Abstract
Introduction
Adiponectin is an adipose tissue-derived anti-inflammatory protein that is down-regulated in obesity. The effects of caloric restriction and exercise induced weight loss on adiponectin are not clear.
Purpose
To determine whether addition of aerobic exercise training to caloric restriction has additive effects over caloric restriction alone on circulating adiponectin concentrations and adiponectin release from abdominal and gluteal adipose tissue.
Methods
Overweight or obese (body mass index=25-40 kg/m2, waist>88 cm) postmenopausal women were randomized to 20-week caloric restriction with and without aerobic exercise (CR+EX, n=48 and CR, n=22). Blood samples were collected for measuring plasma adiponectin concentration, and abdominal and gluteal subcutaneous adipose tissue biopsies were performed in a subgroup to determine in vitro adiponectin release, before and after the interventions.
Results
The interventions elicited similar amounts of weight loss (CR+EX: -11.3±4.6 kg ; CR:-11.2±3.4 kg) and fat loss (CR+EX: -8.0±3.5 kg; CR:-7.4±2.7 kg). The two groups had differential changes in plasma adiponectin concentrations (p for interaction = 0.014); CR+EX increased (6.9±3.9 to 8.5±4.9 μg/ml, p= 0.0001), while CR did not alter (6.4±4.4 to 6.5±4.5 μg/ml, p=0.42), plasma adiponectin. Likewise, adiponectin release from abdominal and gluteal subcutaneous adipose tissue increased with CR+EX (p=0.0076 and 0.089, respectively), but did not change with CR (p=0.13 and 0.95, respectively).
Conclusion
Despite similar reductions in body weight and fat mass, the addition of aerobic exercise to caloric restriction increased plasma adiponectin concentrations, which may be partly explained by increased adiponectin release from abdominal and gluteal subcutaneous adipose tissue.
Keywords: Adiponectin, Aerobic Exercise, Caloric Restriction, Weight Loss, Postmenopausal Women
INTRODUCTION
Adipose tissue is an endocrine organ(20) that produces many factors, among which, adiponectin is a protein that is involved in energy metabolism, is anti-inflammatory and plays a protective role in the development of atherosclerosis(23). Therefore, higher adiponectin concentrations may be associated with lower risks for cardiometabolic diseases. Although adiponectin is primarily produced by adipose tissue, obese individuals have lower circulating concentrations than lean people(5). Lifestyle interventions are commonly recommended for treating obesity(25). However, weight loss, induced by caloric restriction alone or the combination of caloric restriction and exercise, has showed inconsistent effects on circulating adiponectin concentrations; it is increased after weight loss in some studies(1-3, 8, 10, 12, 16-18, 37), but not in others(6, 28, 35, 37). These results do not always support that adiponectin concentration increases with certain amount of weight loss. Thus, there is still confusion regarding the effects of weight loss induced by caloric restriction and exercise on adiponectin concentrations, and it is not clear about their interplay on adiponectin. Also, only a few studies have compared caloric restriction alone with caloric restriction and exercise in the same study(1, 10, 17, 35). Because of the potential role of adiponectin as a causal link between obesity and cardiometabolic diseases(13, 15), it is of interest to clarify and to ascertain how caloric restriction and exercise, the interventions that are commonly used in treating obesity, affect adiponectin.
Previous studies have shown changes in the size and number of adipocytes from abdominal and lower-body fat in response to overfeeding(38) and weight loss(39). But only a few studies examined whether there are changes in adiponectin production by adipose tissue with weight loss and results are not conclusive. Therefore, the purpose of this study was to determine whether addition of aerobic exercise training to caloric restriction has additive effects, over caloric restriction alone, on circulating adiponectin concentrations, and to attempt to explain the inconsistent results from previous studies. Another purpose was to determine whether adiponectin release from subcutaneous adipose tissue changed in response to weight loss induced by caloric restriction alone and in combination with exercise.
METHODS
Participants
Participants in this study were a subset of those enrolled in a randomized clinical trial that was previously published (Clinicaltrials.gov identification: NCT00664729)(33). Briefly, participants completed 5 months of caloric restriction alone (CR) or combined with aerobic exercise (CR+EX). The participants were: 1) postmenopausal women between 50-70 years, 2) overweight or obese (body mass index [BMI]: 25-40 kg/m2) and waist circumference > 88 cm, 3) nonsmoking, 4) not on menopausal hormone therapy, and 5) sedentary (<15 minutes of exercise twice per week) in the past 6 months before enrollment. The study was approved by the Wake Forest University Institutional Review Board. All women signed an informed consent form to participate in the study according to the guidelines for human research.
Data used for the current analyses were from women who completed the 5-month intervention and had circulating adiponectin concentrations measured both at baseline and after intervention. There were 22 women in the CR only group and 48 in the CR+EX group. Among these women, abdominal fat samples were obtained from 10 in the CR group and 15 in the CR+EX group, and gluteal fat samples were obtained from 8 in the CR group and 13 in the CR+EX group, both at baseline and after intervention.
Interventions
The total energy deficits were designed to be approximately 2800 kcal/week for women in both the CR and CR+EX groups. Individual energy needs for weight maintenance were estimated based on each woman's resting metabolic rate and an activity factor based on self-reported daily activity (1.2-1.3 for sedentary individuals). The resting metabolic rate was determined via indirect calorimetry after an overnight fast by using a MedGraphics CCM/D metabolic cart and BREEZE 6.2 software (MedGraphics, St. Paul, MN). The energy deficits for the CR group resulted completely from reduction in dietary intake, whereas deficits for the CR+EX group resulted from the combination of reduction in dietary intake (~2100 kcal/week) and exercise energy expenditure (~700 kcal/week).
Throughout the 5-month interventions, all women were provided with daily lunch, dinner, and snacks prepared by the General Clinical Research Center (GCRC) metabolic kitchen. Women prepared their own breakfast meals from a provided menu plan. They were asked to eat only the food that was given to them or that from the breakfast menu. Energy make-up of the diet was approximately 25% from fat, 15% from protein, and 60% from carbohydrate. Women were allowed to consume noncaloric, noncaffeinated beverages ad libitum. All women were provided with daily calcium supplements (500 mg, 2 times per day). They were asked to keep a log of all foods consumed, and the records were monitored by dietitians to verify compliance.
The exercise intervention was center-based walking on treadmills 3 times per week under the supervision of an exercise physiologist. After flexibility exercise and walking for 3-5 minutes at a slow pace to warm up, women exercised at moderate or vigorous intensity (45-50% to 70-75% of heart rate reserve, which was the maximal heart rate, obtained from each woman's maximal exercise test described below, minus resting heart rate). The duration of the exercise was adjusted according to the intensity so that the estimated exercise energy expenditure was ~700 kcal/week. Blood pressure was taken before and after each exercise session. Heart rate readings (by Polar heart rate monitor; Polar Electro Inc, Lake Success, NY) were taken before, at least 2 times during and after the exercise.
Peak oxygen consumption
Peak oxygen consumption (VO2) was measured on a treadmill (Medical Graphics Corporation, Minneapolis, MN) during a progressive exercise test to voluntary exhaustion(33). The speed was set at a constant rate according to individual ability, and the incline increased at small intervals continuously throughout the test. A valid peak VO2 was obtained when at least 2 of the following criteria were met: 1) plateau in VO2 with increasing work rate, 2) maximal heart rate >90% of age-predicted maximal heart rate, and 3) respiratory exchange ratio of ≥1.1.
Body composition and fat distribution
Height and weight were measured with shoes and outer garments removed. Whole-body fat mass, lean mass, and percentage body fat were measured by dual-energy-X-ray absorptiometry (Hologic Delphi QDR, Bedford, MA).
Adiponectin circulating concentrations and adipose tissue release
Blood samples were collected in EDTA-treated evacuated tubes by venipuncture in the early morning after an overnight fast before and after the interventions. The post-intervention samples were collected at least two days after the previous bout of exercise. Plasma was separated and stored at -80oC until analysis. Plasma adiponectin concentration was measured by enzyme-linked immunosorbent assay using Quantikine kits (R&D System, Minneapolis, MN). The sensitivity for this assay was 0.2 ng/ml. The inter- and inta-assay coefficients of variation were 5.7% and 3.4% in our laboratory.
Abdominal and gluteal subcutaneous adipose tissue samples were taken via aspiration with a 16-gauge needle under local anesthesia after an overnight fast. The tissue was put in warm saline and transported immediately to the laboratory to be washed with warm saline to eliminate blood and connective tissue. Minced adipose tissue fragments (5-10 mg each, total of 200 mg) were placed in 2 ml medium 199 (Invitrogen, Carlsbad, CA) containing 1% Bovine Serum Albumin (Serologicals, Norcross, GA), pH 7.4, and incubated in a shaking water bath at 50 RPM at 37°C under an atmosphere of 95% O2 / 5% CO2 for 3 hours. At the end of the incubation, samples of the incubation medium were frozen at -80°C until final analysis. Adiponectin released from the adipose tissue was measured using aliquots of the incubation medium using an immunoassay (Millipore, St. Charles, MO).
Statistics
All analyses were conducted using the SAS software, version 9.3 (SAS Institute, Cary, NC). Descriptive statistics for normally distributed variables are presented as mean ± SD, median (quartiles) for non-normally distributed variables, or frequency in percentage. Natural logarithm was used to transform concentrations of adiponectin at baseline and post-intervention to achieve normality of distribution. Analysis of variance with repeated measures was used to determine differences between groups, changes after intervention (time), and interaction of the two factors. When the group x time interaction was significant, the main effect of time (changes after intervention) was examined in each group separately. An α level of 0.05 was selected to denote statistical significance.
RESULTS
Physical characteristics and body composition measurements of participants at baseline and post-intervention are included in Table 1. Women in the CR and the CR+EX groups were of a similar age, racial distribution, and height. There were no group differences in body weight, BMI, total lean mass, fat mass, body fat percent, or relative peak VO2 at baseline. Both interventions decreased body weight (12.5±3.7% and 12.7±4.7% for CR and CR+EX, respectively), BMI, fat mass (19.6±7.0% and 21.0±8.4%, respectively), body fat percent, by similar magnitude (p for group*time interactions > 0.10 for all). There was a significant group*time interaction for total lean mass which decreased in both groups (p < 0.0001 for both) and the decrease was slightly greater in CR (4.4±1.8 kg) than the CR+EX (3.3±1.8 kg) group (p = 0.026). There were significantly different changes in peak VO2 relative to body weight (p for group*time interaction = 0.038), with a greater increase in CR+EX (3.0±2.7 ml/kg/min, p < 0.0001) compared to CR (1.5±2.7 ml/kg/min, p = 0.025).
Table 1.
Physical characteristics and body composition at baseline and post-intervention by intervention group
| CR (n=22) | CR+EX (n=48) | |
|---|---|---|
| Age (year) | 58.5±6.1 | 58.4±5.2 |
| Race-ethnicity [n (%)] | ||
| Non-Hispanic white | 13 (59.1) | 34 (70.8) |
| African American | 9 (40.9) | 14 (29.2) |
| Height (cm) | 163.7±5.8 | 164.1±5.3 |
| Weight (kg) | ||
| Baseline | 89.8±9.8 | 88.8±11.7 |
| Post-intervention | 78.6±9.7 | 77.5±10.9 |
| BMI (kg/m2) | ||
| Baseline | 33.5±3.8 | 32.9±3.7 |
| Post-intervention | 29.3±3.6 | 28.7±3.5 |
| Total lean mass (kg)* | ||
| Baseline | 53.3±4.7 | 52.3±6.0 |
| Post-intervention | 48.9±5.1 | 48.9±5.9 |
| Total fat mass (kg) | ||
| Baseline | 38.5±6.7 | 38.4±6.9 |
| Post-intervention | 31.1±6.7 | 30.4±6.4 |
| Body fat (%) | ||
| Baseline | 41.7±3.9 | 42.2±2.9 |
| Post-intervention | 38.5±4.7 | 38.1±3.9 |
| Relative VO2peak (ml/kg/min)† | ||
| Baseline | 20.9±3.1 | 20.7±3.3 |
| Post-intervention | 22.4±4.6 | 23.7±2.9 |
All data are mean±SD.
CR: Caloric restriction; CR+EX, caloric restriction and exercise
No significant differences were observed between CR and CR+EX at baseline. Similar decreases between CR and CR+EX in body weight, BMI, fat mass, and body fat percent.
p = 0.026 for group*time interaction, indicating significant difference in magnitude of changes between CR and CR+EX after interventions.
p = 0.038 for group*time interaction, indicating significant difference in magnitude of changes between CR and CR+EX after interventions.
The mean concentration of circulating adiponectin was similar at baseline between CR [5.3 (2.8, 9.6) μg/ml, median (quartile 1, quartile 3)] and CR+EX [6.4 (3.9, 9.6) μg/ml] groups (p = 0.47). However, changes with intervention were different between groups (p for group*time interaction = 0.014), with the concentrations significantly increased by 34% (p = 0.0001) with CR+EX [7.6 (4.4, 11.3) μg/ml at post-intervention] but unchanged (p = 0.42) with CR alone [5.2 (3.2, 8.9) μg/ml at post-intervention]. Reports in the literature suggest the increase in adiponectin concentration occur~10% weight loss (see discussion). Therefore, we further examined plasma adiponectin concentrations in those who have lost greater and less than the average percentage of weight loss (12.7±4.4%) (Table 2). In the CR group, in those who lost >12.7% of weight, there was a trend for an increase in adiponectin concentration (p=0.085). In contrast, in those in CR group whose weight loss was <12.7%, adiponectin concentration did not change (p=0.96). In the CR+EX group, adiponection concentrations increased in women who lost >12.7% (p=0.002) and <12.7% (p=0.007).
Table 2.
Plasma adiponectin concentrations at baseline and post-intervention by intervention
| Group | CR |
CR+EX |
||||
|---|---|---|---|---|---|---|
| All | Weight loss >12.7% | Weight loss <12.7% | All | Weight loss >12.7% | Weight loss <12.7% | |
| Adiponectin (μg/ml) | (n=22) | (n=10) | (n=12) | (n=48) | (n=28) | (n=20) |
| Baseline | 5.3 (2.8, 9.7) | 3.5 (2.6, 5.6) | 8.4 (3.2, 10.9) | 6.4 (3.9, 9.0) | 7.0 (3.5, 12.3) | 5.3 (3.9, 7.4) |
| Post-intervention | 5.2 (3.0, 9.0) | 4.0 (2.5, 5.8) | 7.0 (3.4, 9.7) | 7.6 (4.4, 11.4) | 9.1 (5.6, 12.8) | 6.4 (3.6, 10.0) |
| P value* | 0.42 | 0.085 | 0.96 | <0.001 | 0.002 | 0.007 |
Data are median (quartiles).
CR: Caloric restriction; CR+EX, caloric restriction and exercise
paired t-test on log-transformed data comparing baseline and post-intervention
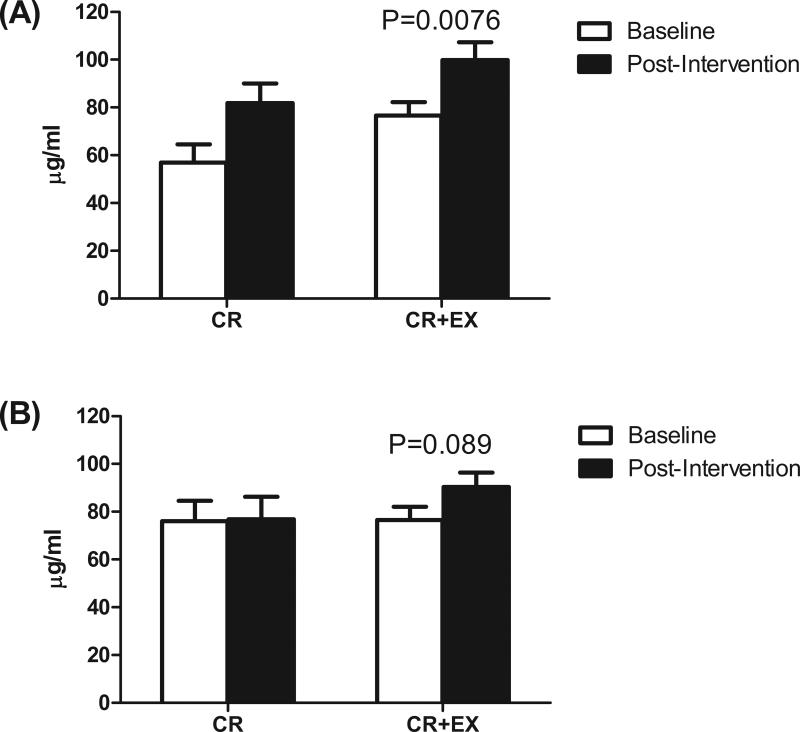
In the subgroup where abdominal and gluteal subcutaneous fat tissue samples were collected, baseline adiponectin release from abdominal fat was lower (p = 0.0025) in the CR (56.9±24.1 μg/ml) than the CR+EX group (76.6±22.0 μg/ml), but adiponectin release from gluteal fat was similar (76.0±24.2 and 76.6±19.7 μg/ml for CR and CR+EX, respectively, p = 0.37) between the two groups. After the interventions, adiponectin release from abdominal fat significantly increased in the CR+EX group (p = 0.0076), but did not change (p = 0.13) in the CR group (Figure 1A). Adiponectin release from gluteal fat showed a trend to increase (p = 0.089) in the CR+EX group, but did not change (p = 0.95) in the CR group (Figure 1B). At baseline, there was significant association between adiponectin concentration and release from abdominal and gluteal fat ((Spearman correlation: r = 0.43, p = 0.032; and r=0.50, p=0.021, respectively); however, the changes in adiponectin concentration and release from abdominal and gluteal fat were not significant in either group (p >0.05 for all).
Figure 1.
Adiponectin release from (A) abdominal subcutaneous adipose tissue and (B) gluteal subcutaneous adipose tissue, before and after weight loss by intervention group (CR: Carloric restriction; CR+EX, caloric restriction and exercise). Data are means ± SE. P values are for paired t-tests within CR+EX group.
DISCUSSION
The primary finding of this study was that, in postmenopausal women, with similar amount of weight loss (~12%), circulating adiponectin concentrations did not change with caloric restriction alone, but significantly increased when the caloric restriction intervention was combined with aerobic exercise training. The changes in adiponectin release from subcutaneous adipose tissue support this in that the amount released from abdominal fat significantly increased and, from gluteal fat, showed a trend to increase, in the CR+EX group; while in the CR group adiponection release from both abdominal and gluteal fat was unchanged. Although the changes in adiponectin concentration and adipose tissue release were not associated, the changes were in the same direction following CR+EX intervention. Therefore, the different changes in adiponectin release from subcutaneous fat in response to CR and CR+EX interventions may partly explain the different changes in circulating concentrations.
The lack of change in adiponectin concentrations in the CR group was somewhat surprising. Several previous studies examined adiponectin changes after caloric restriction interventions. Increased adiponectin concentrations have been reported in most(3, 10, 12, 17, 18, 37), but not all studies(6, 28, 35, 37). The findings of these prior studies support that weight loss close to 10% or more is associated with increased adiponectin concentrations. As an example, in one of these studies, adiponectin concentration did not change with 5% weight loss but increased with 10% weight loss(37). There may also be a sex difference with men having a larger increase in adiponectin than women(3). In fact, the above-mentioned studies that did not show an increase in adiponectin concentration were in women only (5%, 7.5%, and 7% weight loss)(6, 35, 37), or with women as the majority of the study sample (average of ~11% weight loss)(28). Differently, in studies that showed increased adiponectin concentration, either there was a significant percentage of both men and women(2, 10, 12, 17), or there was a large amount of weight loss (>15%) when there were women only(18) or only a small percentage of men was included(8). In our study, the average 12.7% weight loss in the CR group did not increase adiponectin concentration. In women who lost more than 12.7% of weight showed a trend for increased adiponectin concentration, and those who lost less than 12.7% of weight did not show any change. Putting these study findings together, in women, an even larger weight loss may be needed by caloric restriction to induce changes in circulating adiponectin concentration. We suspect that this may be due to the greater percent body fat in women than men; as a result, similar amount of total weight loss is often associated with smaller percentage of fat mass loss in women, and adiponectin is primarily produced by adipose tissue.
As we discussed earlier, we concluded that in women, a larger amount of weight loss may be needed to increase adiponectin concentration where weight loss was induced by caloric restriction only. In line with this, in our study, 12.7% weight loss induced by caloric restriction alone did not result in any change in adiponectin. Yet, an increase was observed in those with the addition of exercise with the same amount of weight loss. In fact, for women in CR+EX, adiponectin concentrations increased no matter whether they lost less or greater than the average weight loss of 12.7%. Therefore, exercise may “sensitize” the adiponectin response to weight loss, because it appears that the same amount of weight loss increased adiponectin concentration in women with exercise added to the caloric restriction intervention, while caloric restriction alone did not increase adiponectin.
The effects of exercise alone on adiponectin concentration are also inconsistent. Most studies do not show a change in adiponectin concentration with exercise training(1, 10, 16, 17, 24, 34). On the contrary, other studies showed that exercise training increased adiponectin despite unchanged body weight(29) or very small weight loss(32). Similar to caloric restriction induced weight loss, these two studies were in men only and those did not show a change in adiponectin concentration included a mixed sample of men and women(10, 16, 17, 24) or women only(1, 34). Among these studies, the timing of adiponectin measurement was reported as at least 24 hours after the last exercise bout in a few(16, 17, 24), but the timing was not reported in others. Therefore, we cannot rule out the possibility that the inconsistency of timing in relation to the last exercise contributes to the inconsistent findings.
Only a few studies examined the effects of caloric restriction and exercise on adipose tissue adiponectin production. Weight loss via caloric restriction appears to increase subcutaneous abdominal adipose mRNA expression of adiponectin(11, 17, 36). However, inconsistent results regarding exercise alone have been reported. One study found 3.5% weight loss induced by aerobic exercise three times per week, with an estimated energy expenditure of 500-600 kcal per session in a mixed sample of men and women increased abdominal subcutaneous adipose tissue adiponectin mRNA expression(17). Another study, however, showed unchanged abdominal adipose adiponectin mRNA in women after 12-week aerobic exercise 5 times per week of 45 min at 50% of maximum VO2, even though there was an average 5.9% of weight loss(34). Strength training did not change abdominal adipose tissue adiponectin mRNA either(27). As with caloric restriction alone, weight loss by exercise combined with caloric restriction appears to increase abdominal adipose tissue mRNA expression of adiponectin(11, 16, 17). Gene expression levels may not always be reflected in protein levels. There is also evidence that culture of human adipose tissue showing alteration in mRNA gene expression(21). Our study was unique because we examined the in vitro adiponectin release from subcutaneous adipose tissue in two fat depots. Our findings support that exercise sensitizes the response of adiponectin to a weight loss intervention. In addition, we are not aware of any studies involving caloric restriction or exercise interventions that have conducted gluteal subcutaneous adipose tissue biopsy. Our study suggests that changes in adiponectin release from gluteal fat were in the same direction as that from abdominal fat, but of less magnitude. We recognize that the adiponectin release method we used is limited to the tissue collected from the biopsy, and Bovine Serum Albumin that was used in the media may influence experimental outcome as it may contain growth factors(14). This method quantifies the amount of adiponectin released from the adipose tissue during the incubation period, which represents synthesis by adipocytes and other cells in the tissue; however, whether cells other than adipocytes contributes to circulating adiponectin in humans is still unanswered(19).
We did not have information regarding adiponectin production by other fat depots, such as deep subcutaneous adipose tissue or visceral fat. It has been shown that deep subcutaneous adipose tissue has different characteristics than subcutaneous adipose tissue(31), and that adiponectin mRNA expression is lower in visceral than subcutaneous adipose tissue(30). Despite our small sample size on adipose adiponectin release data, our findings regarding the different effects between the two interventions were consistent with previous findings of greater reduction in abdominal adipocyte size after CR+EX in comparison with CR only(39).
Exercise and caloric restriction induced weight loss may function via different mechanisms to influence adiponectin concentration. One study showed that mRNA expression of adiponectin receptor 1 and 2 in skeletal muscle significantly increased in exercise trained individuals, but not in those undergoing a hypocaloric diet(17). Moreover, weight loss by caloric restriction appears to be associated with an increase in high molecular weight (HMW) adiponectin(2, 9). Regarding exercise alone, one study showed that irrespective of any associated weight loss, there was a shift in the adiponectin multimer distribution toward a lower molecular weight (LMW)(7); two other studies showed no changes in HMW adiponectin after exercise training(4, 17); yet, another study showed that HMW adiponectin concentration increased(26). Thus, with current evidence, we cannot determine whether exercise training and caloric restriction induced weight loss have different effects on adiponectin multimer complex composition. Unfortunately, we did not measure adiponectin multimer distribution in our study. Because high molecular weight (HMW) adiponectin is more closely (negatively) associated with insulin resistance than total plasma adiponectin concentration(22), it is important to determine how adiponectin multimer distribution changes in response to interventions, and this may lend insight regarding the inconsistent findings from previous studies.
In summary, our study found that circulating adiponectin concentration did not change with weight loss by caloric restriction only but increased with the addition of aerobic exercise training. The changes in in vitro adiponectin release from subcutaneous abdominal and gluteal adipose tissue were in line with changes in circulating adiponectin concentration. Our data and others support that, in women, a greater percentage of weight loss may be needed to increase adiponectin concentration than men, and that exercise strengthens the effects of weight loss on adiponectin.
Acknowledgement
This work was made possible by NIH grant R01AG/DK20583, Wake Forest University Claude D Pepper Older Americans Independence Center (P30AG21332), and Wake Forest University General Clinical Research Center (M01-RR07122).
Footnotes
Clinical Trial Registration: Clinicaltrials.gov identification: NCT00664729
Disclosure Statement: The authors have nothing to disclose.
The results of the present study do not constitute endorsement by ACSM.
References
- 1.Abbenhardt C, McTiernan A, Alfano CM, et al. Effects of individual and combined dietary weight loss and exercise interventions in postmenopausal women on adiponectin and leptin levels. J Intern Med. 2013;274(2):163–75. doi: 10.1111/joim.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya SD, Brooks MM, Evans RW, Linkov F, Burke LE. Weight loss is more important than the diet type in improving adiponectin levels among overweight/obese adults. J Am Coll Nutr. 2013;32(4):264–71. doi: 10.1080/07315724.2013.816607. [DOI] [PubMed] [Google Scholar]
- 3.Ambeba EJ, Styn MA, Kuller LH, Brooks MM, Evans RW, Burke LE. Longitudinal effects of weight loss and regain on cytokine concentration in obese adults. Metabolism. 2013;62:1218–1222. doi: 10.1016/j.metabol.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando D, Hosaka Y, Suzuki K, Yamagata Z. Effects of Exercise Training on Circulating High Molecular Weight Adiponectin and Adiponectin Oligomer Composition: a Randomized Controlled Trial. Journal of Atherosclerosis and Thrombosis. 2010;16(6):733–9. doi: 10.5551/jat.2089. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Arvidsson E, Viguerie N, Andersson I, Verdich C, Langin D, Arner P. Effects of Different Hypocaloric Diets on Protein Secretion From Adipose Tissue of Obese Women. Diabetes. 2004;53(8):1966–71. doi: 10.2337/diabetes.53.8.1966. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach P, Nordby P, Bendtsen LQ, et al. Differential effects of endurance training and weight loss on plasma adiponectin multimers and adipose tissue macrophages in younger, moderately overweight men. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R490–R8. doi: 10.1152/ajpregu.00575.2012. [DOI] [PubMed] [Google Scholar]
- 8.Behre CJ, Gummesson A, Jernås M, et al. Dissociation between adipose tissue expression and serum levels of adiponectin during and after diet-induced weight loss in obese subjects with and without the metabolic syndrome. Metabolism. 2007;56(8):1022–8. doi: 10.1016/j.metabol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Bobbert T, Rochlitz H, Wegewitz U, et al. Changes of Adiponectin Oligomer Composition by Moderate Weight Reduction. Diabetes. 2005;54(9):2712–9. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 10.Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes. 2014;38(3):423–31. doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocr Metab. 2006;290(5):E961–7. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 12.Bruun JM, Lihn AS, Verdich C, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocr Metab. 2003;285(3):E527–33. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 13.Calton EK, Miller VS, Soares MJ. Factors determining the risk of the metabolic syndrome: is there a central role for adiponectin? Eur J Clin Nutr. 2013;67(5):485–91. doi: 10.1038/ejcn.2013.1. [DOI] [PubMed] [Google Scholar]
- 14.Carswell K, Lee M-J, Fried S. Culture of Isolated Human Adipocytes and Isolated Adipose Tissue. In: Mitry RR, Hughes RD, editors. Human Cell Culture Protocols. Methods in Molecular Biology. Vol. 806. Humana Press; 2012. pp. 203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: More Than Just Another Fat Cell Hormone? Diabetes Care. 2003;26(8):2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocr Metab. 2010;298(4):E824–31. doi: 10.1152/ajpendo.00574.2009. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab. 2010;95(2):911–9. doi: 10.1210/jc.2008-2505. [DOI] [PubMed] [Google Scholar]
- 18.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA. 2003;289(14):1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 19.Fain JN, Buehrer B, Tichansky DS, Madan AK. Regulation of adiponectin release and demonstration of adiponectin mRNA as well as release by the non-fat cells of human omental adipose tissue. Int J Obes. 2007;32(3):429–35. doi: 10.1038/sj.ijo.0803745. [DOI] [PubMed] [Google Scholar]
- 20.Fischer-Posovszky P, Wabitsch M, Hochberg Z. Endocrinology of Adipose Tissue - An Update. Horm Metab Res. 2007;39(05):314–21. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- 21.Gesta S, Lolmede K, Daviaud D, Berlan M, Bouloumie A, Lafontan M, et al. Culture of human adipose tissue explants leads to profound alteration of adipocyte gene expression. Horm Metab Res. 2003;35(3):158–63. doi: 10.1055/s-2003-39070. [DOI] [PubMed] [Google Scholar]
- 22.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the High–Molecular Weight Form of Adiponectin in Plasma Is Useful for the Prediction of Insulin Resistance and Metabolic Syndrome. Diabetes Care. 2006;29(6):1357–62. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 23.Hocking S, Samocha-Bonet D, Milner K-L, Greenfield JR, Chisholm DJ. Adiposity and Insulin Resistance in Humans: The Role of the Different Tissue and Cellular Lipid Depots. Endocrol Rev. 2013;34(4):463–500. doi: 10.1210/er.2012-1041. [DOI] [PubMed] [Google Scholar]
- 24.Hulver MW, Zheng D, Tanner CJ, et al. Adiponectin is not altered with exercise training despite enhanced insulin action. Am J Physiol Endocrinol Metab. 2002;283(4):E861–E5. doi: 10.1152/ajpendo.00150.2002. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly KR, Blaszczak A, Haus JM, et al. A 7-d exercise program increases high-molecular weight adiponectin in obese adults. Med Sci Sports Exerc. 2012;44(1):69–74. doi: 10.1249/MSS.0b013e318228bf85. [DOI] [PubMed] [Google Scholar]
- 27.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91(12):5107–12. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 28.Kovacova Z, Vitkova M, Kovacikova M, et al. Secretion of adiponectin multimeric complexes from adipose tissue explants is not modified by very low calorie diet. Eur J Endocrinol. 2009;160(4):585–92. doi: 10.1530/EJE-08-0727. [DOI] [PubMed] [Google Scholar]
- 29.Kriketos AD, Gan SK, Poynten AM, Furler SM, Chisholm DJ, Campbell LV. Exercise Increases Adiponectin Levels and Insulin Sensitivity in Humans. Diabetes Care. 2004;27(2):629–30. doi: 10.2337/diacare.27.2.629. [DOI] [PubMed] [Google Scholar]
- 30.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219(1–2):9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Lundbom J, Hakkarainen A, Lundbom N, Taskinen MR. Deep subcutaneous adipose tissue is more saturated than superficial subcutaneous adipose tissue. Int J Obes. 2013;37(4):620–2. doi: 10.1038/ijo.2012.72. [DOI] [PubMed] [Google Scholar]
- 32.Moghadasi M, Mohebbi H, Rahmani-Nia F, Hassan-Nia S, Noroozi H, Pirooznia N. High-intensity endurance training improves adiponectin mRNA and plasma concentrations. Eur J Appl Physiol. 2012;112(4):1207–14. doi: 10.1007/s00421-011-2073-2. [DOI] [PubMed] [Google Scholar]
- 33.Nicklas BJ, Wang X, You T, et al. Effect of exercise intensity on abdominal fat loss during calorie restriction in overweight and obese postmenopausal women: a randomized, controlled trial. Am J Clin Nutr. 2009;89(4):1043–52. doi: 10.3945/ajcn.2008.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polak J, Klimcakova E, Moro C, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor α in obese women. Metabolism. 2006;55(10):1375–81. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27(9):1066–71. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- 36.Salas-Salvado J, Bullo M, Garcia-Lorda P, et al. Subcutaneous adipose tissue cytokine production is not responsible for the restoration of systemic inflammation markers during weight loss. Int J Obes. 2006;30(12):1714–20. doi: 10.1038/sj.ijo.0803348. [DOI] [PubMed] [Google Scholar]
- 37.Summer SS, Brehm BJ, Benoit SC, D'Alessio DA. Adiponectin Changes in Relation to the Macronutrient Composition of a Weight-Loss Diet. Obesity. 2011;19(11):2198–204. doi: 10.1038/oby.2011.60. [DOI] [PubMed] [Google Scholar]
- 38.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci. 2010;107(42):18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You T, Murphy KM, Lyles MF, Demons JL, Lenchik L, Nicklas BJ. Addition of aerobic exercise to dietary weight loss preferentially reduces abdominal adipocyte size. Int J Obes (Lond) 2006;30(8):1211–6. doi: 10.1038/sj.ijo.0803245. [DOI] [PubMed] [Google Scholar]