Abstract
Marital status was found to be an independent prognostic factor for survival in various cancer types. In this study, we used the Surveillance, Epidemiology and End Results database to analyze the survival difference among different marital status in the United States. Gastric adenocarcinoma patients from 2004–2012 were enrolled for study. The 5-year cause specific survival (CSS) was our primary endpoint. Totally 29,074 eligible patients were identified. We found that more male patients were married than female. Asian patients had the highest percentages of married than the other races. More married patients were covered by the insurance. Married patients had better 5-year CSS than unmarried, 30.6% vs 25.7%, P < 0.001. The median overall CSS was 17.87 and 13.61 months for the married and unmarried patients, hazard ratio: 1.09 (95% confidence interval: 1.01–1.17), P = 0.027. The survival difference was significant in the insured but not in the uninsured patients. Widowed patients had the worst prognosis compared with other groups even though they had more stage I disease and more well / moderate differentiated tumors. These results indicated that unmarried gastric adenocarcinoma patients were at greater risk of cancer specific mortality. We recommend every patient should have access to best available gastric cancer therapy.
Although the incidence and mortality has declined over the last half-century, gastric cancer remains the fourth most common cancer and the second most frequent cause of cancer related death worldwide1,2,3. According to the GLOBOCAN 2012, the estimate new cases of gastric cancer are 631,300 for male and 320,300 for female4. Adenocarcinoma is the most frequent histologic subtype of gastric cancer5. Despite the success of modern chemotherapy, the prognosis for gastric cancer patients is still dismal6. Finding out potential prognostic factors is helpful for us to set up individual therapy schedule and improve the survival.
Greater longevity of married people compared with unmarried persons has been demonstrated7,8,9. However the impact of marital status on disease specific survival among cancer patients has been controversial, including protective7,10,11,12,13,14,15, mixed16,17 and no effect18,19. A systematic review and meta-analysis demonstrated that all non-married conditions (widowed, divorced/ separated and never married) were associated with a significantly greater risk of death, as compared to married individuals20. In a Surveillance, Epidemiology and End Results (SEER) based study, Aizer AA et al. identified 1,260,898 patients diagnosed between 2004 and 2008 with lung, colorectal, breast, pancreatic, prostate, liver/intrahepatic bile duct, non-Hodgkin lymphoma, head / neck, ovarian and esophageal cancer for analysis4. They found that unmarried patients were at significantly higher risk of metastatic diseases, under treatment and cancer related death10. Zhou RP et al. using the SEER database to investigate the relationship between marital status and the survival of gastric cancer patients and they found that unmarried patients were at higher risk of cancer related death15. However, the authors mixed the carcinoid tumor/neuroendocrine, gastrointestinal stromal sarcoma and adenocarcinoma together. In the present study, we would like to focus on the gastric adenocarcinoma, which is the most common histology subtype for gastric malignance diseases.
Results
Patient baseline characteristics
The study identified 29,074 gastric adenocarcinoma patients. Of these patients, 18,284 (62.89%) were male and 10,790 (37.11%) were female. The median age of the whole group was 67 years old. Totally 17,854 (61.41%) patients were married and 11,220 (38.59%) were unmarried including 4,353 (14.97%) widowed, 4,139(14.24%) single and 2,728 (9.38%) separated / divorced. Table 1 showed the relationship between clinicopathologic features and marital status.
Table 1. Basic characteristics.
| Married N (%) N = 17854 | Unmarried N (%) N = 11220 | P value | |||
|---|---|---|---|---|---|
| Widowed N = 4353 | Single N = 4139 | Separated/Divorced N = 2728 | |||
| Sex | <0.001 | ||||
| Male | 12,841(71.92) | 1,325 (30.44) | 2,564 (61.95) | 1,554 56.96) | |
| Female | 5,013 (28.08) | 3,028 (69.56) | 1,575 (38.05) | 1,174 (43.04) | |
| Age (Mean ± SD) | 64.5 ± 13.0 | 78.2 ± 9.4 | 58.3 ± 15.2 | 63.3 ± 11.9 | <0.001 |
| Race/Ethnicity | <0.001 | ||||
| Caucasian | 12,829 (71.85) | 2,971 (68.25) | 2,654 (64.12) | 1,909 (69.98) | |
| African-American | 1,553 (8.70) | 659 (15.14) | 973 (23.51) | 500 (18.33) | |
| Asian | 3,137 (17.57) | 647 (14.86) | 413 (10.00) | 257 (9.42) | |
| Others | 309 (1.73) | 74 (1.70) | 97 (2.34) | 56 (2.05) | |
| Unknown | 26 (0.15) | 2 (0.05) | 2 (0.03) | 6 (0.22) | |
| Insurance status | <0.001 | ||||
| Uninsured | 401 (2.24) | 35 (0.80) | 276 (6.67) | 100 (3.67) | |
| Insured | 11,260 (63.07) | 2,684 (61.66) | 2,579 (62.31) | 1,673 (61.33) | |
| Unknown | 6193 (34.69) | 1634 (37.54) | 1284 (31.02) | 955 (35.00) | |
| Location | <0.001 | ||||
| Cardia | 6,096 (34.14) | 940 (21.59) | 1,198 (28.94) | 900 (33.00) | |
| Fundus | 636 (3.56) | 167 (3.84) | 135 (3.26) | 110 (4.03) | |
| Body | 1,509 (8.45) | 422 (9.69) | 346 (8.36) | 247 (9.05) | |
| Antrum/Pylorus | 3,991 (22.35) | 1,326 (30.46) | 1002 (24.21) | 579 (21.22) | |
| Lesser/Greater curvature | 2,168 (12.14) | 564 (12.96) | 484 (11.69) | 330 (12.10) | |
| Others | 3,454 (19.36) | 934 (21.46) | 974 (23.54) | 562 (20.60) | |
| AJCC 6th TNM stage | <0.001 | ||||
| I | 4,976 (27.87) | 1,601 (36.78) | 982 (23.73) | 728 (26.69) | |
| II | 2,581 (14.46) | 582 (13.37) | 546 (13.18) | 350 (12.83) | |
| III | 2,521 (14.12) | 552 (12.68) | 564 (13.63) | 379 (13.89) | |
| IV | 7,776 (43.55) | 1,618 (37.17) | 2,047 (49.46) | 1,271 (46.59) | |
| Lymph node removed | <0.001 | ||||
| 1–3 | 746 (4.18) | 215 (4.94) | 177 (4.29) | 110 (4.03) | |
| >3 | 8,779 (49.17) | 1,827 (41.97) | 1,707 (41.24) | 1,217 (44.61) | |
| Unknown | 8,329 (46.65) | 2,311 (53.09) | 2,261 (54.47) | 1,401 (51.36) | |
| Histology subgroup | <0.001 | ||||
| Signet Ring cell | 3,910 (21.90) | 724 (16.63) | 1,063 (25.68) | 597 (21.88) | |
| Mucinous adenocarcinoma | 420 (2.35) | 114 (2.62) | 120 (2.90) | 63 (2.31) | |
| Adenocarcinoma | 13,524 (75.75) | 3,515 (80.75) | 2,965 (71.42) | 2,068 (75.81) | |
| Grade | <0.001 | ||||
| Well differentiated | 640 (3.58) | 212 (4.87) | 109 (2.63) | 104 (3.81) | |
| Moderately differentiated | 4,067 (22.78) | 1,137 (26.12) | 884 (21.36) | 620 (22.73) | |
| Poorly differentiated | 10,512 (58.88) | 2,349 (53.96) | 2,480 (59.92) | 1,552 (56.89) | |
| Undifferentiated | 351 (1.97) | 76 (1.75) | 67 (1.62) | 46 (1.69) | |
| Unknown | 2,284 (12.79) | 579 (13.30) | 599 (14.47) | 406 (14.88) | |
| Therapy (Surgery ± radiotherapy) | <0.001 | ||||
| None | 5,794 (32.45) | 1,548 (35.56) | 1,352 (32.66) | 967 (35.45) | |
| Therapy | 10,111 (56.63) | 2,314 (53.16) | 2,221 (53.66) | 1,449 (53.12) | |
| Unknown | 1,949 (10.92) | 491 (11.28) | 566 (13.67) | 312 (11.43) | |
SD: Standard deviation.
NOS: Not otherwise specified.
AJCC: American Joint Committee on Cancer.
TNM: Tumor-Node-Metastasis.
The ratio of male to female was highest in the married group (2.56) while it reversed in the widowed group (0.44). The mean age of patients in the widowed group was significantly higher than in other subgroups. Asian patients had higher percentage of married than other races, and African-American had the lowest. As for the insurance status, we found that more married patients were covered by the insurance and more single patients were uninsured.
Widowed patients had more antrum/pylorus and lesser/greater curvature cancer, more tumors at stage I and well/moderately differentiated tumors. Patients in the married group had more stage II/III diseases. Single patients had more stage IV diseases, more signet ring cell and poorly differentiated tumors.
There is no information of chemotherapy in the SEER database. We only collected the information of surgery and radiotherapy. The percentage of patients who received therapy (surgery or radiotherapy) from stage I to IV was 54.89%, 54.47%, 55.78% and 55.81%, P = 0.438. Similarly, no significant difference was found for patients with different histologic subtype to receive treatment, 55.48% for adenocarcinoma, 54.53% for mucinous adenocarcinoma and 55.02% for signet ring cell carcinoma, P = 0.663. More patients in the married group received surgery with or without radiation than those in the unmarried group.
Survival
In this study, 17197 deaths (59.13%) were observed including 10289 (57.63%) in the married group (N = 17854), 2712 (62.30%) in the widowed group (N = 4353), 2522 (60.93%) in the single group and 1668 (61.14%) in the separated/divorced group. The median survival for the whole population was 16.08 months with a 5-year cause specific survival (CSS) of 28.7% [95% confidence interval (CI): 28.1–29.4%]. The median overall CSS was 17.87 and 13.61 months for the married and unmarried patients, P < 0.001. The 5-year CSS was higher in the married group than in the other groups, 30.6% in married group, 25.3% for the widowed group, 25.4% for the single group and 26.2% for the separated/divorced group, Fig. 1. Since the survival difference among patients in the widowed, single and separated/divorced groups was small, we combined these three groups into a new group called unmarried. The median survival for patients in unmarried group was 13.61 months and the 5-year CSS was 25.7% (95% CI: 24.7%–26.7%). The survival difference between married and unmarried group was significant, P < 0.001, Fig. 2.
Figure 1. The survival difference among different marital status.
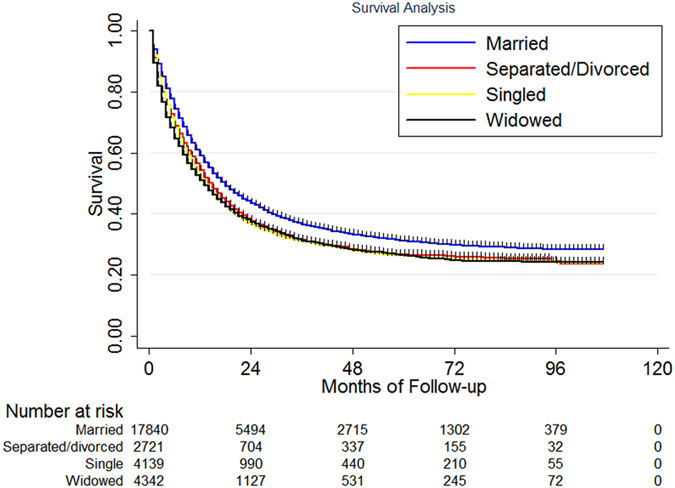
Figure 2. The survival difference between married and unmarried patients.
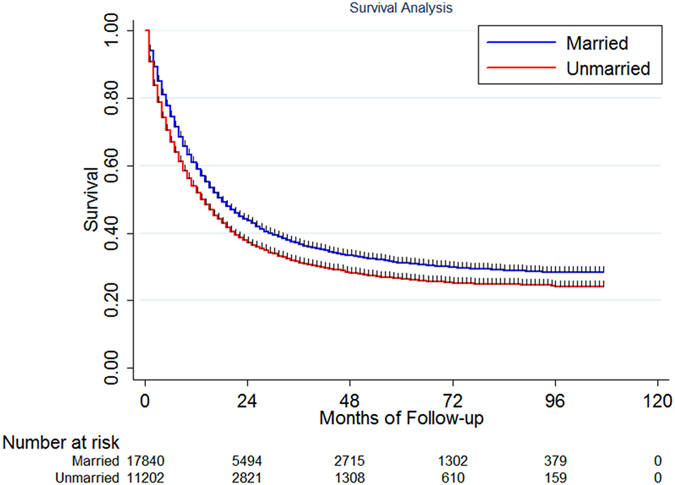
Table 2 demonstrated the comparison of median survival and 5-year CSS in different variables. Compared with female patients, male patients had a slightly better survival. The 5-year CSS was 28.8% vs 28.5%, P = 0.0285. Patients with tumor in the lesser/greater curvature had the best survival with a 5-year CSS of 39.0%. Asian patients had a significantly better survival than patients in other race/ethnicity. The 5-year CSS was 40.1% in Asian patients, 26.7% in African-American patients and 26.6% in the Caucasian patients, P < 0.001. We also analyzed the influence of insurance on the survival and found that the 5-year CSS was 8.5% higher in the insured group than uninsured group, 29.9% vs 21.4%, P < 0.001. The 5-year CSS for patients from AJCC 6th TNM stage I to IV patients was 61.9%, 40.9%, 23.3% and 4.8% respectively, P < 0.001. The median survival for patients in stage I has not yet reached. For patients who received resection, the number of lymph nodes resected also had an effect on the survival. Patients with the number of lymph nodes resected over 3 had a significantly better survival than those with 1–3 lymph nodes resected, 45.7% vs 39.5%, P < 0.001. The survival became poorer as the tumor grade progressed from well to undifferentiated, 58.7% for well differentiated, 39.4% for moderately differentiated, 24.9% for poorly differentiated and 22.4% for undifferentiated tumors, P < 0.001.
Table 2. Univariate analysis of cause specific survival.
| Median CSS | 5-year CSS (95% CI) | Univariate analysis | P value | |
|---|---|---|---|---|
| Log rank | ||||
| Sex | 4.8 | 0.0285 | ||
| Male | 16.48 | 28.8 (28.0–29.6) | ||
| Female | 15.35 | 28.5 (27.5–29.6) | ||
| Age | 10.6 | 0.0011 | ||
| <67 | 16.41 | 28.7 (27.5–29.3) | ||
| >66 | 15.77 | 29.1 (28.1–30.0) | ||
| Marital status | 149.37 | <0.001 | ||
| Married | 17.87 | 30.6 (29.7–31.4) | ||
| Widowed | 13.21 | 25.3 (24.2–27.4) | ||
| Single | 13.29 | 25.4 (23.7–27.1) | ||
| Separate/Divorced | 14.69 | 26.2 (24.2–28.2) | ||
| Race/Ethnicity | 293.33 | <0.001 | ||
| Caucasian | 15.10 | 26.6 (25.9–27.4) | ||
| African-American | 14.50 | 26.7 (25.0–28.5) | ||
| Asian | 28.00 | 40.1 (38.3–41.8) | ||
| Others | 17.53 | 34.6 (25.5–43.9) | ||
| Insurance status | 28.0 | <0.001 | ||
| Uninsured | 11.43 | 21.4 (17.3–25.8) | ||
| Insured | 16.95 | 29.9 (28.9–30.8) | ||
| Location | 999.05 | <0.001 | ||
| Cardia | 16.38 | 25.4 (24.2–26.5) | ||
| Fundus | 13.51 | 24.0 (20.8–27.3) | ||
| Body | 16.77 | 30.9 (28.6–33.1) | ||
| Antrum/Pylorus | 23.10 | 37.0 (35.6–38.3) | ||
| Lesser/Greater curvature | 26.32 | 39.0 (37.0–40.9) | ||
| Others | 9.22 | 17.6 (16.4–18.8) | ||
| AJCC 6th TNM stage | 9814.31 | <0.001 | ||
| I | – | 61.9 (60.6–63.2) | ||
| II | 36.82 | 40.9 (39.0–42.9) | ||
| III | 19.47 | 23.3 (21.6–25.0) | ||
| IV | 7.11 | 4.8 (4.3–5.3) | ||
| Lymph node removed | 34.11 | <0.001 | ||
| 1–3 | 30.43 | 39.5 (36.3–42.7) | ||
| >3 | 44.13 | 45.7 (44.6–46.7) | ||
| Histology subgroup | 173.40 | <0.001 | ||
| Signet Ring cell | 12.73 | 21.6 (20.3–22.8) | ||
| Mucinous adenocarcinoma | 17.59 | 29.4 (25.5–33.4) | ||
| Adenocarcinoma | 17.45 | 30.8 (30.0–31.5) | ||
| Grade | 682.88 | <0.001 | ||
| Well differentiated | – | 58.7 (55.1–62.2) | ||
| Moderately differentiated | 27.71 | 39.4 (37.9–40.8) | ||
| Poorly differentiated | 14.55 | 24.9 (24.1–25.7) | ||
| Undifferentiated | 14.20 | 22.4 (18.1–26.9) | ||
| Therapy (Surgery ± radiotherapy) | 9457.52 | <0.001 | ||
| Therapy | 44.02 | 45.7 (44.8–46.6) | ||
| None | 6.24 | 4.4 (3.8–5.0) | ||
| Unknown | 10.37 | 0 |
CSS: Cause specific survival.
CI: Confidence interval.
AJCC: American Joint Committee on Cancer.
TNM: Tumor-Node-Metastasis.
Male versus Female
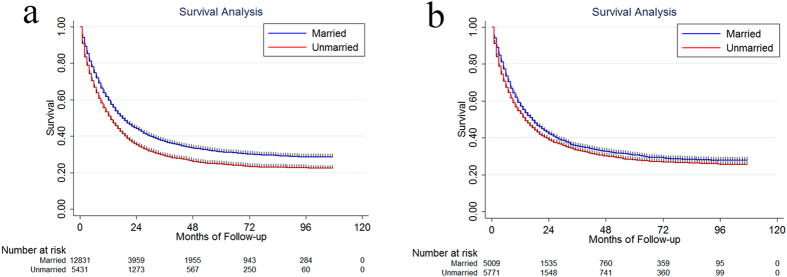
Since some studies have identified a differential effect of marriage in men versus women, we also made a comparison of the prognostic effect of marital status between male and female. The median survival and 5-year CSS for married male were 18.22 months and 30.8% (95% CI: 29.8–31.8%). For unmarried male, they were 13.08 months and 24.0% (95% CI: 22.6–25.5%), P < 0.001 (Fig. 3a). For married female, the median survival and 5-year CSS were 17.13 months and 30.0% (95% CI: 28.5–31.5%). They were 14.11 months and 27.30% (95% CI: 25.9–28.7%) for unmarried female, P < 0.001 (Fig. 3b).
Figure 3.
The survival difference between married and unmarried patients in male (a) and female (b).
Insurance and Race/Ethnicity
In order to find out potential reasons for the survival disparity between married and unmarried patients, we further explored the effect of insurance and race/ethnicity on the survival.
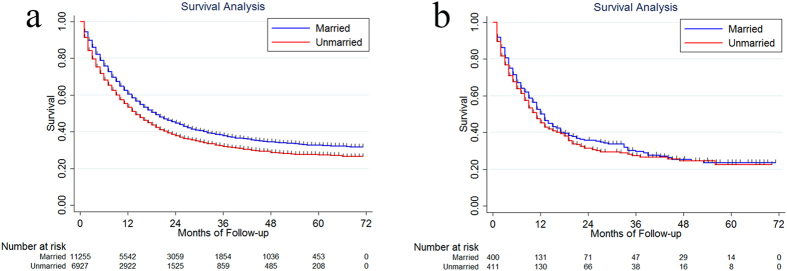
For patients who were covered by the insurance, married patients had a significantly better median survival and 5-year CSS than unmarried patients, which were 18.07 months, 30.8% (95%CI: 29.5–32.1%) and 13.30 months, 24.8% (95% CI: 23.2–26.4%), P < 0.001 (Fig. 4a). While for those uninsured, the marital status had no effect on the survival, which were 11.99 months, 20.9% (95% CI: 15.6–26.7%) and 9.82 months, 18.5% (95% CI: 12.9–24.9%), P = 0.2627 (Fig. 4b).
Figure 4.
The survival difference between married and unmarried patients in insured (a) and uninsured patients (b).
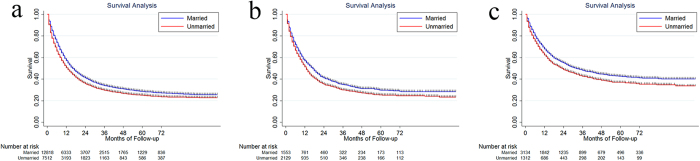
The median survival and 5-year CSS for married Caucasian patients were 15.69 months and 27.2% (95% CI: 26.2–28.2%). For unmarried Caucasian patients, they were 12.20 months and 23.0% (95% CI: 21.7–24.3%), P < 0.001 (Fig. 5a). The median survival and 5-year CSS were 16.12 months, 28.7% (95% CI: 25.8–31.6%) for married African-American, and 12.19 months, 22.3% (95% CI: 20.0–24.7%) for unmarried African-American, P = 0.002 (Fig. 5b). The median survival and 5-year CSS were 28.23 months, 41.1% (95% CI: 37.9–42.3%) and 20.31 months, 32.3% (95% CI: 28.9–35.7%) for married and unmarried Asian patients, respectively, P = 0.0001 (Fig. 5c).
Figure 5.
The survival difference between married and unmarried patients in Caucasian (a), African-American (b) and Asian (c).
Multivariate analysis
Variables showing a trend for association with survival (P < 0.05) were selected in the cox proportional hazards model. Sex, location and insurance status was not independent prognostic factors. Age, marital status, race/ethnicity, TNM stage, number of lymph nodes resected, histologic subtypes and grade were all independent prognostic factors in the multivariable analysis (Table 3). Compared for married patients, the HR for unmarried patients was 1.09 (95% CI: 1.01–1.17), P = 0.027.
Table 3. Multivariate analysis of survival.
| Hazard ratio (95% confidence interval) | P value | |
|---|---|---|
| Sex | ||
| Male | Reference | |
| Female | 0.96 (0.89–1.04) | 0.313 |
| Age | ||
| <67 | Reference | |
| >66 | 1.45 (1.35–1.56) | <0.001 |
| Marital status | ||
| Married | Reference | |
| Unmarried | 1.09 (1.01–1.17) | 0.027 |
| Race/Ethnicity | ||
| Caucasian | Reference | |
| African-American | 1.03 (0.93–1.14) | 0.580 |
| Asian | 0.78 (0.71–0.86) | <0.001 |
| Others | 0.96 (0.73–1.26) | 0.759 |
| Insurance status | ||
| Insured | Reference | |
| Uninsured | 1.08 (0.88–1.31) | 0.472 |
| Location | ||
| Cardia | Reference | |
| Fundus | 0.90 (0.73–1.10) | 0.297 |
| Body | 0.81 (0.70–0.93) | 0.002 |
| Antrum/Pylorus | 0.83 (0.75–0.91) | <0.001 |
| Lesser/greater curvature | 0.77 (0.69–0.87) | <0.001 |
| Others | 0.97 (0.87–1.09) | 0.641 |
| AJCC 6th TNM stage | ||
| I | Reference | |
| II | 2.58 (2.29–2.90) | <0.001 |
| III | 4.43 (3.96–4.95) | <0.001 |
| IV | 7.75 (6.94–8.67) | <0.001 |
| Lymph node removed | ||
| 1–3 | Reference | |
| >3 | 0.64 (0.56–0.72) | <0.001 |
| Histology subgroup | ||
| Adenocarcinoma | Reference | |
| Signet Ring cell | 1.14 (1.04–1.24) | 0.003 |
| Mucinous adenocarcinoma | 1.06 (0.87–1.30) | 0.563 |
| Grade | ||
| Well differentiated | Reference | |
| Moderately differentiated | 1.39 (1.08–1.80) | 0.012 |
| Poorly differentiated | 1.95 (1.52–2.50) | <0.001 |
| Undifferentiated | 2.18 (1.60–2.98) | <0.001 |
| Therapy (Surgery ± radiotherapy) | ||
| Therapy | Reference | |
| None | 2.06 (1.92–2.23) | <0.001 |
| Unknown | 1.51 (1.43–1.59) | <0.001 |
AJCC: American Joint Committee on Cancer.
TNM: Tumor-Node-Metastasis.
Discussion
We found that unmarried gastric adenocarcinoma patients (including widowed, single and separated/divorced), were at significantly greater risk of cause-specific death than married patients. After adjusting for demographics, stage and histologic subtypes, marital status remained independent prognostic factors. The insurance status reflected the socioeconomic status of patients and was reported to influent the overall survival of gastric cancer patients21. According to our result, more married patients were covered by insurance. Moreover, for insured patients, patients who were married had significantly better survival than those unmarried. While, this difference was not significant in uninsured patients. This indicated that insurance and financial status might play the key roles in the survival difference between married and unmarried patients. Moreover, Asian patients had higher percentages of married than other races. Lots of studies showed that the prognosis for cancer was better for Asian than for Caucasian patients22,23,24,25. In our present study, we also found that race/ethnicity was an independent prognostic factor in the multivariable analysis. Comparing with Caucasian, there was a 22% decreased of cause-specific death in Asian patients. The survival difference between married and unmarried patients persisted in Caucasian, African-American and Asian patients. Except for the race/ethnicity issue, the earlier stage at presentation may also contribute to the better survival for married patients. Some studies showed that delayed diagnosis was one of the reasons for poor prognosis in unmarried patients10,14. Spouse might provide social supports and encourage the patients to seek medical treatments26,27. Though there is no information of chemotherapy in the SEER database, in our study, we found that more married patients received surgery with or without radiation than unmarried patients. Cancer patients may suffer from distress, depression and some other psychologic problems28. Married patients were reported to suffer less from distress, depression and anxiety than their unmarried counterparts, especially for male patients29,30. Partly because the spouse could share the emotional burden and provide supports to the patients10.
The comparison of clinicopathologic features among different marital status showed that patients in the widowed groups had more stage I and well or moderately differentiated tumors. Even with these good prognostic factors, patients in widowed group still ended up with the poorest 5-year CSS. The exact reasons need to be furtherly explored. From our present data, we found that widowed patients were older than patients in other groups. Comparing with the younger patients, elderly gastric cancer patients were reported to have a poorer survival31,32. Meanwhile, some studies showed that excess mortality after the death of a spouse was partly caused by stress33. Stress and depression might cause noncompliance to the medical treatment34,35.
Compared with female patients, male patients had a slightly better survival, the 5-year CSS was 28.8% vs 28.5%, P = 0.0285. Though the difference was statistically significant, the absolute difference was small. This P value may be caused by the big sample size.
Though marriage showed a significant protective effect in both male and female patients, male patients benefitted more from marriage than female patients did. The same phenomenon was reported by Aizer AA et al. in lung, colorectal, breast, pancreatic, prostate, liver/intrahepatic bile duct, non-Hodgkin lymphoma, head/neck, ovarian as well as esophageal cancer patients10. The author explained that maybe unmarried women received greater social support from their relatives, friends, or the community than unmarried men10. However, a systematic review and meta-analysis did not confirm the differential effect of marriage in men versus women20. As for the gender difference, there was one more point merited discussion. The ratio of male to female was about 2:1 in the whole population which was consistent to the previous reports36,37. However, the ratio of male to female reversed in the widowed subgroup which was about 1:2.28. One of the possible explanations was that the mortality rate was higher among widowers than widows38. The loss of social support and the inability to deal with stress might also explain why men suffer from bereavement more than women33. In the whole population, male patients had a slightly better survival than female patients. The association between marital status and gender was a complicated issue. Further data are required to support this hypothesis.
From the present knowledge, the benefits of marriage on survival might be mediated through social and psychologic support. It is therefore important for the physicians to screen for depression among gastric adenocarcinoma patients and consider closer observation as well as necessary psychologic support to these patients.
In 2015, Zhou RP et al. had reported that unmarried GC patients, especially widowed patients, were at a high risk of gastric cancer specific survival15. Though our present study is not the first study to analyze the survival disparity between married and unmarried gastric cancer patients, we not just replicated their main conclusion. Actually, we made a further analysis to seek out possible explanations for the survival difference. We used data to elucidate that insurance and financial status as well as race/ ethnicity may play important roles in the survival disparity. The another big difference between our present study and Zhou RP’s previous report is that we focus on the adenocarcinoma, which is the most common histologic subtype for gastric cancer. While Zhou RP et al. put carcinoid tumor/neuroendocrine, gastrointestinal stromal sarcoma and adenocarcinoma together.
Potential limitations of our study should be taken into consideration. Firstly, there may be some other factors that contribute to the survival disparity among different marital status patients, such as chemotherapy. However, data related to chemotherapy are not available in SEER database. Secondly, there is no data about the HP and EBV infection in the SEER database, we cannot make a comparison about infection among different marital status. Finally, the quality of marriage could not be assessed in this study. Since the support and accompany of spouse seem to be responsible for the good survival in married patients, the quality of marriage is an important issue.
In conclusion, we used the SEER database to evaluate the survival disparity of gastric adenocarcinoma patients with different marital status. Our data revealed that married patients had a survival advantage in both male and female patients. The relationship between marriage and survival can be explained hypothetically by psychosocial and socioeconomic factors. We recommend that every subject should have access to best available gastric cancer therapy.
Methods
Database
The SEER database is the largest publicly available cancer dataset. It is a population-based cancer registry across several disparate geographic regions. The SEER research data include cancer incidence and prevalence as well as demographic information tabulated by age, sex, race / ethnicity, year of diagnosis, marital status, insurance, Tumor-Node-Metastasis (TNM) stage and geographic region. The exact dataset we used for this analysis was SEER Program (www.seer.cancer.gov) Research Data (1973–2012), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission, “Incidence-SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2014 Sub (1973–2012 varying)”.
Outcome variables
Variable definitions information on age at diagnosis, sex, year of diagnosis, race/ethnicity, marital status, primary site, tumor grade and differentiation, histology, lymph node involvement, AJCC 6th TNM stage, insurance status and overall survival were coded and available in SEER database (Appendix S1).
For the Race/Ethnicity, we reclassified the patients into 5 groups: “Caucasian”, “African American”, “Asian”, “Others” and “Unknown”.
Patients were classified as married and unmarried. Since the group of “Unmarried or domestic partner” is misleading and we removed this group of patients from analysis. Unmarried patients included single, separated/divorced and widowed.
Since the AJCC 7th TNM staging system was released in 2010 and if we used this staging system, there would be no 5 year survival due to insufficient follow up and less patients, so we picked up the AJCC 6th TNM staging systems. Meanwhile, since the AJCC 6th TNM staging system was released in 2004, we restricted our study from 2004–2012. Patients were divided into “insured group” and “uninsured group” according to their insurance status.
Patient Population
The study population was based on the SEER cancer registry. We restricted eligibility to adults (aged 18 years or older) who were diagnosed with gastric adenocarcinoma (also including mucinous adenocarcinoma and signet ring cell carcinoma) from 2004 to 2012. We excluded cases without follow-up records (survival time code of 0 months). Patients without record of marital status and TNM stage were also excluded.
Statistical Methods
The patients’ demographic and tumor characteristics were summarized with descriptive statistics. Comparisons of categorical variables among different marital status were performed using the Chi square test, and continuous variables were compared using Student’s t test. The primary endpoint of this study was 5-year CSS, which was calculated from the date of diagnosis to the date of cancer specific death. Deaths attributed to gastric cancer were treated as events and deaths from other causes were treated as censored observations. Survival function estimation and comparison among different variables were performed using Kaplan–Meier estimates and the log-rank test. The independence of the prognostic effect of the marital status was evaluated by adjusting for other known factors including age at diagnosis and tumor stage. The multivariate Cox proportional hazard model was used to evaluate the hazard ratio (HR) and the 95% CI for all the known prognostic factors, including sex, age, marital status, location, race/ethnicity, time of diagnosis, TNM stage, lymph node removed, histology, grade, insurance status and therapy (Surgery with or without radiotherapy). All of statistical analyses were performed using the Intercooled Stata 13.0 (Stata Corporation, College Station, TX). Statistical significance was set at two-sided P < 0.05.
Additional Information
How to cite this article: Qiu, M. et al. Impact of marital status on survival of gastric adenocarcinoma patients: Results from the Surveillance Epidemiology and End Results (SEER) Database. Sci. Rep. 6, 21098; doi: 10.1038/srep21098 (2016).
Supplementary Material
Acknowledgments
This work was supported by: The third outstanding young talents training plan of Sun Yat-sen University cancer center, Medical Scientific Research of Guangdong province B2014161, Scientific and Technological projects Guangdong Esophageal Cancer Institute Q201408, Science and Technology Planning Project of Guangdong Province (No. 2013A022100023), National High Technology Research and Development Program of China (863 Program), China (No. 2015AA020103), National Natural Science Foundation of China (No.81372570, 81572392), Natural Science Foundation of Guangdong Province (No.2014A030312015), Science and Technology Program of Guangzhou (No. 15570006, 158100066). The authors acknowledge the staff members of the National Cancer Institute and their colleagues across the United States and at Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology and End Results (SEER) Program.
Footnotes
Author Contributions M.Q. and R.X. planned the study. M.Q. and D.Y. calculated statistics and analyzed the data. M.Q. and R.X. wrote the manuscript. D.Y. and R.X. supervised the entire project. All authors reviewed the manuscript.
References
- Brenner H., Rothenbacher D. & Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 472, 467–477 (2009). [DOI] [PubMed] [Google Scholar]
- Kamangar F., Dores G. M. & Anderson W. F. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 24, 2137–2150 (2006). [DOI] [PubMed] [Google Scholar]
- Chen W., Zheng R., Zeng H. & Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer . 34, 53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin . 65, 87–108 (2015). [DOI] [PubMed] [Google Scholar]
- Hu Y., Fang J. Y. & Xiao S. D. Can the incidence of gastric cancer be reduced in the new century? J Dig Dis. 14, 11–15 (2013). [DOI] [PubMed] [Google Scholar]
- Qiu M. Z. & Xu R. H. The progress of targeted therapy in advanced gastric cancer. Biomark Res . 1, 32 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. R. & Goldman N. Mortality differentials by marital status: an international comparison. Demography. 27, 233–250 (1990). [PubMed] [Google Scholar]
- Ikeda A. et al. Marital status and mortality among Japanese men and women: the Japan Collaborative Cohort Study. BMC Public Health. 7, 73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R. M. & Kronick R. G. Marital status and longevity in the United States population. J Epidemiol Community Health. 60, 760–765 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer A. A. et al. Marital status and survival in patients with cancer. J Clin Oncol. 31, 3869–3876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossa S. D. et al. Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol. 29, 963–970 (2011). [DOI] [PubMed] [Google Scholar]
- Sammon J. D. et al. Marital status: a gender-independent risk factor for poorer survival after radical cystectomy. BJU Int. 110, 1301–1309 (2012). [DOI] [PubMed] [Google Scholar]
- Wang L., Wilson S. E., Stewart D. B. & Hollenbeak C. S. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol . 35, 417–422 (2011). [DOI] [PubMed] [Google Scholar]
- Li Q., Gan L., Liang, L., Li X. & Cai S. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget. 6, 7339–7347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yan S. & Li J. Influence of marital status on the survival of patients with gastric cancer. J Gastroenterol Hepatol Oct 29 (2015). doi: 10.1111/jgh.13217. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Hunt W. C., Key C. R. & Samet J. M. The effect of marital status on stage, treatment, and survival of cancer patients. Jama. 258, 3125–3130 (1987). [PubMed] [Google Scholar]
- Nelles J. L., Joseph S. A. & Konety B. R. The impact of marriage on bladder cancer mortality. Urol Oncol. 27, 263–267 (2009). [DOI] [PubMed] [Google Scholar]
- Greenberg E. R. et al. Social and economic factors in the choice of lung cancer treatment. A population-based study in two rural states. N Engl J Med. 318, 612–617 (1988). [DOI] [PubMed] [Google Scholar]
- Jatoi A. et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 12, 1456–1463 (2007). [DOI] [PubMed] [Google Scholar]
- Manzoli L., Villari P., G M. P. & Boccia A. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med . 64, 77–94 (2007). [DOI] [PubMed] [Google Scholar]
- Jang J. S. et al. Differences in the Survival of Gastric Cancer Patients after Gastrectomy according to the Medical Insurance Status. J Gastric Cancer. 13, 247–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C. S. & Mayer R. J. Gastric carcinoma. N Engl J Med. 333, 32–41 (1995). [DOI] [PubMed] [Google Scholar]
- Lin J., Qiu M., Xu R. & Dobs A. S. Comparison of survival and clinicopathologic features in colorectal cancer among African American, Caucasian, and Chinese patients treated in the United States: Results from the surveillance epidemiology and end results (SEER) database. Oncotarget . 6, 33935–33943 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong V. E. et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 251, 640–646 (2010). [DOI] [PubMed] [Google Scholar]
- Wang J., Sun Y. & Bertagnolli M. M. Comparison of Gastric Cancer Survival Between Caucasian and Asian Patients Treated in the United States: Results from the Surveillance Epidemiology and End Results (SEER) Database. Ann Surg Oncol. 22, 2965–2971 (2015). [DOI] [PubMed] [Google Scholar]
- Aizer A. A. et al. Multidisciplinary care and pursuit of active surveillance in low-risk prostate cancer. J Clin Oncol. 30, 3071–3076 (2012). [DOI] [PubMed] [Google Scholar]
- Cohen S. D. et al. Social support and chronic kidney disease: an update. Adv Chronic Kidney Dis. 14, 335–344 (2007). [DOI] [PubMed] [Google Scholar]
- Kaiser N. C., Hartoonian N. & Owen J. E. Toward a cancer-specific model of psychological distress: population data from the 2003-2005 National Health Interview Surveys. J Cancer Surviv . 4, 291–302 (2010). [DOI] [PubMed] [Google Scholar]
- Goldzweig G. et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. 21, 877–883 (2010). [DOI] [PubMed] [Google Scholar]
- Goldzweig G. et al. Gender and psychological distress among middle- and older-aged colorectal cancer patients and their spouses: an unexpected outcome. Crit Rev Oncol Hematol. 70, 71–82 (2009). [DOI] [PubMed] [Google Scholar]
- Seo J. Y. et al. Clinicopathologic and molecular features associated with patient age in gastric cancer. World J Gastroenterol. 21, 6905–6913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu H., Wang H., Li C., Kang Y. & Xue Y. Clinico-pathological features and prognostic analysis of gastric cancer patients in different age groups. Hepatogastroenterology. 62, 225–230 (2015). [PubMed] [Google Scholar]
- Martikainen P. & Valkonen T. Mortality after the death of a spouse: rates and causes of death in a large Finnish cohort. Am J Public Health. 86, 1087–1093 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo M. R., Lepper H. S. & Croghan T. W. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 160, 2101–2107 (2000). [DOI] [PubMed] [Google Scholar]
- Weissman M. M. et al. Cross-national epidemiology of major depression and bipolar disorder. Jama. 276, 293–299 (1996). [PubMed] [Google Scholar]
- Qiu M. Z. et al. Prognostic analysis in node-negative gastric cancer patients in China. Tumour Biol. 32, 489–492 (2011). [DOI] [PubMed] [Google Scholar]
- Qiu M. Z. et al. Clinicopathological characteristics and prognostic analysis of gastric cancer in the young adult in China. Tumour Biol. 32, 509–514 (2011). [DOI] [PubMed] [Google Scholar]
- Bowling A. Mortality after bereavement: a review of the literature on survival periods and factors affecting survival. Soc Sci Med. 24, 117–124 (1987). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.