Abstract
Liver kinase B1 (LKB1) is a tumor suppressor, and its loss might lead to activation of the mammalian target of rapamycin (mTOR) and tumorigenesis. This study aimed to determine the clinical relevance of LKB1 gene and protein expression in breast cancer patients. LKB1 protein expression was evaluated using immunohistochemistry in tumors from early breast cancer patients in two Taiwanese medical centers. Data on LKB1 gene expression were obtained from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) data set. The correlations between LKB1 expression, clinicopathologic factors, and patient outcome were analyzed. LKB1 expression was significantly associated with estrogen receptor (ER) expression in 2 of the 4 cohorts, but not with other clinicopathologic factors. LKB1 expression was not a predictor for relapse-free survival, overall survival (OS), or breast cancer-specific survival. In a subgroup analysis of the two Taiwanese cohorts, high LKB1 protein expression was predictive of high OS in human epidermal growth factor receptor 2 (HER2)-positive breast cancer patients (P = 0.013). Our study results indicate that LKB1 expression is not prognostic in the whole population of breast cancer patients, but it is a potential predictor of OS in the subset of HER2-positive patients
Liver kinase B1 (LKB1) is a tumor suppressor gene identified in hereditary Peutz-Jeghers syndrome1. It is involved in the control of metabolism2 and tumorigenesis3. When LKB1 interacts with AMP-activated protein kinase (AMPK), it abrogates signal transduction through the mammalian target of rapamycin (mTOR) to inhibit cell growth4, thus functioning as a tumor suppressor2. In previous studies on breast cancer, reduced LKB1 expression was associated with increased metastatic and invasive potential5. Therefore, LKB1-related pathways might provide potential targets for mitigating the invasive and metastatic characteristics of breast cancer.
A previous study evaluated LKB1 expression in metastatic estrogen receptor (ER)-positive breast cancer samples (n = 55) from the TAMRAD trial6,and observed that low LKB1 expression was predictive of everolimus efficacy. This study is the only human study to indicate the predictive value of LKB1 in targeted therapy for breast cancer. Other studies evaluating the role of LKB1 in breast cancer patients have provided inconsistent results. Two studies have evaluated the prognostic value of LKB1 in breast cancer patients. In Shen et al.7, low LKB1 expression was associated with low relapse-free survival (RFS) and overall survival (OS) in 116 breast cancer patients. By contrast, in Bouchekioua-Bouzaghou et al.8, high cytoplasmic LKB1 expression was associated with low disease-free survival (DFS) in a cohort of 154 breast cancer patients. In other studies, LKB1 status correlated with ER but in opposite directions. In a study on MCF-7 human breast cancer cells9, ERα downregulated LKB1 expression by regulating its promoter regions. In 2 human breast cancer studies, ER expression was associated with low LKB18 and high LKB15 expression. However, these studies have had relatively small samples (n = 154 and 80, respectively), thus limiting the ability to stratify patients into prognostic groups for LKB1 assessment. Nevertheless, the results have indicated that LKB1 might play a key role in breast cancer biology; therefore, its relationships with survival and ER status warrant further investigation.
In this study, we investigated the role of LKB1 in breast cancer by evaluating LKB1 protein expression in 2 Asian cohorts using immunohistochemistry (IHC) stain, and by evaluating LKB1 gene expression in microarray data sets from 2 Western cohorts9. We analyzed the associations of LKB1 protein and gene expression with clinicopathologic factors, such as ER and human epidermal growth factor receptor 2 (HER2) statuses, and survival. We assessed the predictive value of LKB1 for survival outcomes according to various risk and prognostic groups.
Results
Patient characteristics
The demographic data of the 4 study cohorts are listed in Supplementary Table 1. We collected 730 and 307 archival breast cancer patient samples from the NTUH and MMH cohorts, from which LKB1 IHC analysis results were evaluable in 600 and 290 samples, respectively. The METABRIC discovery and validation cohorts consisted of 997 and 995 patients, respectively. The numbers of stage I–III patients from the 4 cohorts included in final analyses of clinicopathologic factors and survival status were, sequentially, 569 (NTUH), 277 (MMH), 988 (METABRIC discovery), and 975 (METABRIC validation).
The median ages of the NTUH and the MMH cohorts were 48.0 and 54.0 years, respectively; those of the METABRIC discovery and validation cohorts were 61.3 and 62.6 years, respectively. The major histological subtypes were invasive ductal carcinoma (80.7–94.9%) and invasive lobular carcinoma (1.6–12.4%). Of the cancer stages, stage II was dominant in all 4 cohorts. The NTUH and MMH cohorts contained higher numbers of HER2-positive patients than the METABRIC cohorts did, whereas the METABRIC cohorts contained higher numbers of ER-positive patients than the NTUH and MMH cohorts did.
Correlation between liver kinase B1 protein expression and clinicopathologic factors or survival
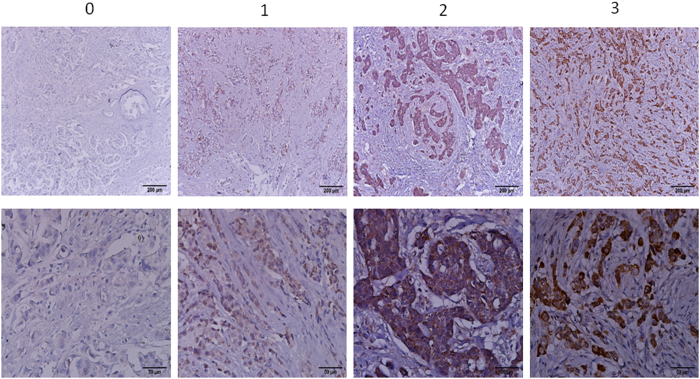
Figure 1 and Supplementary Fig. 1 showed the representative LKB1 IHC staining (scored as 0, 1, 2, and 3) in the NTUH and the MMH cohorts, respectively. LKB1 expression was high in 71.7% and 68.2% of the stage I–III breast cancer patients from the NTUH and the MMH cohorts (Table 1). Low LKB1 protein expression was significantly associated with high ER positivity (P = 0.002) and high PR positivity (P = 0.018) in the MMH cohort, but not in the NTUH cohort. LKB1 protein expression was not associated with clinicopathologic factors such as HER2 status, tumor size, lymph node status, stage, grade, and menopause in the NTUH and MMH cohorts.
Figure 1. LKB1 IHC stains: NTUH cohort Immunohistochemical staining of LKB1 in breast cancer patients in NTUH cohort.
Table 1. LKB1 and Clinicopathologic Factors.
| NTUH |
MMH |
|||||
|---|---|---|---|---|---|---|
| LKB1 High | LKB1 Low | Total | LKB1 High | LKB1 Low | Total | |
| Patient Number | 408(71.7%) | 161(28.3%) | 569(100%) | 189(68.2%) | 88(31.8%) | 277(100%) |
| ER | ||||||
| Negative | 129(31.6%) | 54(33.5%) | 183(32.2%) | 94(49.7%) | 26(29.5%) | 120(43.3%) |
| Positive | 279(68.4%) | 107(66.5%) | 386(67.8%) | 95(50.3%) | 62(70.5%) | 157(56.7%) |
| p | 0.691 | 0.002* | ||||
| PR | ||||||
| Negative | 231(56.6%) | 94(58.3%) | 325(57.1%) | 122(64.6%) | 43(48.9%) | 165(59.6%) |
| Positive | 177(43.4%) | 67(41.6%) | 244(42.9%) | 67(35.4%) | 45(51.1%) | 112(40.4%) |
| p | 0.708 | 0.018* | ||||
| HER2 | ||||||
| Negative | 319(78.2%) | 127(78.9%) | 446(78.4%) | 131(70.8%) | 61(70.1%) | 87(32.0%) |
| Positive | 89(21.8%) | 34(21.1%) | 123(21.6%) | 54(29.2%) | 26(29.9%) | 185(68.0%) |
| p | 0.910 | 1.000 | ||||
| Menopause | ||||||
| Premenopause | 222(54.4%) | 100(62.1%) | 322(56.6%) | 72(38.1%) | 36(40.9%) | 108(39.0%) |
| Postmenopause | 186(45.6%) | 61(37.9%) | 247(43.4%) | 117(61.9%) | 52(59.1%) | 169(61.0%) |
| p | 0.110 | 0.692 | ||||
| p | 0.575 | 0.691 | ||||
| T | ||||||
| 1 | 183(44.9%) | 71(44.1%) | 254(44.8%) | 27(14.3%) | 22(25.0%) | 49(17.7%) |
| 2 | 189(46.3%) | 74(46.0%) | 263(46.4%) | 147(77.8%) | 60(68.2%) | 207(74.7%) |
| 3/4 | 34(8.3%) | 16(9.9%) | 50(8.8%) | 15(7.9%) | 6(6.8%) | 21(7.6%) |
| P | 0.922 | 0.094 | ||||
| N | ||||||
| 0 | 253(62.5%) | 91(57.6%) | 344(61.0%) | 83(43.9%) | 32(36.4%) | 115(41.5%) |
| 1 | 111(27.4%) | 54(34.2%) | 165(29.3%) | 49(25.9%) | 25(28.4%) | 74(26.7%) |
| 2 | 25(6.2%) | 8(5.1%) | 33(5.9%) | 33(17.5%) | 12(13.6%) | 45(16.2%) |
| 3 | 16(4.0%) | 5(3.2%) | 21(3.7%) | 24(12.7%) | 19(21.6%) | 43(15.5%) |
| P | 0.551 | 0.207 | ||||
| Stage | ||||||
| 1 | 139(34.1%) | 53(32.9%) | 192(33.7%) | 19(10.1%) | 12(13.6%) | 31(11.2%) |
| 2 | 212(52.0%) | 85(52.8%) | 297(52.2%) | 104(55.0%) | 40(45.5%) | 144(52%) |
| 3 | 57(14.0%) | 23(14.3%) | 80(14.1%) | 66(34.9%) | 36(40.9%) | 102(36.8%) |
| P | 0.966 | 0.312 | ||||
| Grade | ||||||
| 1 | 81(19.9%) | 25(15.5%) | 12(6.3%) | 2(2.4%) | 14(5.1%) | |
| 2 | 212(52.0%) | 86(53.4%) | 99(52.4%) | 55(64.7%) | 154(56.2%) | |
| 3 | 99(24.3%) | 40(24.8%) | 78(41.3%) | 28(32.9%) | 106(38.7%) | |
| Unknown | 16(3.9%) | 10(6.2%) | 0 | 0 | ||
| p | 0.467 | 0.107 | ||||
| METBRIC Discovery Cohort | METBRIC Validation Cohort | |||||
| LKB1 High | LKB1 Low | Total | LKB1 High | LKB1 Low | Total | |
| Patient Number | 494(50.0%) | 494(50.0%) | 988(100%) | 487(50.0%) | 488(50.0%) | 975(100%) |
| ER | ||||||
| Negative | 86(17.4%) | 113(22.9%) | 199(20.1%) | 124(26.2%) | 131(27.2%) | 255(26.7%) |
| Positive | 408(82.6%) | 381(77.1%) | 789(79.9%) | 350(73.8%) | 350(73.8%) | 700(73.3%) |
| p | 0.039* | 0.715 | ||||
| PR | ||||||
| Negative | 226(45.7%) | 242(49.0%) | 468(47.4%) | 223(47.0%) | 224(46.6%) | 447(46.8%) |
| Positive | 268(54.3%) | 252(51.0%) | 520(52.6%) | 251(53.0%) | 257(53.4%) | 508(53.2%) |
| p | 0.339 | 0.897 | ||||
| HER2 | ||||||
| Negative | 440(89.1%) | 432(87.4%) | 494(88.2%) | 416(87.8%) | 419(85.9%) | 829(86.8%) |
| Positive | 54(10.9%) | 62(12.6%) | 116(11.7%) | 58(12.2%) | 68(14.1%) | 126(13.2%) |
| p | 0.489 | 0.391 | ||||
| Menopause | ||||||
| Premenopause | 106(21.7%) | 129(26.4%) | 235(24.1%) | 91(19.2%) | 100(20.8%) | 191(20.0%) |
| Postmenopause | 382(78.3%) | 360(73.6%) | 742(75.9%) | 383(80.8%) | 381(79.2%) | 764(80.0%) |
| p | 0.100 | 0.571 | ||||
| T | ||||||
| 1 | 205(41.5%) | 230(46.6%) | 435(44.0%) | 208(44.3%) | 207(43.1%) | 415(43.7%) |
| 2 | 263(53.2%) | 244(49.4%) | 507(51.3%) | 238(50.6%) | 244(50.8%) | 482(50.7%) |
| 3/4 | 26(5.3%) | 20(4.0%) | 46(4.7%) | 24(5.1%) | 29(6.0%) | 53(5.6%) |
| p | 0.231 | 0.801 | ||||
| N | ||||||
| 0 | 259(52.4%) | 253(51.2%) | 512(51.8%) | 260(55.0%) | 238(49.7%) | 498(52.3%) |
| 1 | 156(31.6%) | 161(32.6%) | 317(32.1%) | 136(28.8%) | 165(34.4%) | 301(31.6%) |
| 2 | 61(12.3%) | 64(13.0%) | 125(12.7%) | 50(10.6%) | 49(10.2%) | 99(10.4%) |
| 3 | 18(3.6%) | 16(3.2%) | 34(3.4%) | 27(5.7%) | 27(5.6%) | 54(5.7%) |
| p | 0.953 | 0.291 | ||||
| Stage | ||||||
| 1 | 141(28.5%) | 155(31.4%) | 296(30.0%) | 133(28.1%) | 124(25.8%) | 257(26.9%) |
| 2 | 265(53.6%) | 254(51.4%) | 519(52.5%) | 255(53.8%) | 269(55.9%) | 524(54.9%) |
| 3 | 88(17.8%) | 85(17.2%) | 173(17.5%) | 85(18.1%) | 88(18.3%) | 174(18.2%) |
| p | 0.623 | 0.719 | ||||
| Grade | ||||||
| 1 | 35(3.5%) | 37(3.7%) | 72(7.2%) | 46(9.7%) | 49(10.2%) | 95(9.9%) |
| 2 | 212(21.5%) | 198(20.0%) | 410(41.5%) | 182(38.4%) | 168(34.9%) | 350(36.6%) |
| 3 | 247(25.0%) | 259(26.2%) | 506(51.2%) | 216(45.6%) | 224(46.6%) | 440(46.1%) |
| Unknown | 0 | 0 | 30(6.3%) | 40(8.3%) | 70(7.3%) | |
| P | 0.534 | 0.535 | ||||
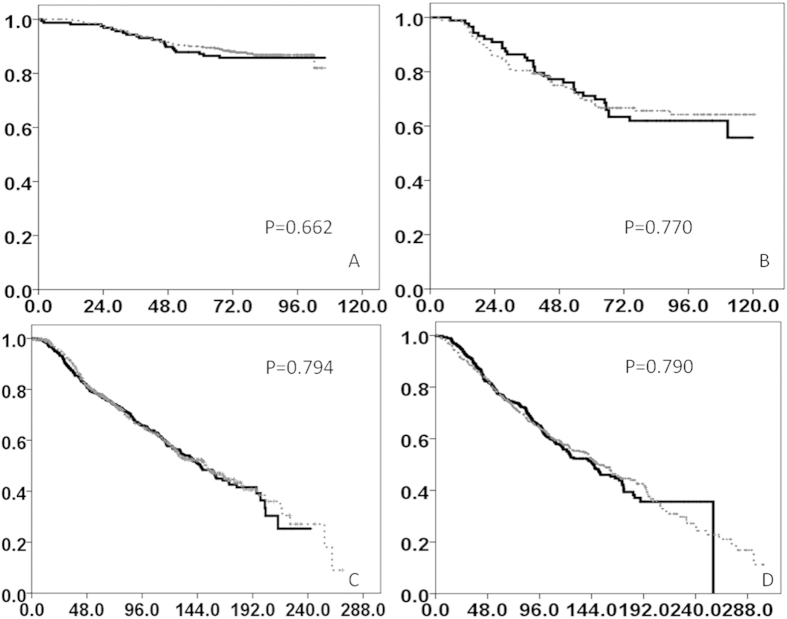
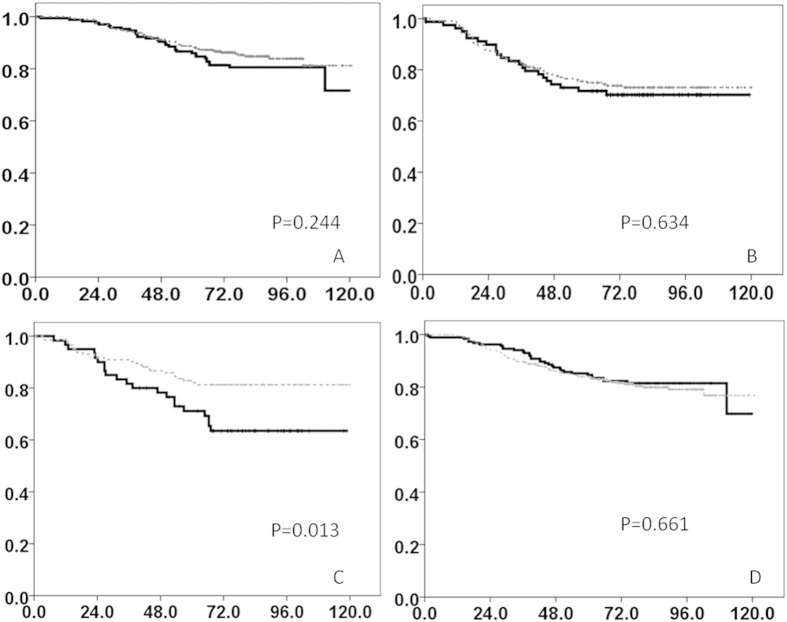
In the NTUH and MMH cohorts, the median duration of follow-up was 77.8 and 62.6 months, respectively. LKB1 protein expression was not predictive of RFS or OS (Fig. 2A,B) in the 2 cohorts. To minimize the effects of sample size on predictive value in survival analyses, we combined the patients from the NTUH and MMH cohorts in a Cox regression model analysis. Table 2 lists the major prognostic factors for OS in the 2 cohorts: ER positivity (HR = 0.523, P < 0.0001), tumor size (P < 0.0001), lymph node status (P < 0.0001), and menopause (HR = 1.631, P = 0.006). ER positivity (HR = 0.644, P = 0.008), tumor size (P < 0.001), lymph node status (P < 0.001), and grade (P = 0.014) were predictive of RFS in both cohorts (Supplementary Table 2). For subgroup analyses based on ER and HER2 statuses, we stratified the patients from the NTUH and MMH cohorts into ER-positive/negative and HER2-positive/negative groups (Fig. 3). High LKB1 protein expression was prognostic for high OS in the HER2-positive subgroup (P = 0.013) (Fig. 3C), but not in the remaining 3 subgroups (Fig. 3A,B,D).
Figure 2. OS The overall survival is shown in months.
(A)NTUH cohort (B) MMH cohort (C) Discovery cohort (D) Validation cohort (Dotted line: LKB1 high, solid line: LKB1 low).
Table 2. Cox Regression Model: Overall survival.
| NTUH |
MMH |
NTUH/MMH Summary Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| LKB1(IHC) | 0.321 | 0.823 | 0.419 | ||||||
| Negative | 1.000 | 1.000 | 1.000 | ||||||
| Positive | 0.766 | 0.453–1.296 | 1.054 | 0.665–1.671 | 0.868 | 0.615–1.224 | 0.419 | ||
| ER | 0.001* | 0.064 | <0.0001* | ||||||
| Negative | 1.000 | 1.000 | 1.000 | ||||||
| Positive | 0.369 | 0.210–0.648 | 0.620 | 0.374–1.028 | 0.523 | 0.365–0.748 | |||
| HER2 | 0.233 | 0.247 | 0.211 | ||||||
| Negative | 1.000 | 1.000 | 1.000 | ||||||
| Positive | 0.690 | 0.375–1.269 | 0.753 | 0.466–1.217 | 0.790 | 0.547–1.142 | |||
| T | 0.193 | <0.0001* | <0.0001* | ||||||
| T1 | 1.000 | 1.000 | 1.000 | ||||||
| T2 | 1.657 | 0.910–3.016 | 0.098 | 0.732 | 0.424–1.263 | 0.262 | 1.169 | 0.785–1.742 | 0.442 |
| T3 | 2.114 | 0.838–5.334 | 0.113 | 1.901 | 0.820–4.404 | 0.134 | 1.716 | 0.951–3.096 | 0.073 |
| T4 | 2.881 | 0.811–10.232 | 0.102 | 3.499 | 1.502–8.150 | 0.004 | 3.976 | 2.038–7.757 | <0.0001 |
| N | 0.007* | <0.0001* | <0.0001* | ||||||
| N0 | 1.000 | 1.000 | 1.000 | ||||||
| N1 | 1.349 | 0.742–2.450 | 0.326 | 2.325 | 1.231–4.390 | 0.009 | 1.744 | 1.139–2.671 | 0.011 |
| N2 | 3.088 | 1.444–6.601 | 0.004 | 4.186 | 2.130–8.226 | <0.0001 | 4.587 | 2.895–7.269 | <0.0001 |
| N3 | 3.292 | 1.356–7.991 | 0.008 | 5.329 | 2.730–10.399 | <0.0001 | 6.004 | 3.712–9.711 | <0.0001 |
| Grade | 0.809 | 0.361 | 0.105 | ||||||
| Grade 1 | 1.000 | 1.000 | 1.000 | ||||||
| Grade 2 | 1.257 | 0.574–2.752 | 0.567 | 2.692 | 0.630–11.510 | 0.182 | 1.955 | 1.005–3.805 | 0.048 |
| Grade 3 | 1.327 | 0.557–3.161 | 0.523 | 2.946 | 0.668–13.002 | 0.154 | 2.127 | 1.051–4.306 | 0.036 |
| Menopause | 0.083 | 0.105 | 0.006* | ||||||
| Negative | 1.000 | 1.000 | 1.000 | ||||||
| Positive | 1.586 | 0.941–2.674 | 1.501 | 0.918–2.453 | 1.631 | 1.149–2.317 | |||
| METBRIC Discovery Cohort | METBRIC Validation Cohort | ||||||||
| HR | 95%CI | P | HR | 95%CI | P | ||||
| LKB1(mRNA) | 0.512 | 0.816 | |||||||
| Negative | 1.000 | 1.000 | |||||||
| Positive | 0.937 | 0.772–1.138 | 0.512 | 1.024 | 0.839–1.250 | 0.816 | |||
| ER | 0.050* | 0.080 | |||||||
| Negative | 1.000 | 1.000 | |||||||
| Positive | 0.770 | 0.593–1.000 | 0.794 | 0.613–1.028 | |||||
| HER2 | 0.008* | 0.521 | |||||||
| Negative | 1.000 | 1.000 | |||||||
| Positive | 1.479 | 1.110–1.972 | 1.101 | 0.821–1.476 | |||||
| T | 0.007* | <0.0001* | |||||||
| T1 | 1.000 | 1.000 | |||||||
| T2 | 1.334 | 1.076–1.653 | 0.009 | 1.701 | 1.369–2.114 | <0.0001 | |||
| T3 | 1.798 | 1.129–2.864 | 0.013 | 2.381 | 1.600–3.543 | <0.0001 | |||
| N | <0.0001* | <0.0001* | |||||||
| N0 | 1.000 | 1.000 | |||||||
| N1 | 1.307 | 1.038–1.647 | 0.023 | 1.385 | 1.102–1.740 | 0.005 | |||
| N2 | 2.112 | 1.594–2.799 | <0.0001 | 2.060 | 1.508–2.814 | <0.0001 | |||
| N3 | 4.343 | 2.682–7.032 | <0.0001 | 3.433 | 2.370–4.973 | <0.0001 | |||
| Grade | 0.713 | 0.240 | |||||||
| Grade 1 | 1.000 | 1.000 | |||||||
| Grade 2 | 1.044 | 0.664–1.640 | 0.852 | 1.381 | 0.926–2.060 | 0.113 | |||
| Grade 3 | 1.134 | 0.719–1.788 | 0.590 | 1.411 | 0.937–2.124 | 0.099 | |||
| Menopause | 0.001* | 0.008* | |||||||
| Negative | 1.000 | 1.000 | |||||||
| Positive | 1.543 | 1.194–1.994 | 1.443 | 1.099–1.894 | |||||
Figure 3. OS of NTUH and MMH cohorts in subgroups.
(A) ER positive (B) ER negative (C) HER2 positive (D) HER2 negative (Dotted line:LKB1 high, solid line:LKB1 low).
LKB1 gene expression, clinicopathologic factors, and survival
We divided the 2 METABRIC cohorts into low and high LKB1 gene expression groups. High ER positivity was associated with low LKB1 gene expression in the METABRIC discovery cohort (P = 0.039) (Table 1), but not in the validation cohort. Other clinicopathologic factors were not associated with LKB1 gene expression in the METABRIC discovery and validation cohorts.
The median duration of follow-up in the METABRIC discovery and validation cohorts was 83.8 and 87.8 months, respectively. When we analyzed OS by using a Cox regression model, LKB1 status was not predictive of OS in all stage I–III patients from the 2 cohorts (HR = 0.937 and 1.024, P = 0.512 and 0.816, respectively) (Table 2, Fig. 2C,D). We observed no difference in OS between the high LKB1 and low LKB1 groups (Fig. 3). The major predictors for high OS (Table 2) and high BSS (Table 3) in the 2 METABRIC cohorts were small tumor size and low lymph node involvement. Menopause was predictive of low OS in both cohorts, but not predictive of BSS. ER positivity (HR = 0.770, P = 0.050) and HER2 status (HR = 1.479, P = 0.008) were predictive of OS in the discovery cohort, but not in the validation cohort. In subgroup analyses, LKB1 gene expression was nonsignificantly associated with OS and BSS in the ER-positive/negative and HER-positive/negative subgroups (Supplementary Figs 2–5).
Table 3. Cox Regression Model: Breast Cancer Specific Survival.
| METBRIC Discovery Cohort |
METBRIC Validation Cohort |
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| LKB1(mRNA) | 0.856 | 0.282 | ||||
| Negative | 1.000 | 1.000 | ||||
| Positive | 0.977 | 0.761–1.255 | 0.865 | 0.665–1.126 | ||
| ER | 0.054 | 0.021* | ||||
| Negative | 1.000 | 1.000 | ||||
| Positive | 0.726 | 0.524–1.005 | 0.680 | 0.490–0.943 | ||
| HER2 | 0.003* | 0.056 | ||||
| Negative | 1.000 | 1.000 | ||||
| Positive | 1.669 | 1.186–2.348 | 1.393 | 0.991–1.959 | ||
| T | 0.049* | <0.0001* | ||||
| T1 | 1.000 | 1.000 | ||||
| T2 | 1.316 | 0.991–1.747 | 0.058 | 1.709 | 1.271–2.299 | <0.0001 |
| T3 | 1.841 | 1.051–3.223 | 0.033 | 2.287 | 1.390–3.761 | 0.001 |
| N | <0.0001* | <0.0001* | ||||
| N0 | 1.000 | 1.000 | ||||
| N1 | 1.628 | 1.201–2.208 | 0.002 | 1.787 | 1.299–2.459 | <0.0001 |
| N2 | 2.771 | 1.945–3.948 | <0.0001 | 3.223 | 2.163–4.803 | <0.0001 |
| N3 | 5.483 | 3.057–9.836 | <0.0001 | 5.244 | 3.380–8.135 | <0.0001 |
| Grade | 0.182 | 0.042* | ||||
| Grade 1 | 1.000 | 1.000 | ||||
| Grade 2 | 1.266 | 0.651–2.459 | 0.487 | 2.655 | 1.223–5.765 | 0.014 |
| Grade 3 | 1.585 | 0.817–3.075 | 0.173 | 2.690 | 1.227–5.898 | 0.013 |
| Menopause | 0.977 | 0.633 | ||||
| Negative | 1.000 | 1.000 | ||||
| Positive | 0.996 | 0.738–1.344 | 0.926 | 0.674–1.271 | ||
Surrogate makers of LKB1 catalytic function
The catalytic function of LKB1 could not be directly analyzed by IHC in the formalin fixed paraffin embedded slides or by gene expressions. We tested phosphorylated AMP- activated protein kinase (pAMPK) and phosphorylated acetyl-CoA carboxylase(pACC) status as potential surrogate markers of LKB1 catalytic function in breast cancer. We randomly selected 108 tumor samples from the NTUH cohort and conducted IHC for pAMPK and pACC. The representative figures for pAMPK and pACC staining were shown in Supplementary Fig. 7 and their correlations with LKB1 expression were shown in Supplementary Table 3. LKB1 expression was positively associated with pACC expression (p = 0.0003), but it was not associated with pAMPK expression (p = 0.700). Neither pACC nor pAMPK expression was associated with other clinical factors assessed in this study (data not shown).
Discussion
Our study evaluated 2809 stage I–III breast cancer patients in 4 cohorts to investigate the relationships between LKB1 expression and clinicopathologic factors or patient outcome. Our results indicated nonsignificant associations between LKB1 protein and gene expression and OS, BSS, or RFS in the stage I–III breast cancer patients. However, in subgroup analyses, high LKB1 protein expression was associated with high OS in the HER2-positive population from the 2 Asian cohorts.
LKB1 expression correlated with ER positivity in 2 of the study cohorts but in opposite directions. Consistent with Linher-Melville et al.10, high ER positivity was associated with low LKB1 expression in the MMH cohort. Linher-Melville et al. evaluated LKB1 expression a MCF-7 cell line, and observed that ERα is a downregulator of LKB1 gene expression. Thus, when ER is highly expressed, it leads to low LKB1 expression and low LKB1 protein expression. In the METABRIC discovery cohort, high LKB1 gene expression was associated with high ER positivity. This observation might be explained by the results from a previous study on LKB1 and ERα signaling11 that evaluated the role of LKB1 as a coactivator for ERα according to its catalytic function in the nucleus. When the authors used siRNA to knock down LKB1, ERα activity was downregulated, indicating that LKB1 and ERα might interact reciprocally as a control mechanism. In addition, LKB1 might be associated with its regulator ER in some, but not all, conditions, which might explain the inconsistencies in results on the relationship between LKB1 and ER among our and previous studies. In Brown et al.12, they have demonstrated that ERα would bind to LKB1 promoter region in the presence of estradiol in MCF7 cells. This provides an explanatory mechanism for potentially low LKB1 in ER positive breast cancer. Individual differences in the control mechanisms for LKB1 and ER in breast cancer patients might also exist, but we could not evaluate the possible differences in this study.
In addition to the presence of LKB1 in ERα functional modulation, LKB1 catalytic function also lead to enhanced transactivation of ERα11. This catalytic function might be lost after point mutation in LKB1 or loss of LKB113 resulting in altered ERα function. LKB1 is also a pivotal kinase to control AMPK subfamily by catalyzing AMPK phosphorylation14 and therefore pAMPK and its downstream pACC might be surrogate markers for functional LKB1. In the present study, the pAMPK status was not associated with LKB1 expression. A prior study showed that AMPK can also be phosphorylated by calmodulin-dependent protein kinase kinase-beta in LKB1 deficient conditions15. Therefore, the association of pAMPK with LKB1 might not be as evident as pACC in breast cancer. In contrast, the positive association between LKB1 and pACC suggested that pACC could be a surrogate marker for LKB1 catalytic function in breast cancer. This finding is consistent with the study by Carretero et al. that LKB1-negative primary lung adenocarcinoma had very low level of pACC protein16.
In our subgroup analyses, we stratified the 4 cohorts into 4 groups according to ER and HER2, based on the prognostic17,18,19,20,21 and treatment grouping of breast cancer patients. High LKB1 protein expression was predictive of high OS in the HER2-positive population in the 2 Asian cohorts. In previous LKB1 human studies, HER2-positive patients were not well described. Shen et al.7,8 did not specify the percentage of HER2-positive patients. In Bouchekioua-Bouzaghou et al.8, the HER2-positive population was between 7.7% and 15%. A biomarker analysis from the FinHER trial22 detected one case of LKB1 mutation in 687 genotyped HER2-positive patient tumors. However, because the study focused on somatic mutational status in HER2-positive patients, the presence or loss of LKB1 was not analyzed.
Although previous human studies did not establish an association between HER2 and LKB1, loss of LKB1 led to reduced elapsed time for HER2-mediated tumorigenesis2,23 in previous studies on LKB1 knockout mice. The HER2-positive tumor in LKB1−/− mice has an altered metabolic pathway, suggesting that loss of LKB1 might be an indicator of hyperactive mTOR in HER2-positive breast cancer. Andrade-Vieira et al.24 treated HER2-positive tumor samples from LKB1−/− mice with an mTOR inhibitor and 2-deoxyglucose and observed favorable tumor control. In the BOLERO-3 study25, adding the mTOR inhibitor everolimus to trastuzumab and vinorelbine increased progression-free survival in HER2-positive trastuzumab-resistant breast cancer patients. Our subgroup analysis result of an association between high LKB1 and high OS in HER2 patients might support these BOLERO-3 results. However, this association requires further validation in a large HER2-enriched cohort.
The major differences between our and the described previous human LKB1 studies are sample size, stratification, definition of LKB1, and duration of follow-up. Our study evaluated 2809 patients in 4 cohorts, which is an approximately 24-fold and 18-fold larger sample than the samples evaluated in Shen et al.7 and Bouchekioua-Bouzaghou et al.8, respectively. In these 2 studies, stratification of the patients according to biological factors and treatment was not feasible because of limited patient numbers. In our study, when we analyzed the entire population, LKB1 was not prognostic. However, when we stratified the patients according to HER2 status, high LKB1 protein expression was predictive of high OS in the HER2-positive subgroup. LKB1 might thus play differing biological roles in different breast cancer groups. Our and the described human LKB1 studies also differ in the methods used for LKB1 analysis. Shen et al.7 evaluated LKB1 expression in 116 breast cancer patients by using Western blot analysis with a LKB1 polyclonal antibody. The authors defined low or high LKB1 expression according to the levels of LKB1 expression in the liver. When the ratio of tumor LKB1:liver LKB1 was lower than 0.5, it was defined as low LKB1 expression. According to this definition, the authors categorized 34.2% of the patients (38 of 111 evaluable LKB1 patients) as low LKB1 expression. Although our methods for defining LKB1 expression differed from those used in the described study, the percentages of low LKB1 expression patients in our NTUH and MMH cohorts were comparable. Bouchekioua-Bouzaghou et al.8 evaluated LKB1 in 154 breast cancer patients by using IHC analysis and observed that low cytoplasmic LKB1 expression was associated with ER positivity. Our IHC methods for defining LKB1 are consistent with the methods used in the study. In the 2 METABRIC cohorts, we defined LKB1 expression based on levels of mRNA expression. The final methodological difference between our and previous studies is the duration of follow-up. A sufficient follow-up is mandatory in outcome studies to observe survival events. In our study, the median duration of follow-up was between 62.6 and 87.8 months in the 4 cohorts. In Shen et al.7, the median duration of follow-up was 44.5 months. In Bouchekioua-Bouzaghou et al.8, the median duration of follow-up was not mentioned. Therefore, the strengths of our study include its relatively large cohort and long duration of follow-up, which enable the associations between LKB1 and survival status to be more accurately assessed than in the described small-scale studies.
Our study has limitations. First, when the median level of mRNA expression is used as a cutoff for discriminating high from low LKB1 status, a non-normal distribution can lead to statistical biases. However, we analyzed LKB1 expression and survival by dividing LKB1 expression into 3 or 4 groups (Supplementary Fig. 5), and our results indicated no correlation between LKB1 and survival. Second, the two Asian cohorts are retrospectively collected samples which will need larger validation cohorts to confirm our observation6. The prospectively collected METABRIC cohorts served as one of the good validation resource, but further mechanistic or prospective studies are complimentary to confirm the phenomena discovered in this study. The last limitation is that relapse status was unknown in the 2 Western cohorts. Therefore, we analyzed only LKB1 expression and RFS in the 2 Asian cohorts. However, the samples from the 2 Asian cohorts were considerably larger than those from previous studies and should provide sufficient information for RFS analysis.
Conclusion
LKB1 might be a prognostic factor in HER2-positive breast cancer patients, but is not consistently associated with other clinicopathologic variables. Our exploratory findings from subgroup analyses warrant further validation in a large HER2-enriched breast cancer population. Our study results might indicate a potential additional targetable mechanism for treatment of HER2-positive breast cancer patients.
Materials and Methods
Patient cohorts
Four cohorts were evaluated in this study. The first cohort consisted of breast cancer patients diagnosed between 2004 and 2007 at National Taiwan University Hospital (NTUH cohort), from whom archival paraffin slides were collected for LKB1 IHC analysis. The second cohort was composed of breast cancer patients diagnosed between 2004 and 2010 at Mackay Memorial Hospital (MMH cohort). Tissue microarray slides from the MMH cohort were obtained for LKB1 IHC analysis. The human sample collection in National Taiwan University Hospital (NTUH) and Mackay Memorial Hospital (MMH) are approved by the institutional research committees in both institutions. The informed consents were obtained from all of the subjects enrolled in NTUH and MMH. All the methods carried out in this study are in accordance with the approved protocols by the Institutional Review Board of NTUH. The third and fourth cohorts were from the publicly available Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) data sets (discovery and validation cohorts). Their raw data were analyzed for LKB1 expression. Supplementary Table 1 lists the demographic data of the 4 cohorts.
Immunohistochemical analysis of liver kinase B1, phosphorylated AMP-activated protein kinase, and phosphorylated acetyl-CoA carboxylase expression
Rabbit antihuman LKB1 antibody (ab58786) was purchased from Abcam (Cambridge MA, USA). Phospho-AMPKα (Thr172) (40H9) rabbit monoclonal antibody (#2535) and phospho-Acetyl-CoA Carboxylase (Ser79) (D7D11) rabbit monoclonal antibody (#11818) were purchased from Cell Signaling (Danvers MA, USA). Four-micrometer slides from formalin-fixed paraffin blocks were deparaffinized using xylene and rehydrated using serial gradient ethanol. The anti-LKB1 antibody was diluted to 1:100. The remaining standard staining procedure was performed according to manufacturer manual. Omission of the primary antibody incubation was served as negative control for every batch of LKB1 staining, and normal breast epithelial cells on the slides were served as internal positive control26.
LKB1 protein expression was categorized into 4 groups according to the intensity and extent of IHC staining, using the standards described in Bouchekioua-Bouzaghou et al.8. When the cells showed no positivity or <10% positive staining, they were scored as 0 and 1, respectively. When 10%–50% or >50% positive staining was observed, they were scored as 2 and 3, respectively. Low LKB1 expression was defined as a score of 0 or 1, and high LKB1 expression was defined as a score of 2 or 3.
LKB1 gene expression from the Molecular Taxonomy of Breast Cancer International Consortium database
Data on LKB1 gene expression were available online for all patients in the METABRIC cohorts. Because the recurrence status was not recorded in the original METABRIC database, it was not analyzed in these 2 cohorts. In the METABRIC database, LKB1 gene expression was normalized to the median expression level in the data set. LKB1 gene expression levels higher than the median LKB1 gene expression level were categorized as high, whereas those lower than the median LKB1 gene expression level were categorized as low.
Statistical analysis
Stage I–III patients from the 4 cohorts were included in analyses of the correlations between LKB1 status and clinicopathologic factors and outcomes. A crosstable analysis was applied to correlate LKB1 status with age, menopause, ER status, progesterone receptor (PR) status, HER2 status, stage, grade, and OS in the 4 cohorts. RFS was analyzed in the NTUH and the MMH cohorts. To determine the influence of sample size on the NTUH and MMH cohorts, a Cox regression model analysis for OS and RFS was performed using the combined data from the 2 cohorts. Breast cancer-specific survival (BSS) was analyzed in the 2 METABRIC cohorts. Patients were stratified according to ER and HER2 status for subgroup analysis of OS, RFS, and BSS. A chi-squared test was applied to determine significance.
Additional Information
How to cite this article: Chen, I.-C. et al. Clinical Relevance of Liver Kinase B1(LKB1) Protein and Gene Expression in Breast Cancer. Sci. Rep. 6, 21374; doi: 10.1038/srep21374 (2016).
Supplementary Material
Acknowledgments
This study used the data sets from METABRIC and it was funded by Cancer Research UK and the British Columbia Cancer Agency Branch. The co-author, Tzu-Pin Lu, has contributed a lot during data extraction and analysis for METABRIC cohort.
Footnotes
Author Contributions I.C.C. and C.H.L. participated in the study design, data analysis, and manuscript drafting. Y.C.C., C.S.H., W.H.K., K.T.K. and M.Y.W. participated in the sample collection. Y.S.L., K.P.C., P.F.W., C.Y.S. and A.L.C. participated in the data analysis process and manuscript drafting. T.P.L. helped downloading and analyzing METABRIC data sets. T.H.H. performed the immunohistochemical stain and C.Y.S. provided the technical support. All authors read and approved the final manuscript.
References
- Hemminki A. et al. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet 15, 87–90 (1997). [DOI] [PubMed] [Google Scholar]
- Andrade-Vieira R., Xu Z., Colp P. & Marignani P. A. Loss of LKB1 expression reduces the latency of ErbB2-mediated mammary gland tumorigenesis, promoting changes in metabolic pathways. PLoS One 8, e56567 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. B. & Shaw R. J. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9, 563–575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Inukai K., Ikegami Y., Awata T. & Katayama S. LKB1, an upstream AMPK kinase, regulates glucose and lipid metabolism in cultured liver and muscle cells. Biochem Biophys Res Commun 351, 595–601 (2006). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Loss of LKB1 disrupts breast epithelial cell polarity and promotes breast cancer metastasis and invasion. J Exp Clin Cancer Res 33, 70 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treilleux I. et al. Translational studies within the TAMRAD randomized GINECO trial: evidence for mTORC1 activation marker as a predictive factor for everolimus efficacy in advanced breast cancer. Ann Oncol 26, 120–125 (2015). [DOI] [PubMed] [Google Scholar]
- Shen Z., Wen X. F., Lan F., Shen Z. Z. & Shao Z. M. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res 8, 2085–2090 (2002). [PubMed] [Google Scholar]
- Bouchekioua-Bouzaghou K. et al. LKB1 when associated with methylatedERalpha is a marker of bad prognosis in breast cancer. Int J Cancer. 135, 1307–1318 (2014). [DOI] [PubMed] [Google Scholar]
- Curtis C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linher-Melville K., Zantinge S. & Singh G. Liver kinase B1 expression (LKB1) is repressed by estrogen receptor alpha (ERalpha) in MCF-7 human breast cancer cells. Biochem Biophys Res Commun 417, 1063–1068 (2012). [DOI] [PubMed] [Google Scholar]
- Nath-Sain S. & Marignani P. A. LKB1 catalytic activity contributes to estrogen receptor alpha signaling. Mol Biol Cell 20, 2785–2795 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. A. et al. LKB1 expression is inhibited by estradiol-17beta in MCF-7 cells. J Steroid Biochem Mol Biol 127, 439–443 (2011). [DOI] [PubMed] [Google Scholar]
- Ui A. et al. Possible involvement of LKB1-AMPK signaling in non-homologous end joining. Oncogene 33, 1640–1648 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano J. M. et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23, 833–843 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S. A. et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2, 9–19 (2005). [DOI] [PubMed] [Google Scholar]
- Carretero J. et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene 26, 1616–1625 (2007). [DOI] [PubMed] [Google Scholar]
- Dawood S., Broglio K., Buzdar A. U., Hortobagyi G. N. & Giordano S. H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28, 92–98 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B., Redmond C., Fisher E. R. & Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. J Clin Oncol 6, 1076–1087 (1988). [DOI] [PubMed] [Google Scholar]
- Parl F. F., Schmidt B. P., Dupont W. D. & Wagner R. K. Prognostic significance of estrogen receptor status in breast cancer in relation to tumor stage, axillary node metastasis, and histopathologic grading. Cancer 54, 2237–2242 (1984). [DOI] [PubMed] [Google Scholar]
- Menard S., Fortis S., Castiglioni F., Agresti R. & Balsari A. HER2 as a prognostic factor in breast cancer. Oncology 61 Suppl 2, 67–72, (2001). [DOI] [PubMed] [Google Scholar]
- Cooke T., Reeves J., Lanigan A. & Stanton P. HER2 as a prognostic and predictive marker for breast cancer. Ann Oncol 12 Suppl 1, S23–28 (2001). [DOI] [PubMed] [Google Scholar]
- Loi S. et al. Somatic mutation profiling and associations with prognosis and trastuzumab benefit in early breast cancer. J Natl Cancer Inst 105, 960–967 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy F. et al. LKB1 is a central regulator of tumor initiation and pro-growth metabolism in ErbB2-mediated breast cancer. Cancer Metab 1, 18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Vieira R., Goguen D., Bentley H. A., Bowen C. V. & Marignani P. A. Pre-clinical study of drug combinations that reduce breast cancer burden due to aberrant mTOR and metabolism promoted by LKB1 loss. Oncotarget 5, 12738–12752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre F. et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. Oncol 15, 580–591 (2014). [DOI] [PubMed] [Google Scholar]
- Fenton H. et al. LKB1 protein expression in human breast cancer. Appl Immunohistochem Mol Morpho 14, 146–153 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.