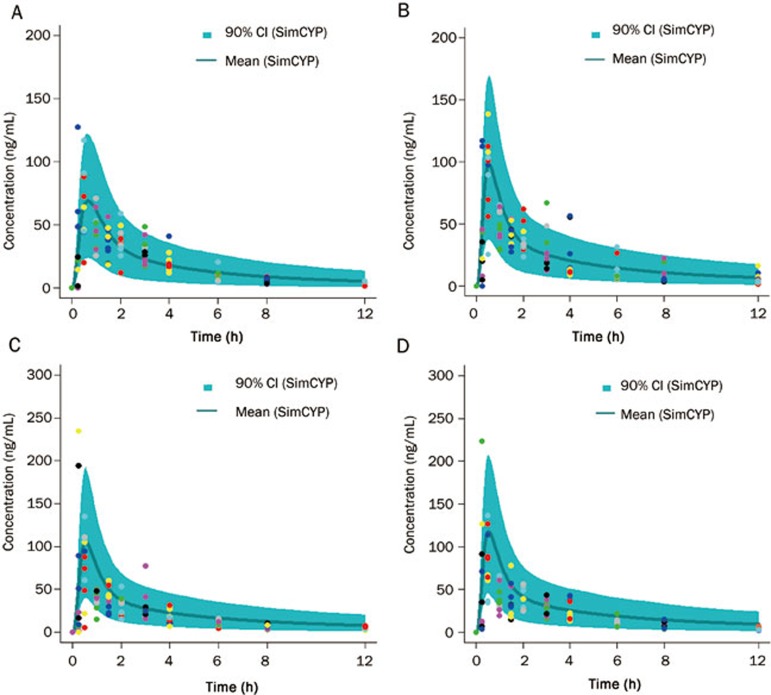
The simulated and observed plasma concentration-time profiles of midazolam in Chinese after oral administration are shown in Figure 2, and Table 4 summarizes the observed and predicted PK parameters. These data indicate that use of the SimCYP ADAM and full PBPK model resulted in the recovery of reasonable plasma concentration-time profiles in virtual Chinese. For the 20–50 years subjects, the predicted Cmax and AUC0-t were 63.4 ng/mL and 184.1 h·ng/mL, respectively. These values were in good agreement with the observed data of 68.7 ng/mL and 192.7 h·ng/mL. Moreover, for the middle- and old age subjects (51–65 years and 66–80 years), the predictions of Cmax and AUC were also comparable to the observed values (Table 4).
Figure 2.
Simulated mean plasma concentration–time (line) and observed plasma concentration–time (points) profiles of midazolam in Chinese after oral administration. (A) 20–50 years; (B) 51–65 years; (C) 66–75 years; (D) >76 years. The blue line represents the 90th percentile of the virtual population.
Table 4. Geometric mean of observed and predicted PK parameters (95%CI in brackets) in Chinese.
| PK Parameter | Observed | Predicted | Ratio* | |
|---|---|---|---|---|
| Age 20–50 years | Cmax (ng/mL) | 68.7 (51.6–91.3) | 63.4 (61.5–65.4) | 0.94 |
| AUC0-t (h·ng/mL) | 192.7 (159.7–232.2) | 184.1 (177.3–191.1) | 0.96 | |
| CLPO# (L/h) | 73.0 (60.6–88.1) | 81.5 (78.5–84.6) | 1.12 | |
| Age 51–65 years | Cmax (ng/mL) | 94.5 (76.7–116.3) | 90.3 (87.7–92.9) | 0.96 |
| AUC0-t (h·ng/mL) | 218.8 (164.6–290.8) | 225.6 (217.8–233.8) | 1.03 | |
| CLPO# (L/h) | 59.8 (43.5–82.3) | 66.5 (64.2–68.9) | 1.11 | |
| Age 66–75 years | Cmax (ng/mL) | 93.2 (64.6–134.2) | 97.1 (94.3–99.6) | 1.04 |
| AUC0-t (h·ng/mL) | 199.6 (163.1–244.5) | 247.3 (238.8–256.2) | 1.23 | |
| CLPO#(L/h) | 66.5 (53.6–83.6) | 60.7(58.6–62.8) | 0.91 | |
| Age >76 years | Cmax (ng/mL) | 83.1 (54.6–126.3) | 108.8 (105.7–111.2) | 1.19 |
| AUC0-t (h·ng/mL) | 215.3 (167.8–276.2) | 278.7 (268.9–288.9) | 1.29 | |
| CLPO#(L/h) | 62.4 (48.5–80.3) | 53.8 (53.9–58.8) | 0.86 |
#CLpo for oral administration;
*ratios of predicted to observed.
Midazolam clearance prediction
After simulation in 1000 virtual Chinese for each age group (20–50, 51–65, 66–75 and >76 years), the predicted oral clearances were 81.5, 66.5, 60.7 and 53.8 L/h, respectively, and the observed values were 73.0, 59.8, 66.5 and 62.4 L/h, respectively. The ratios of predicted to observed clearances were between 0.8 and 1.25 (Table 4). These simulations demonstrated that the present midazolam PBPK model could recover midazolam clearance very well in Chinese.
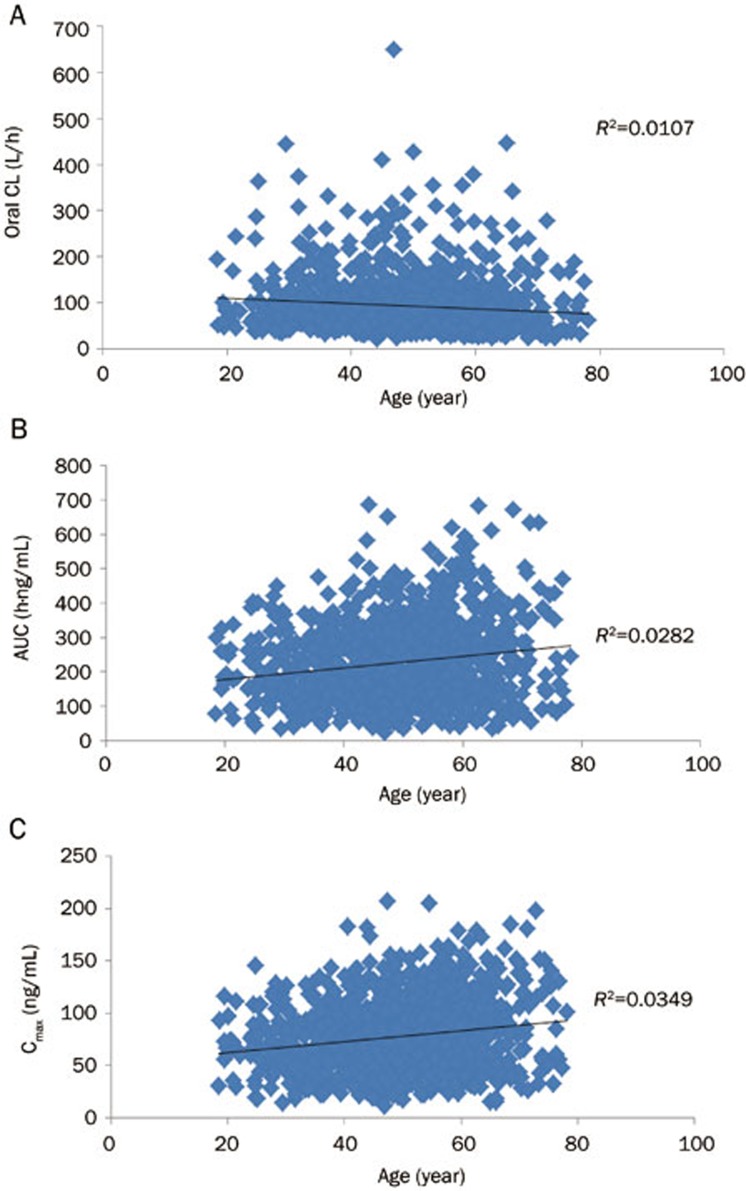
Furthermore, we attempted to apply the PBPK model to investigate the effects of age and gender on midazolam pharmacokinetics in Chinese. After simulation in virtual Chinese subjects (n=1000, with a proportion of females of 0.5), we plotted the regression of AUC, Cmax and CL versus age (Figure 3). These data indicated that predicted AUC, Cmax and CL in virtual Chinese subjects were not closely related to age, consistent with our observations from the clinical study. Moreover, one-way ANOVA analysis demonstrated that there were no significant differences between female and male virtual subjects with regards to the predicted AUC, Cmax and CL (P>0.05).
Figure 3.
Relationships of the predicted oral CL, AUC and Cmax to age (n=1000).
Discussion
CYP3A is one of the most important metabolic pathways and accounts for the metabolism of approximately 50%–60% of the drugs on the market28. Recently, Barter et al reported an ethnic-specific PBPK model for predicting CYP3A-mediated pharmacokinetics in Chinese and Caucasian populations11. Unfortunately, the predicted oral clearance of midazolam in Chinese was 2-fold higher than that in previous publications, and the scarcity of quality of clinical data and appreciable interstudy variability in the outcome was assumed to potentially account for the overprediction.
In this study, we developed a midazolam PBPK model for CYP3A4/5 using the SimCYP Chinese population database, and we validated our model using a top-down study in which we performed a clinical study of forty Chinese subjects (23–80 years).
Furthermore, the PBPK model was used to investigate the effects of age and gender on midazolam pharmacokinetics in Chinese after oral administration.
Midazolam pharmacokinetics in Chinese after oral administration
The pharmacokinetics of orally administered midazolam were studied in 40 Chinese subjects from a wide age range (23–80 years). This is the first study to characterize midazolam PK properties in Chinese during middle and old age (>50 years). In this study, we demonstrated that midazolam was absorbed rapidly, with an overall time of peak concentration of 0.7 h after oral administration, consistent with the highly lipophilic properties of midazolam leading to rapid absorption. Statistical analysis indicated that there were no significant differences in system plasma exposure (AUC and Cmax), CL/F or V/F among the 4 age groups (20–50, 51–65, 66–75 and >76 years; P>0.05). The same results were obtained with regards to gender (P>0.05). These data suggest that midazolam pharmacokinetics in Chinese after oral administration are unaffected by either age or gender, and this conclusion is consistent with studies of Caucasian subjects34,35,36,37.
As an oral hypnotic, midazolam has been in the market for many years in China; however, there have been few pharmacokinetic studies25,26,27, and there is significant inter-study variability (Table 5). Surprisingly, double-peaks were observed in the midazolam plasma concentration-time profiles of some Chinese subjects given domestic midazolam tablets (NHwa Pharm Co China)26; however, this was not observed in other studies in which the drug was formulated as a solution or as a tablet from other suppliers25,27. These studies suggested that effects of formulation should be included in PBPK modeling for oral midazolam.
Table 5. Summary of the midazolam PK in Chinese after oral administration.
| PK parameter | Current study Age 20–45 years (n=10) | Study 1 Age 23±2.3 years (n=10) | Study 2 Age 20–28 years (n=22) | Study 3 Age 22–32 years (n=42) |
|---|---|---|---|---|
| Dose 15 mg | Dose 15 mg | Dose 7.5 mg | Dose 7.5 mg | |
| Cmax (ng/mL) | 73.5±27.3 | 103.1±26,4 | 53.4±22.1 (POR*28, CC) | 30–40 |
| 57.6±14.6 (POR*28, CT) | ||||
| 60.7±19.3 (POR*28, TT) | ||||
| Tmax (median) | 0.5 (0.25-1.5) | 1.5±0.7 | 0.5±0.2 (POR*28, CC) | 0.5–2 |
| 0.4±0.1 (POR*28, CT) | ||||
| 0.4±0.1 (POR*28, TT) | ||||
| T1/2 (h) | 3.3±0.9 | 3.0±0.8 | 2.3±0.8 (POR*28, CC) | NA |
| 2.0±0.4 (POR*28, CT) | ||||
| 2.1±0.4 (POR*28, TT) | ||||
| AUC0-t (h·ng/mL) | 198.7±51.1 | 369.8±103.6 | 95.7±34.4 (POR*28, CC) | 154.0±17.5 (CYP3A5*3) |
| 88.6±20.5 (POR*28, CT) | 132.2±12.8 (CYP3A5*1*3) | |||
| 94.6±14.0 (POR*28, TT) | ||||
| AUC0-∞(h·ng/mL) | 211.6±52.6 | 397.3±124.1 | 104.8±39.6 (POR*28, CC) | NA |
| 93.7±23.4 (POR*28, CT) | ||||
| 100.1±14.6 (POR*28, TT) | ||||
| CL/F (L/h) | 75.3±20.5 | 41.2±13.1 | 80.1±27.1 (POR*28, CC) | NA |
| 84.3±19.7 (POR*28, CT) | ||||
| 76.2±10.1 (POR*28, TT) | ||||
| Reference | 26 | 25 | 27 |
NA: not available.
Midazolam PBPK modeling
The Chinese database within the SimCYP population-based simulator was used to predict midazolam pharmacokinetics. Because the tablet used in the clinical study was formulated for immediate release, the SimCYP simulator first order model that treats the gut as a single compartment was used as the starting absorption model. Assuming that the Fa value (fraction of the drug available from dosage) was 1, the first-order absorption rate constant (ka) was input as 0.7 L/h and the lag time was 0.25 h. These values were estimated from the observed plasma concentration data; however, the simulation indicated that the first-order absorption model could not capture the midazolam plasma concentration–time profile. In contrast, the ADAM absorption model, which considered the impact of formulation, provided better predictions, especially when the midazolam dissolution profile was included. Given the high lipophilicity of midazolam and large volume of distribution, a full PBPK model was applied to improve the recovery of the plasma concentration-time profile. In contrast with the previous model for oral midazolam (2-fold over-prediction)11, this study contained two major modifications: (1) the single adjusting compartment (SAC) used previously was replaced by the full PBPK model with consideration of the high lipophilicity and large volume of distribution of midazolam, and (2) given the impact of formulation, in vitro midazolam dissolution profiles were acquired and incorporated into the ADAM model. Despite other potential factors (eg, intestinal transit time, stomach emptying time and variations in organ size and blood flow) that may influence drug absorption and distribution but were not considered in the PBPK model, the simulated plasma concentration-time profiles derived using the SimCYP Chinese population database were in good agreement with the observed data, as were the AUC and Cmax. Furthermore, the ratio of the geometric mean of predicted clearance to observed clearance was between 0.8 and 1.25. These results demonstrated that despite lack of Chinese hepatic and gut CYP3A4/5 information, the PBPK model generated using the SimCYP Chinese population database could effectively recover the pharmacokinetics of midazolam, a typical probe of CYP3A4/5. Therefore, it is suggested that the current SimCYP Chinese population model incorporating limited CYP3A4/5 enzyme data from Chinese11,38,39 might offer an opportunity for predicting CYP3A-mediated pharmacokinetics in Chinese.
Investigation of the effects on midazolam pharmacokinetics using the PBPK model
Previous studies in Caucasians demonstrated that the pharmacokinetics of midazolam were unaffected by age34,35,36,37; however, this topic has never been examined in Chinese. In this study, the verified PBPK model was used to investigate the effects of age on midazolam pharmacokinetics in Chinese from a physiological perspective.
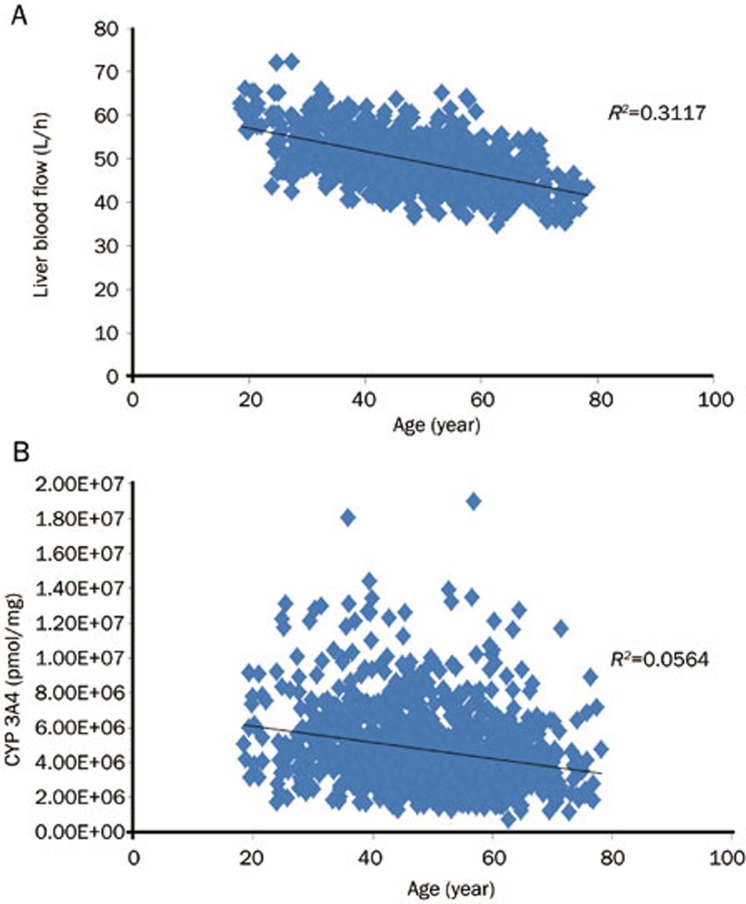
Midazolam is a moderate extraction ratio drug and its clearance depends on hepatic blood flow and intrinsic clearance40,41. Based on the simulation, liver blood flow (calculated as a 19% of the cardiac output11) appeared to be related to age (Figure 4A). In contrast, intrinsic CYP3A midazolam clearance was not expected to differ among elderly and young subjects as CYP3A activity did not change with age42,43, and the PBPK model simulation supported this assumption (Figure 4B). Assuming that both Fa and Fg (fraction of drug entering enterocytes and escaping the first-pass gut wall metabolism) were unaffected by age, only protein binding was expected to affect midazolam bioavailability. As midazolam moderately binds to proteins35, we expected no difference in midazolam exposure between young and elderly subjects. These hypotheses were supported by our simulations using virtual Chinese subjects; therefore, the current PBPK model can identify the effects of age on midazolam pharmacokinetics from a physiological perspective.
Figure 4.
Relationship between simulated liver blood flow and CYP3A4 abundance (n=1000).
In summary, the SimCYP Chinese population model for midazolam was verified by comparing its predictions with our observations. Although there are limitations and assumptions regarding the abundances of hepatic and gut CYP3A4/5 in Chinese, it is believed that the Chinese population model of the SimCYP simulator can adequately predict CYP3A4/5-mediated pharmacokinetics in Chinese. Consequently, this capacity may lead to the early identification of ethnic sensitivity for the Chinese population, as well as the need for and design of real studies for drugs that are predominantly metabolized by CYP3A4/5. Notably, because limited data for the abundances of CYP3A4/5 in Chinese were used, this PBPK model is still evolving model and should be updated with additional physiological and genetic data. Moreover, use of this model for additional drugs may further demonstrate its strengths and weaknesses.
Author contribution
Pei HU, Ji JIANG and Jun SHI designed the research; Xia CHEN and Hong-yun WANG performed the research; Hong-yun WANG analyzed the data and wrote the paper; Jun SHI revised the paper.
Acknowledgments
This study was supported by the National Major Scientific and Technological Special Project: Innovative Drugs Development (2012–2015, No 2012ZX09303-006-002), and the PUMCH-Roche Quantitative Pharmacology Fellowship Program (2012–2014).
References
- 1Evolving R&D for emerging markets. Nat Rev Drug Discov 2010; 9: 417–20. [DOI] [PubMed] [Google Scholar]
- 2Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov 2008; 7: 13–4. [Google Scholar]
- 3Karlberg JP. Globalization of sponsored clinical trials. Nat Rev Drug Discov 2009; 7: 458–60. [Google Scholar]
- 4Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 2008; 84: 417–23. [DOI] [PubMed] [Google Scholar]
- 5Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol 2004; 44: 1083–105. [DOI] [PubMed] [Google Scholar]
- 6Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 2011; 51: 45–73. [DOI] [PubMed] [Google Scholar]
- 7Lalonde RL, Kowalski KG, Hutmacher MM, Ewy W, Nichols DJ, Milligan PA, et al. Model-based drug development. Clin Pharmacol Ther 2007; 82: 21–32. [DOI] [PubMed] [Google Scholar]
- 8Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther 2011; 89: 259–67. [DOI] [PubMed] [Google Scholar]
- 9Huang SM, Rowland M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clin Pharmacol Ther 2012; 91: 542–9. [DOI] [PubMed] [Google Scholar]
- 10Zhao P, Rowland M, Huang SM. Best practice in the use of physiologically based pharmacokinetic modeling and simulation to address clinical pharmacology regulatory questions. Clin Pharmacol Ther 2012; 92: 17–20. [DOI] [PubMed] [Google Scholar]
- 11Barter ZE, Tucker GT, Rowland-Yeo K. Differences in cytochrome p450-mediated pharmacokinetics between Chinese and Caucasian populations predicted by mechanistic physiologically based pharmacokinetic modeling. Clin Pharmacokinet 2013; 52: 1085–100. [DOI] [PubMed] [Google Scholar]
- 12Li G, Yu G, Liu H, Zheng Q. Ethnic-specific in vitro–in vivo extrapolation and physiologically based pharmacokinetic approaches to predict cytochrome P450-mediated pharmacokinetics in Chinese population: opportunities and challenges. Clin Pharmacokinet 2014; 53: 197–202. [DOI] [PubMed] [Google Scholar]
- 13Lu Y, Yang J, Zhang H, Yang J. Prediction of warfarin maintenance dose in Han Chinese patients using a mechanistic model based on genetic and non-genetic factors. Clin Pharmacokinet 2013; 52: 567–81. [DOI] [PubMed] [Google Scholar]
- 14Wang B, Liu Z, Li D, Yang S, Hu J, Chen H, et al. Application of physiologically based pharmacokinetic modeling in the prediction of pharmacokinetics of bicyclol controlled-release formulation in human. Eur J Pharm Sci 2015; 77: 265–72. [DOI] [PubMed] [Google Scholar]
- 15Feng S, Cleary Y, Parrott N, Hu P, Weber C, Wang Y, et al. Evaluating a physiologically based pharmacokinetic model for prediction of omeprazole clearance and assessing ethnic sensitivity in CYP2C19 metabolic pathway. Eur J Clin Pharmacol 2015; 71: 617–24. [DOI] [PubMed] [Google Scholar]
- 16Zhu L, Yang J, Zhang Y, Wang Y, Zhang J, Zhao Y, et al. Prediction of pharmacokinetics and penetration of moxifloxacin in human with intra-abdominal infection based on extrapolated PBPK model. Korean J Physiol Pharmacol 2015; 19: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Yu MM, Gao ZW, Chen XY, Zhong DF. Predicting pharmacokinetics of anti-cancer drug, famitinib in human using physiologically based pharmacokinetic model. Yao Xue Xue Bao 2014; 49: 1684–8. [PubMed] [Google Scholar]
- 18Chen J, Liu D, Zheng X, Zhao Q, Jiang J, Hu P. Relative contributions of the major human CYP450 to the metabolism of icotinib and its implication in prediction of drug-drug interaction between icotinib and CYP3A4 inhibitors/inducers using physiologically based pharmacokinetic modeling. Expert Opin Drug Metab Toxicol 2015; 11: 857–68. [DOI] [PubMed] [Google Scholar]
- 19Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrating general knowledge of physical chemistry, biology, anatomy, physiology and genetics: A tale of 'bottom-up' vs 'top-down' recognition of covariates. Drug Metab Pharmacokinet 2009; 24: 53–75. [DOI] [PubMed] [Google Scholar]
- 20Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp population-based ADME simulator. Expert Opin Drug Metab Toxicol 2009; 5: 211–23. [DOI] [PubMed] [Google Scholar]
- 21Kanto JH. Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy 1985; 5: 138–55. [DOI] [PubMed] [Google Scholar]
- 22Thummel K, O'shea D, Paine M, Shen D, Kunze K, Perkins J, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharm Ther 1996; 59: 491–502. [DOI] [PubMed] [Google Scholar]
- 23Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol 1989; 36: 89–96. [PubMed] [Google Scholar]
- 24FDA Drug interaction studies-study design, data analysis, implications for dosing, and labeling recommendations (draft). 2012 [DOI] [PubMed]
- 25Yang G, Fu Z, Chen X, Yuan H, Yang H, Huang Y, et al. Effects of the CYP oxidoreductase Ala503Val polymorphism on CYP3A activity in vivo: a randomized, open-label, crossover study in healthy Chinese men. Clin Ther 2011; 33: 2060–70. [DOI] [PubMed] [Google Scholar]
- 26Guo T, Mao GF, Xia DY, Su XY, Zhao LS. Pharmacokinetics of midazolam tablet in different Chinese ethnic groups. J Clin Pharm Ther 2011; 36: 406–11. [DOI] [PubMed] [Google Scholar]
- 27Shih PS, Huang JD. Pharmacokinetics of midazolam and 10-hydroxymidazolam in Chinese with different CYP3A5 genotypes. Drug Metab Dispos 2002; 30: 1491–6. [DOI] [PubMed] [Google Scholar]
- 28Luo G, Guenthner T, Gan LS, Humphreys WG. CYP3A4 induction by xenobiotics: biochemistry, experimental methods and impact on drug discovery and development. Curr Drug Metab 2004; 5: 483–505. [DOI] [PubMed] [Google Scholar]
- 29CHNS. China Health and Nutrition Survey. http://www.cpc.unc.edu/projects/china. Accessed 23 May 2013.
- 30Howgate EM, Rowland Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: Impact of inter-individual variability. Xenobiotica 2006; 36: 473–97. [DOI] [PubMed] [Google Scholar]
- 31Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther 1997; 283: 1552–62. [PubMed] [Google Scholar]
- 32Jamei M, Turner D, Yang J, Neuhoff S, Polak S, Rostami-Hodjegan A, et al. Population-based mechanistic prediction of oral drug absorption. AAPS J 2009; 11: 225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res 2007; 24: 918–33. [DOI] [PubMed] [Google Scholar]
- 34Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, et al. The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther 1999; 65: 630–9. [DOI] [PubMed] [Google Scholar]
- 35Platten HP, Schweizer E, Dilger K, Mikus G, Klotz U. Pharmacokinetics and the pharmacodynamic action of midazolam in young and elderly patients undergoing tooth extraction. Clin Pharmacol Ther 1998; 63: 552–60. [DOI] [PubMed] [Google Scholar]
- 36Tomoki N, Takashi M, Kazuo H. The effects of age and gender on the optimal premeditation dose of intramuscular midazolam. Anesth Analg 1998; 86: 1103–8. [DOI] [PubMed] [Google Scholar]
- 37Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology 1984; 61: 25–35. [PubMed] [Google Scholar]
- 38Shu Y, Wang LS, Xiao WM, Wang W, Huang SL, Zhou HH. Probing CYP2C19 and CYP3A4 activities in Chinese liver microsomes by quantification of 5-hydroxyomeprazole andomeprazole sulphone. Acta Pharmacol Sin 2000; 21: 753–8. [PubMed] [Google Scholar]
- 39Shu Y, Cheng ZN, Liu ZQ, Wang LS, Zhu B, Huang SL, et al. Interindividual variations in levels and activities of cytochrome P-450 in liver microsomes of Chinese subjects. Acta Pharmacol Sin 2001; 22: 283–8. [PubMed] [Google Scholar]
- 40Rogers J, Rocci M, Haughey D, Bertino J. An evaluation of the suitability of IV midazolam as an in vivo marker for hepatic CYP3A activity. Clin Pharmacol Ther 2003; 273: 153–8. [DOI] [PubMed] [Google Scholar]
- 41Wilkinson G, Shand D. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther 1975; 18: 377–90. [DOI] [PubMed] [Google Scholar]
- 42Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res 2004; 10: 8341–50. [DOI] [PubMed] [Google Scholar]
- 43de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 1999; 37: 485–505. [DOI] [PubMed] [Google Scholar]