Abstract
Disorazol, a macrocyclic polykitide produced by the myxobacterium Sorangium cellulosum So ce12 and it is reported to have potential cytotoxic activity towards several cancer cell lines, including multi-drug resistant cells. The disorazol biosynthetic gene cluster (dis) from Sorangium cellulosum (So ce12) was identified by transposon mutagenesis and cloned in a bacterial artificial chromosome (BAC) library. The 58-kb dis core gene cluster was reconstituted from BACs via Red/ET recombineering and expressed in Myxococcus xanthus DK1622. For the first time ever, a myxobacterial trans-AT polyketide synthase has been expressed heterologously in this study. Expression in M. xanthus allowed us to optimize the yield of several biosynthetic products using promoter engineering. The insertion of an artificial synthetic promoter upstream of the disD gene encoding a discrete acyl transferase (AT), together with an oxidoreductase (Or), resulted in 7-fold increase in disorazol production. The successful reconstitution and expression of the genetic sequences encoding for these promising cytotoxic compounds will allow combinatorial biosynthesis to generate novel disorazol derivatives for further bioactivity evaluation.
Natural products from microorganisms, fungi, plants and insects display a broad spectrum of biological activities. Currently, approximately 49% of anti-infectives compounds and 61% of anticancer pharmaceutical agents in clinical use are natural products or their derivatives1. Over the last decades, myxobacteria have become well known producer organisms, offering a rich and valuable source of natural products2,3. Most of these compounds are biosynthesized by multifunctional megasynthetases, such as polyketide synthases (PKSs)4, nonribosomal peptide synthetases (NRPSs)5 and hybrids thereof 6. Genes encoding these PKSs and NRPSs in bacteria are often clustered together on the chromosome, so a gene cluster can be cloned into a vector and then transferred to a heterologous host for functional expression7. Recent studies demonstrate the usefulness of heterologously expressed secondary metabolite pathways for the production of natural products8. Heterologous expression can improve fermentation yields and generate new natural or synthetic products that can be evaluated as potential pharmacological agents in the course of targeted derivatization or structure-activity relationship studies9.
Reconstructing biosynthetic gene clusters in various vectors for heterologous expression in more productive hosts can help show how newly discovered biosynthetic gene clusters function. Derivatives of the new available pharmacologically active compounds can then be produced by biomolecular re-engineering and combinatorial biosynthesis10.
Large clusters of genes that encode the enzymes for natural product biosynthesis have been difficult to engineer using conventional technology. Red/ET recombineering in combination with TAR (Transformation-associated recombination) cloning is necessary for large biosynthetic gene clusters to be engineered conveniently11,12.
Red/ET recombineering is based on in vivo homologous recombination in E. coli13,14,15. The greatest advantage of the technology is that it works regardless of restriction enzyme binding sites or the size of DNA fragments to be shuffled. This technology has made it much easier to genetically manipulate complex biosynthetic pathways in bacteria. Over the past decade, several complete biosynthetic pathways from fastidious bacteria have been heterologously expressed via Red/ET recombineering, e.g. myxochromide S and myxothiazol in Pseudomonas putida and M. xanthus16,17,18,19, epothilones in M. xanthus19, human alpha-defensin 5 mature peptide in Pichia pastoris20, nikkomycin in Streptomyces ansochromogenes21, pretubulysin in P. putida and M. xanthus22, luminmycin and glidobactin in E. coli Nissle191723,24, salinomycin in Streptomyces coelicolor25 and even a minimal set of genes for magnetosome biosynthesis from the magnetotactic bacterium in Rhodospirillum rubrum26.
Secondary metabolite gene clusters in microbes express natural products with potential medicinal and values agricultural qualities27. However, many of the microorganisms hosting these biosynthetic pathways grow slowly even in optimized laboratory conditions and can not be genetically manipulated2. Heterologous expression of complete secondary metabolite pathways plays a significant role in hunting for new natural products and developing them into useful drugs16. Many heterologous expression instruments for secondary metabolite pathways have been reported so far, ranging from targeted expression by shuttle vectors to the random expression of large DNA fragments from chromosomes by transposition9.
Disorazols, a family of structually complex macrocyclic polyketides, are produced by the myxobacterium Sorangium cellulosum So ce12 (Fig. S1) and firstly isolated in 199428. Disorazols inhibit cancer cell proliferation at low picomolar concentrations by preventing tubulin polymerization and inducing destabilization of microtubules, which ultimately leads to the induction of apoptosis29,30,31. The extraordinary potency of disorazols fostered their development as peptide-conjugates for cancer therapy32,33 and encouraged the generation of new and simplified disorazol derivatives by means of chemical synthesis34,35,36. However, there are no reports to date on genetic engineering approaches for the production of new analogs of the disorazol compound class.
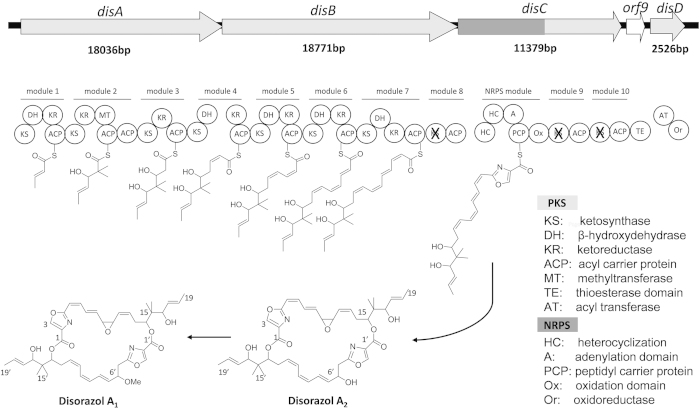
The dis biosynthetic gene cluster was identified by transposon mutagenesis. In 2005, the cluster was cloned into a BAC or cosmid library of S. cellulosum So ce12 by two independent groups37,38. The clusters showed the anticipated disA-C genes encoding hybrid trans-AT type I PKS/NRPS megaenzymes, and also another gene, disD, that encoded an additional acyl transferase protein (Fig. 1).
Figure 1. Domain organization of the dis biosynthetic gene cluster from S. cellulosum So ce12 and a model for biosynthesis of disorazol A1 (scheme according to Kopp et al.).
According to the biosynthetic model, seven malonyl-CoA units and one serine are incorporated as extender units, forming half of the disorazol bis-lactone core unit. Two polyketide monomers may dimerize to form disorazol via the thioesterase (TE) domain38, possibly requiring an esterase encoded by orf3′ 37 (Table S4).
The native strain produces only small amounts of disorazols (~1 mg per liter fermentation medium) and is difficult to cultivate28. Consequently, it is challenging to produce large quantities of disorazol for further development. Using an amenable heterologous host should be a rational way to assure higher and stable disorazol yields and possibly optimize its structure by molecular engineering.
Here we report the Red/ET recombineering of the dis biosynthetic gene cluster into a stable vector containing a p15A replication origin and a MycoMar transposase element. When the dis gene cluster was transposed into the chromosome of the heterologous host M. xanthus DK1622 several disorazol derivatives were produced. Subsequent gene deletions proved that only the disA-D genes and not orf9 or the putative esterase gene orf3′ were needed for disorazol production37. Further, we also improved disorazol production in the heterologous host M. xanthus DK1622 by replacing the native promoter of the disD gene encoding a discrete AT protein with an artificial synthetic promoter.
Results and Discussion
Reconstitution of the disorazol A biosynthetic gene cluster
The disorazol A biosynthetic gene cluster has been cloned, sequenced and identified previously from a BAC library of So ce1237. The BAC contained most of the dis gene cluster from disA to disD. However, the BAC pBeloBAC11-dis was a large and low copy vector and very difficult to transfer between hosts for heterologous expression. To construct a more efficient expression vector and to insert elements for transfer and expression into different heterologous hosts, we sequentially modified the original BAC (pBeloBAC11-dis) by Red/ET recombineering13,14. The backbone of pBeloBAC11-dis was replaced by a cassette containing the p15A replication origin (p15A ori), the origin of transfer (oriT) for conjugation purposes, two inverted repeats (IRs), a MycoMar transposase gene (Tps) for transposition, an inducible promoter tetR-Ptet for driving the dis gene cluster upstream of disA and a kanamycin resistance gene for selection in M. xanthus DK1622.
In the resulting construct p15A-dis, the dis gene cluster (containing disA-D and orf9) is in a relatively high copy number vector (20–30 copies per cell in E. coli). Instead of the native promoter, expression in this vector is controlled by a tetracycline inducible promoter in this vector works in several heterologous hosts, e.g. E. coli, M. xanthus and P. putida39 (Figs 2 and S2).
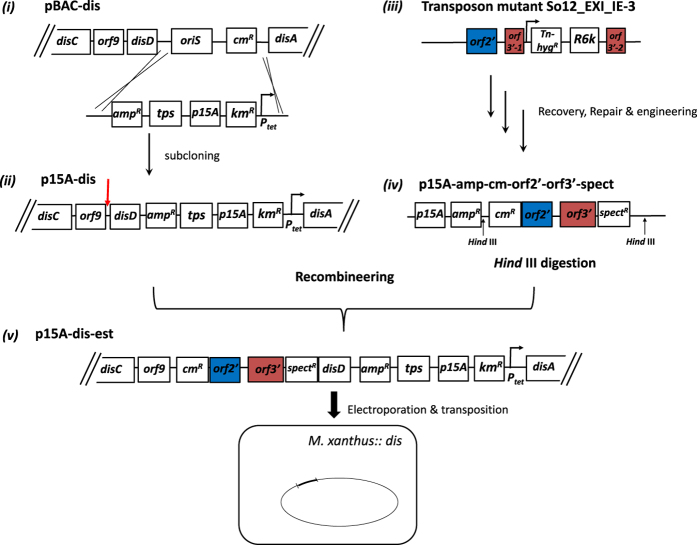
Figure 2. Diagram of disorazol A gene cluster engineering.
Firstly, the backbone of plasmid pBeloBAC11-dis (i) was replaced by p15A ori-tps casstte to form p15A-dis (ii) which containing an original MycoMar transposon by Red/ET recombineering. In this way dis gene cluster was driven by Ptet promoter. Then, the interrupted esterase gene orf3’ in pTn-Rec_IE2 plasmid (iii) from transposon mutant So12_EXI_IE-336 was recovered, repaired and engineered to form the vector p15A-amp-cm-orf2’-orf3’-spect (iv) that contained the whole length of the esterase gene orf3’. Next, linear DNA fragment released by Hind III was integrated into disorazol vector p15A-dis (ii) to get the final construct p15A-dis-est (v) via Red/ET recombinantion. Finally, two types of modified vectors p15A-dis (ii) and p15A-dis-est (v) were electroporated into M. xanthus respectively and kanamycin-resistant colonies were selected for further analysis. Hind III restriction sites used for releasing linear fraction “cmR-orf2’-orf3’-spectR” were indicated in ↑. The insertion site of the linear fragment DNA containing orf2’ and orf3’ gene was marked with ↓.
We previously found that disorazol production was no longer detectable when an esterase gene (orf3′) was mutated by transposon insertion in mutant strain So12_EXI_IE-337. This mutated esterase gene was implicated in bis-lactone formation during disorazol biosynthesis. We recovered plasmid pTn-Rec_IE2 (Fig. S6), which contained several genes near the transposition in the mutant So12_EXI_IE-3. The transposon was found in the middle of the carboxyl esterase gene orf3′ (only 6.7 kb upstream of the disA start codon).
The plasmid pTn-Rec_IE2 also included a S-adenosyl methionine (SAM) dependent methyl transferase gene orf2′37. As the product of orf2′ may O-methylate the OH group at C-6′ adjacent to the orf3′ gene, it might also be essential for disorazol biosynthesis (Fig. S6, Table S4). Hence, we inserted both, the repaired carboxyl esterase gene orf3′ and the SAM-dependent methyl transferase gene orf2′ together into p15A-dis to form p15A-dis-est by Red/ET recombineering. To gain the fusion plasmid p15A-dis-est, firstly, two separate PCR cassette “cmR” and “spectR” with suitable homologous arms to the region (containing two Hind III restriction sites in both sides) between the orf9 and the disD genes were introduced into the vector, respectively. After digestion by Hind III restriction enzyme in correct clones, the linear fragment “cmR-orf2′-orf3′-spectR” was integrated to obtain the final construct p15A-dis-est. By this, the cmR gene was introduced to drive orf2′ and orf3′ genes. Likewise, the spectR gene was introduced to drive the disD gene (Figs 2 and S2).
Certain gene products may be toxic to the host cell, potentially limiting the nature of downstream applications when introduced into E. coli directly at high copy number40. All E. coli strains containing the dis gene cluster with the native promoter were found to carry mutations after recombineering. Therefore, it was very challenging to obtain the expression construct containing the dis gene cluster directly in E. coli because the growth of the host was impeded. We reasoned that one of the dis proteins interfered with a primary metabolic pathway in E. coli to disrupt growth. To address this issue, an inducible promoter Ptet, was used to regulate gene expression. Ptet is a versatile tetracycline-based regulatory system that is usually used to selectively control expression of downstream genes39. No other promoter system is suitable for so many diverse hosts, including E. coli, M. xanthus and P. putida10,22,23,41. Besides, Ptet had already enabled several mixed PKS/NRPS natural products to be produced in heterologous hosts unrelated to the native producing organisms, such as myxochromide S from myxobacterium Stigmatella aurantiaca, which has been engineered into P. putida19.
The transposon method, which was also applied in this study, is clearly more stable and efficient than using shuttle vectors19. Several indispensable elements were inserted into the target vectors, for instance Tps and oriT. The mariner transposon MycoMar is frequently used in Gram-negative hosts for genetic modification42,43 and to transfer and integrate a gene cluster into the chromosome of heterologous host strains19,22. The transformation efficiency of large gene sets is higher when using the MycoMar transposon than using homologous recombination, as has been described for the heterologous expression of epothilone and myxochromide S19. This powerful tool for transforming large genes was used in the disorazol heterologous expression system to make it easier to integrate the dis gene cluster into the genome of host strains. The oriT was also incorporated for conjugation in other heterologous hosts strains, such as P. putida44.
Heterologous expression of dis gene cluster in M. xanthus DK1622
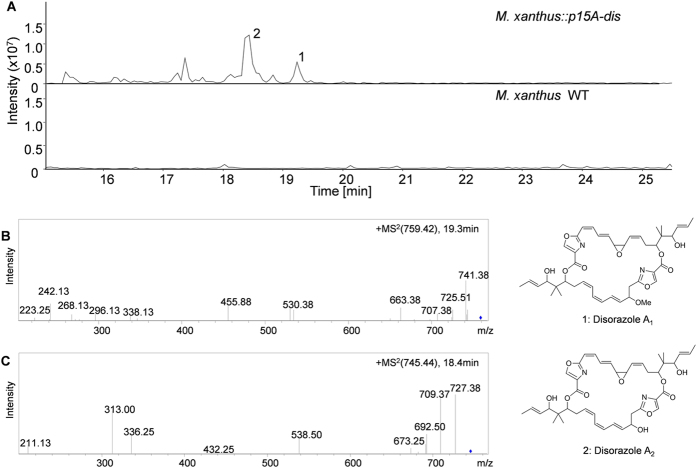
Both expression constructs p15A-dis and p15A-dis-est (Fig. S2) were introduced into the heterologous host M. xanthus DK1622 by electroporation as previously described19. The dis gene cluster was randomly transposed into the chromosome of M. xanthus. Transformants were screened on CTT agar containing kanamycin to select for M. xanthus::p15A-dis and M. xanthus::p15A-dis-est mutants. Six randomly chosen colonies of each mutant were verified by PCR19, which confirmed that the dis gene cluster had been integrated into the M. xanthus chromosome in each case. All the checked mutants contained the whole disorazol gene clusters. Several resulting mutants M. xanthus::p15A-dis and M. xanthus::p15A-dis-est were cultivated (both induction by anhydrotetracycline (AHT)) for compound extraction and detection. All the mutants produced detectable amounts of disorazols by the analysis of high performance liquid chromatography-tandem mass spectrometry (HPLC-MS)45. We have found small amounts of various disorazol compounds (including disrazols A1, A2, A3, A4, B2, B4 and F2) in both extracts of M. xanthus:: p15A-dis and M. xanthus:: p15A-dis-est (Figs 3 and S3, Table S2), upon comparing the secondary metabolite profiles from M. xanthus wild type strain and mutants. As expected, these results indicate that the chosen set of genes is sufficient to produce the polyketide-nonribosomal peptide skeleton of the disorazols.
Figure 3. Analysis of disorazol production in M. xanthus wildtype (WT) and M. xanthus::p15A-dis grown at 30 °C and induced with 0.5 μg/ml AHT.
(A) HPLC-MS analysis (base peak chromatogram [BPC] m/z 720–780) of M. xanthus::p15A-dis and M. xanthus WT. (B) MS2 fragmentation pattern of disorazol A1 (1). (C) MS2 fragmentation pattern of disorazol A2 (2).
Unexpectedly, without the orf2′ and the orf3′ genes, M. xanthus:: p15A-dis can also produce disorazols. The overall yields of disorazols in M. xanthus:: p15A-dis (averagely were 0.4 mg/L) match with that in M. xanthus:: p15A-dis-est (averagely were 0.42 mg/L). Result exhibited that the orf3′ gene is dispensable in the disorazol biosynthesis in the chosen heterologous host. There might be an enzyme that can substitute for the similar function of the orf3′ gene product in M. xanthus host. The dis gene cluster could be inactivated in the transposon mutant So12_EXI_IE-3 due to a strong polar effect37 because it is adjacent to the disA gene, possibly preventing downstream genes in an operon from being transcribed46,47.
The HPLC-MS and NMR data showed that the major compound in both mutants M. xanthus:: p15A-dis and M. xanthus:: p15A-dis-est was disorazol A2 which constituted 55% of final product after purification from crude extracts (Figs S3 and S4, Table S3), whereas disorazol A1 was 20%. But in the native host So ce12, disorazol A1 was the chief component (nearly 70% after purification, 10 times higher than disorazol A2) produced among the 29 derivatives28. The most probable explanation was that an O-methyl transferase that methylates the OH group at C-6′ was absent in the heterologous expression of dis gene cluster. This methyl transferase gene could be possibly located elsewhere in the chromosome of the native producer So ce12, which still needs further investigation. Only small amounts of the C-6′ methylated disorazols A1, A3 and A4 were produced in M. xanthus (Fig. S3), which might be due to partial methylation by a nonspecific M. xanthus O-methyl transferase. After 5 L fermentation of mutant strain M. xanthus::p15A-dis, the yield of disorazol A2 was approximately 0.24 mg/L, which is 5-fold higher than described in the native producer strain So ce1228,48. The result unambiguously demonstrated again that secondary metabolites can be produced in heterologous hosts under the control of the versatile Ptet promoter which encouraged further investigation of disorazol formation.
Biological activity of disorazol compounds
After having isolated disorazols from our heterologous host M. xanthus, biological studies revealed exceptional high cytotoxicity of disorazol A2 on eukaryotic cells. We determined IC50 values against several established human cancer cell lines from different origin and disorazol A2 strongly inhibited the growth of these cell lines with IC50 values between 0.05 and 4.9 nM (Table 1). However, compared to disorazol A1, the antiproliferative activity of disorazol A2 was less pronounced on most cell lines, except for human U-937 histiocytic lymphoma. Most likely, the higher IC50 values for disorazol A2 are due to the lack of a methyl group at C-6′ compared to disorazol A1, which in turn might lead to a less favourable binding to the traget structure tubulin. Nevertheless, when compared to other anticancer drugs, such as epothilone B or vinblastine, disorazol A2 is still much more effective in vitro29,49.
Table 1. Activity of disorazol A1 and disorazol A2 against human cancer cell lines.
| Human Cell line | Origin | IC50 [nM] |
|
|---|---|---|---|
| Disorazol A1 | Disorazol A2 | ||
| A-431 | epidermoid carcinoma | 1.866 | 4.908 |
| A-549 | lung carcinoma | 0.072 | 0.408 |
| HCT-116 | colon carcinoma | 0.032 | 0.071 |
| HepG2 | hepatocellular carcinoma | 0.002 | 0.051 |
| HL-60 | acutemyeloid leukemia | 0.058 | 0.084 |
| K-562 | chronicmyeloid leukemia | 0.074 | 0.140 |
| KB-3.1 | cervix carcinoma | 0.025 | 0.106 |
| SW480 | colonadeno carcinoma | 0.030 | 0.128 |
| U-2 OS | osteosarcoma | 0.038 | 0.206 |
| U-87 MG | glioblastoma-astrocytoma | 0.072 | 0.119 |
| U-937 | histiocytic lymphoma | 0.293 | 0.210 |
IC50 values refer to antiproliferative activities.
Optimized production with biomolecular technology
An unusual feature of the disorazol biosynthetic gene cluster is that it has only one discrete AT domain on the DisD module, and hence it is called a trans-AT type of PKS50. In recent years, trans-AT PKSs have been found in an important group of biosynthetic enzymes that produce bioactive natural products, including pederin, rhizoxin, leinamycin, myxovirescin, chivosazol and psymberin51,52. Accessing functionally-optimized polyketides by modifying PKSs through targeted synthase re-engineering is an encouraging approach to optimize natural products for application52. However, in contrast to ATs from cis-AT PKSs, the mechanisms and structures of trans-acting ATs are still unexplored.
The disD gene has been modified here to show how trans-acting ATs affect the disorazol biosynthesis pathway. In order to enhance the expression of the solitary AT domain, we introduced another strong promoter Pcp25 upstream of the disD gene. Pcp25 is a highly active, constitutive lactococcal consensus promoter, whose sequence has already been reported53,54. Previous studies have illustrated that overexpression of single genes or multigene transcriptional units by promoter exchange in myxobacteria can improve the production of secondary metabolites19,55,56,57.
On the other hand, the role of orf9 gene (showing similarity to hypothetical proteins), which separates the disC and disD genes, in the dis gene cluster has not been defined37. To discover the actual function of the orf9 gene in disorazol biosynthesis, we inactivated it on the expression construct p15A-dis and then performed heterologous production in M. xanthus.
The PCR cassette “Pcp25-spectR” (P18–P20 in Table S1), containing promoter Pcp25 and a spectinomycin resistance gene (spectR), with two different pairs of homologous arms, was inserted into p15A-dis by Red/ET recombineering to form two plasmids p15A-dis-Pcp25 and p15A-dis-Pcp25Δorf9 (Figs 4A and S5). In the first plasmid p15A-dis-Pcp25, the promoter Pcp25 was inserted directly upstream of the disD gene. In the second plasmid, the orf9 gene was deleted by using a synthetic promoter cassette with selection for spectinomycin resistance to obtain p15A-dis-Pcp25Δorf9. The disD gene was thereby controlled by the Pcp25 promoter in both expression constructs. The recombinants were analyzed after growth on low-salt Luria-Bertani (LB) broth plates plus spectinomycin. The verified constructs were transformed into M. xanthus DK1622 and three randomly picked positive transformants of each type of strain were cultivated to analyze the production by HPLC-MS. To clearly identify disorazol, retention times (RT) and the MS2 fragmentation pattern were compared to authentic reference substances. The concentration of disorazol A2 in the culture was determined by UPLC-HRMS. A standard curve between peak area and concentration was established from serial dilutions for disorazol A2 down to 0.01μg/mL. The peak area of disorazol A2 (base peak chromatograms, BPC + 759.3 ± 0.1, RT = 18.2 min) was calculated by BrukerDaltonics compass data analysis 4.0. The yields of all disorazols were estimated from their relative peak areas in the HPLC-MS chromatogram by comparison with the standard curve for each derivative.
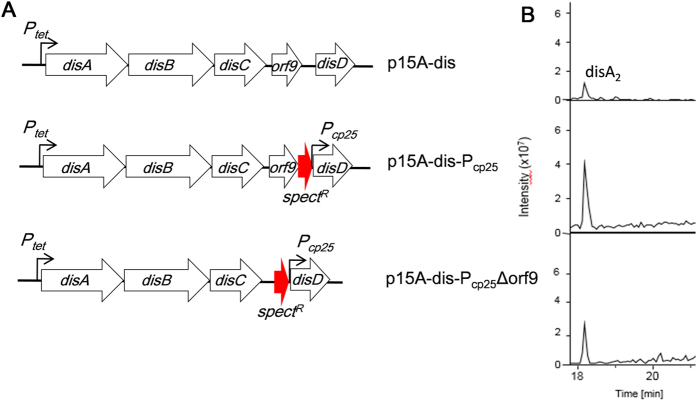
Figure 4. Promoter insertion in front of disD gene.
(A) Three different types of expression constructs used for disorazol heterologous production. The first one is the original plasmid p15A-dis. The second one was modified via Red/ET recombineering by insertion of promoter Pcp25 in front of disD directly. The third one was deletion orf9 gene by Pcp25-spectR so that Pcp25 was also upstream disD. (B) Quantification of heterologous disorazol production by HPLC-MS analysis of the culture extracts from different M. xanthus DK1622 mutant strains. Sections of extracted ion chromatograms at m/z = 745.45 corresponding to the [M + H]+ ion of disorazol A2 are illustrated as representative readout of productivity. The M. xanthus DK1622 host strains contain one of the three expression constructs shown in (A).
All the resulting host strains still produced disorazols with growing production titres based on HPLC-MS analysis. The generated M. xanthus:: p15A-dis-Pcp25 expression host produced on average seven times more disorazol A2 compared to M. xanthus:: p15A-dis and mutant strain M. xanthus:: p15A-dis-Pcp25Δorf9 produced approximately 2.5-fold when compared to M. xanthus:: p15A-dis (Figs 4B and 5). Hence, the orf9 gene ablation did reduce disorazol production although it was described as having “no functional prediction” in BLAST analysis37. The orf9 gene, following the TE domain, might affect the biosynthetic formation of the final product by incorporation and cyclization of two sides of the disorazol bis-lactone. The successful enhancement of disorazol heterologous production suggested that re-engineering trans-AT PKSs domains on the molecular level was a feasible and practicable approach in investigating the characteristic enzymes.
Figure 5. Production of disorazol A after insertion of Pcp25 promoter.
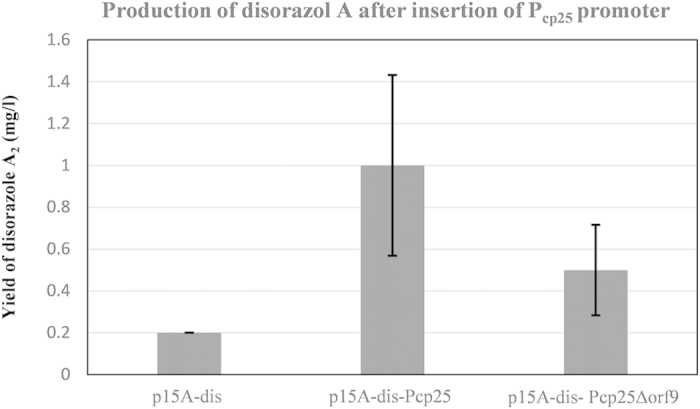
Quantification of disorazol heterologous production by HPLC-MS analysis of the culture extracts from different M. xanthus DK1622 mutant strains. All LC-MS- derived area values are normalized to the crude extracts of each sample by method of standard curves. The depicted values are mean values from three independent mutants. Error bars show calculated SDs, yield, control strain.
Trans-AT PKSs are an important but still less known family of biosynthetic systems in comparison to cis-AT PKSs58,59. There are significant differences in the existing biosynthetic protocols between trans-AT and cis-AT PKSs. A single discrete AT DisD recognize and load all molonyl-CoAs for all the dis PKS modules. Here we change the native promoter of disD gene with a stronger and artifical synthetic Pcp25 promoter which it would increase the transcription of disD gene and most likely raises the amount of DisD protein. Sufficient ATs could provide abundant substrates, thus promote the PKS module efficiency for polyketide chain extension of disorazol biosynthesis leading to improved production52,60,61. As trans-AT PKSs are a special group of enzymes responsible for natural product biosynthesis in the organisms, it is essential to understand their functions in order to develop more heterologous expression systems for these special polyketides. Here, we have established a pioneer protocol to overexpress an independent AT resulted in increased yield of the final product, which can be used for the production optimzation of trans-AT directed natural products in the native or heterologous hosts.
Methods
Bacterial strains and culture conditions
All recombineering was performed in E. coli strain GB2005 and its derivatives cultured in LB medium and antibiotics (kanamycin [km], 15 μg/ml; ampicillin [amp], 100 μg/ml; spectinomycin [spect], 40 μg/ml; chloramphenicol [cm], 30 μg/ml and tetracycline [tet], 5 μg/ml). The strains used were: GB2005, derived from DH10B by deletion of fhuA, ybcC and recET19,62; GB05-red, derived from GB2005 by insertion of the PBAD-gbaA cassette at the ybcC locus41,62; GB05-dir, derived from GB2005 by the PBAD-ETgA operon, which was integrated into the ybcC locus in GB200541. The integration ablates expression of ybcC, which encoded a putative exonuclease similar to that encoded by Redα. The heterologous host for PKS/NRPS gene cluster expression was M. xanthus DK1622 grown at 30 °C in CTT medium (1% casitone, 8mM MgSO4, 10mM Tris-HCl, pH 7.6, and 1mM potassium phosphate, pH 7.6)63 with or without km (50 μg/ml) before or after introduction of the disorazol gene cluster.
Red/ET recombineering
All methods were essentially as described previously62. By using Red/ET recombineering, 0.3 μg of one linear DNA fragment (either a PCR product or a fragment obtained from restriction enzyme digestion) was electroporated into 50 μl Red/ET-competent E. coli cells (such as GB-red cells). After electroporation, colonies grew on the LB-agar plates under selection for the antibiotic resistance gene and then were examined for the intended Red/ET recombination product by restriction analysis with a set of different suitable enzymes.
All PCR reactions carried out using Taq polymerase (Invitrogen GmbH, Karlsruhe, Germany) according to the manufacturer’s protocol. For the amplification of the ~1000 bp cassette with high GC content, DMSO was added to a final concentration of 3%. The conditions using an Eppendorf master cycler were as follows: 10 min at 95 °C to activate the polymerase, denaturation at 95 °C (30 s), annealing at 58 °C (30 s), and extension at 72 °C (35 s); 35 cycles. The PCR product was directly used without any purification.
Reconstitution of dis gene cluster
To harvest the full length of the esterase gene orf3′ in plasmid pTn-Rec_IE2, we first changed the backbone of pTn-Rec_IE2 into p15A-amp-orf2′-Tn-hyg in order to get more stable and higher copies of DNA (P3, P4 in Table S1). Then two linear fragments, p15A-amp-orf2′-Tn-hyg digested by Sal I and primer P5 (see Table S1), were co-transformed into E. coli GB05-dir cells41 to remove R6k-Tn-hygR genes and recover the whole size of orf3′ gene. Thus, we obtained plasmid p15A-amp-orf3′ harboring the full-length esterase gene. In order to insert the whole length orf2′ and orf3′ genes into the disorazol plasmid, we inserted two single PCR cassettes “spectR” and “cmR” with suitable homologous arms (P6-P9 Table S1) into the vector to engineer plasmid p15A-amp-cm-orf2′-orf3′-spect and then digested the new construct with Hind III to release the linear cassette “cmR-orf2′-orf3′-spectR” whose Hind III restriction site were homologous to p15A-dis vector. In the last step, the “cmR-orf2′-orf3′-spectR” cassette was transformed into strain GB-red::p15A-dis strain to generate the final plasmid (Figure S7). Two expression constructs p15A-dis and p15A-dis-est were obtained, containing four core-large genes from the disorazol A pathway (ten PKSs and one NRPS, ~58kb), with the Ptet promoter located upstream of the first PKS domain (Fig. 2).
Electroporation of M. xanthus DK 1622
The engineered gene clusters were introduced into the chromosome of M. xanthus DK1622 by electroporation. Briefly, M. xanthus cells from 1.7 ml of overnight culture with OD600 ~ 0.6 were collected and electrocompetent cells were prepared after washing twice with ice-cold water. A mixture of 50 μl cell suspension in cold water and 3 μg DNA was electroporated (Electroporator 2500, Eppendorf AG, Hamburg, Germany) at 1300V using a 0.1 cm cuvette. After electroporation, the cells were resuspended in 1.7 ml fresh CTT medium, and incubated at 30 °C in a 2 ml Eppendorf tube with a hole punched in the lid on a Thermomixer (Eppendorf) at 11000 r.p.m. for 6 h. Then 1 ml 1.5% CTT agar solution at 42 °C was added to the tube and the cells were plated in soft agar for selection on CTT agar plates supplemented with km (50 μg/ml). Km-resistant colonies appeared after 4 days and were checked by colony PCR as follows. Part of a single colony was washed once in 1 ml H2O and resuspended in 100 μl H2O. Then, 2 μl of the resulting suspension was used as a PCR template using Taq polymerase according to the manufacturer’s protocol. The disorazol-specific primers used to check the integration of the disC gene into the M. xanthus chromosome were the same as used in a previous study16. For PCR amplification, primers 10 and 11 were used (see Table S1).
Expression and analysis of disorazol production
Plasmids harboring a core-region or reconstituted dis gene cluster were introduced into M. xanthus DK1622 by electroporation. The resulting mutants (M. xanthus DK1622:: p15A-dis) were cultivated in 100ml shake flasks containing 30 ml CTT medium. The medium was inoculated with 0.5 mL of the overnight culture and incubated at 30 °C on a rotary shaker at 180 rpm. After induction (anhydrotetracycline, final concentration 0.5 μg/mL) and addition of XAD adsorber resin (2%, 24 h), incubation was continued for 2 more days. The cells and the resin were harvested by centrifugation and extracted with methanol. The extracts were evaporated and then redissolved in 1 mL MeOH. A 5 μL solution was analyzed by HPLC-MS and analysis was performed on an Agilent 1100 series solvent delivery system that was equipped with a photodiode array detector and coupled to a Burker HCTultra ion trap mass spectrometer. Chromatographic conditions were as follows: Luna RP-C18 column, 100 × 2 mm, 2.5 μm particle size, and precolumn C18, 8 × 3 mm, 5 μm. Solvent gradient (with solvents A [water and 0.1% formic acid] and B [CH3CN and 0.1% formic acid]): 20% B from 0 to 20 min, 20% B-95% B within 10 min, followed by 5 min with 95% B at a flow rate of 0.4 mL/min. Detection was carried out in positive ion mode, auto MSn. Disorazols were identified by comparison to the retention times and the MS2 data of disorazols identified from the original producer in our myxo-database (target screening, Table S2)28. The relative production of disorazols was calculated from the peak areas of the extracted ion chromatograms (EICs) of each derivative.
High-resolution mass spectrometry was performed on an Accela UPLC-system (Thermo-Fisher) coupled to a linear trap-FT-Orbitrap combination (LTQ-Orbitrap), operating in positive ionization mode. Separation was achieved on a Waters BEH RP- C18 column (50 × 2.1 mm; 1.7 μm particle diameter; flow rate 0.6 mL/min, Waters), with a mobile phase of H2O/CH3CN (each containing 0.1% formic acid) and a gradient of 5–95% CH3CN over 9 mins. UV and MS detection were performed simultaneously. Coupling of HPLC to MS was supported by an Advion Triversa Nanomate nano-ESI system attached to a Thermo Fisher Orbitrap. Mass spectra were acquired in centroid mode at 200–2000 m/z with a resolution of R = 30000.
Target screening method
The HPLC-HR-MS data of crude extracts were further analyzed to identify the known compounds present in the extracts using the software Target Analysis (Bruker Daltonik GmbH). The known compounds were identified on the basis of their high resolution mass, isotope pattern and retention time according to the known method45. With this approach, re-isolation of known but less interesting compounds could be avoided whereas unknown compounds with potential bioactivity could be identified easily.
Isolation of disorazol A2
M. xanthus containing p15A-dis was cultivated in 5 L CTT medium supplemented with 30 μg/mL kanamycin and 2% XAD 16 resin (after 2 days of incubation) at 30 °C for 5 days63. The resin was collected by sieving, washed with H2O twice, and then extracted stepwise with acetoacetate (5 L). The extract was concentrated in vacuo, followed by suspension in MeOH and extraction with n-hexane to defat. The resulting MeOH extract (0.87 g) was fractionated initially on a Sephadex LH-20 column (100 × 2.5 cm) using MeOH as a mobile phase, and 55 fractions were obtained. Fractions containing disorazol A2 were subjected to semi-preparative reversed-phase HPLC system (Jupiter Proteo C12, 250 × 10 mm, 4 μm, DAD at 254 nm) with an isocratic system of 75% MeOH/H2O with 0.05% TFA to yield (1.2 mg, tR ≈ 22 min).
NMR
NMR spectra were recorded in CD3OD on a DRx 500 MHz spectrometer (1H at 500 MHz, 13C at 125 MHz) equipped with a 5-mm probe and a Bruker Ascend 700 MHz spectrometer (1H at 700 MHz, 13C at 175 MHz) equipped with a 5-mm TXI cryoprobe system (Bruker Biospin GmbH, Germany). Chemical shift values of 1H- and 13C-NMR spectra are reported in ppm relative to the residual solvent signal given as an internal standard. Multiplicities are described using the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, b = broad; corrected coupling constants are reported in Hz.
Additional Information
How to cite this article: Tu, Q. et al. Genetic engineering and heterologous expression of the disorazol biosynthetic gene cluster via Red/ET recombineering. Sci. Rep. 6, 21066; doi: 10.1038/srep21066 (2016).
Supplementary Material
Acknowledgments
The authors would like to thank Eva Luxenburger, Dr. Stephan Hüttel and Dr. Thomas Hoffmann (HIPS) for expert assistance with various analytical techniques, Viktoria Schmitt for assistance in biological function experiments and Dr. Jun Fu (Dresden University of Technology) for expert technical assistance in Red/ET recombineering. Research in the laboratory of R.M. was funded by the Deutsche Forschungsgemeinschaft (DFG) and the Bundesministerium für Bildung und Forschung (BMBF). The work in the laboratory of Y. Z. was supported by funding from the Recruitment Program of Global Experts. The authors acknowledge Dr. MA Meredyth Stewart and Dr. Vinothkannan Ravichandran’s help in proofreading this manuscript.
Footnotes
Author Contributions Q.T., S.H. and Y.Z. planned and performed cloning experiments. Q.T. and X.B. performed genetic transfers, cultivation experiments and data analysis. Q.T. and R.R. performed HPLC and compound isolation. R.R. performed NMR experiments and data analysis. J.H. performed biological functional studies. Q.T., Y.Z. and R.M. designed the study and wrote the paper. All authors discussed the results and commented on the manuscript.
References
- Luo Y., Cobb R. E. & Zhao H. Recent advances in natural product discovery. Curr. Opin. Biotechnol. 30, 230–237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S. C. & Müller R. The impact of genomics on the exploitation of the myxobacterial secondary metabolome. Nat. Prod. Rep. 26, 1385–1407 (2009). [DOI] [PubMed] [Google Scholar]
- Schäberle T. F., Lohr F., Schmitz A. & König G. M. Antibiotics from myxobacteria. Nat. Prod. Rep. 31, 953–972 (2014). [DOI] [PubMed] [Google Scholar]
- Gomes E. S., Schuch V. & de Macedo Lemos E. G. Biotechnology of polyketides: new breath of life for the novel antibiotic genetic pathways discovery through metagenomics. Braz. J. Microbiol. 44, 1007–1034 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieker M., Tanović A. & Marahiel M. A. Nonribosomal peptide synthetases: structures and dynamics. Curr. Opin. Struct. Biol. 20, 234–240 (2010). [DOI] [PubMed] [Google Scholar]
- Fischbach M. A. & Walsh C. T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem. Rev. 106, 3468–3496 (2006). [DOI] [PubMed] [Google Scholar]
- Ongley S. E. et al. High titer heterologous production of Lyngbyatoxin in E. coli, a protein kinase C activator from an uncultured marine Cyanobacterium. ACS Chem. Biol. 8, 1888–1893 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer B. A., Admiraal S. J., Gramajo H., Cane D. E. & Khosla C. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291, 1790–1792 (2001). [DOI] [PubMed] [Google Scholar]
- Ongley S. E., Bian X., Neilan B. A. & Müller R. Recent advances in the heterologous expression of microbial natural product biosynthetic pathways. Nat. Prod. Rep. 30, 1121–1138 (2013). [DOI] [PubMed] [Google Scholar]
- Bian X. et al. Direct cloning, genetic engineering, and heterologous expression of the Syringolin biosynthetic gene cluster in E. coli through Red/ET recombineering. ChemBioChem. 13, 1946–1952 (2012). [DOI] [PubMed] [Google Scholar]
- Larionov V., Kouprina N., Solomon G., Barrett J. C. & Resnick M. A. Direct isolation of human BRCA2 gene by transformation-associated recombination in yeast. Proc. Natl. Acad. Sci. USA 94, 7384–7387 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouprina N. et al. Functional copies of a human gene can be directly isolated by transformation-associated recombination cloning with a small 3′ end target sequence. Proc. Natl. Acad. Sci. USA 95, 4469–4474 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Buchholz F., Muyrers J. P. P. & Stewart A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20, 123–128 (1998). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Muyrers J. P. P., Testa G. & Stewart A. F. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18, 1314–1317 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Muyrers J. P. P., Rientjes J. & Stewart A. F. Phage annealing proteins promote oligonucleotide-directed mutagenesis in Escherichia coli and mouse ES cells. Mol. Biol. 4, 1 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S. C. & Müller R. Recent developments towards the heterologous expression of complex bacterial natural product biosynthetic pathways. Curr. Opin. Biotechnol. 16,594–606 (2005). [DOI] [PubMed] [Google Scholar]
- Gross F. et al. Metabolic engineering of Pseudomonas putida for methylmalonyl-CoA biosynthesis to enable complex heterologous secondary metabolite formation. Chem. Biol. 13, 1253–1264 (2006). [DOI] [PubMed] [Google Scholar]
- Perlova O. et al. Reconstitution of the myxothiazol biosynthetic gene cluster by Red/ET recombination and heterologous expression in Myxococcus xanthus. Appl. Environ. Microbiol. 72, 7485–7494 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. et al. Efficient transfer of two large secondary metabolite pathway gene clusters into heterologous hosts by transposition. Nucleic Acids Res. 36, e113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. et al. High level expression and purification of bioactive human alpha-defensin 5 mature peptide in Pichia pastoris. Appl. Microbiol. Biotechnol. 84, 877–884 (2009). [DOI] [PubMed] [Google Scholar]
- Liao G. J. et al. Cloning, reassembling and integration of the entire nikkomycin biosynthetic gene cluster into Streptomyces ansochromogenes lead to an improved nikkomycin production. Microb. Cell Fact. 9, 6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y. et al. Heterologous expression and genetic engineering of the tubulysin biosynthetic gene cluster using Red/ET recombineering and inactivation mutagenesis. Chem. Biol. 19, 361–371 (2012). [DOI] [PubMed] [Google Scholar]
- Bian X., Plaza A., Zhang Y. & Müller R. Luminmycins A–C, cryptic natural products from Photorhabdus luminescens identified by heterologous expression in Escherichia coli. J. Nat. Prod. 75(9), 1652–1655 (2012). [DOI] [PubMed] [Google Scholar]
- Bian X. et al. Heterologous Production of Glidobactins/Luminmycins in Escherichia coli Nissle Containing the Glidobactin Biosynthetic Gene Cluster from Burkholderia DSM7029. ChemBioChem. 15, 2221–2224 (2014). [DOI] [PubMed] [Google Scholar]
- Yin J. et al. Direct cloning and heterologous expression of the salinomycin biosynthetic gene cluster from Streptomyces albus DSM41398 in S. coelicolor A3(2). Sci. Rep. 5, 15081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolinko I. et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat. Nanotech. 9, 193–197 (2014). [DOI] [PubMed] [Google Scholar]
- Weissman K. J. & Müller R. Myxobacterial secondary metabolites: bioactivities and modes-of-action. Nat. Prod. Rep. 27, 1276–1295 (2010). [DOI] [PubMed] [Google Scholar]
- Jansen R., Irschik H., Reichenbach H., Wray V. & Höfle G. Disorazols: highly cytotoxic metabolites from the sorangicin-producing bacterium Sorangium cellulosum, strain So ce12. Liebigs Ann. Chem. 759–773 (1994). [Google Scholar]
- Elnakady Y. A., Sasse F., Lünsdorf H. & Reichenbach H. Disorazol A1, a highly effective antimitotic agent acting on tubulin polymerization and inducing apoptosis in mammalian cells. Biochem. Pharmacol. 67, 927–935 (2004). [DOI] [PubMed] [Google Scholar]
- Schäckel R., Hinkelmann B., Sasse F. & Kalesse M. The synthesis of novel disorazols. Angew. Chem. Int. Ed. 49, 1619–1622 (2010). [DOI] [PubMed] [Google Scholar]
- Lee C., An D., Lee H. & Cho K. Correlation between Sorangium cellulosum subgroups and their potential for secondary metabolite production. J. Microbiol. Biotechnol . 23, 297–303 (2013). [DOI] [PubMed] [Google Scholar]
- Hopkins C. D. & Wipf P. Isolation, biology and chemistry of the disorazols: new anti-cancer macrodiolides. Nat. Prod. Rep. 26, 585–601 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz S. et al. Triple negative breast cancers express receptors for LHRH and are potential therapeutic targets for cytotoxic LHRH-analogs, AEZS 108 and AEZS 125. BMC Cancer 14, 847 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipf P. & Graham T. H. Total synthesis of (-)-disorazol C1. J. Am. Chem. Soc. 126, 15346–15347 (2004). [DOI] [PubMed] [Google Scholar]
- Xu F. L. et al. Mitotic slippage in non-cancer cells induced by a microtubule disruptor, disorazol C1. BMC Chem. Biol. 10, 1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo J. S. et al. Identifying a resistance determinant for the antimitotic natural products disorazol C1 and A1. J. Pharmacol. Exp. Ther. 332, 906–911 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M., Irschik H., Pradella S. & Müller R. Production of the tubulin destabilizer disorazol in Sorangium cellulosum: biosynthetic machinery and regulatory genes. ChemBioChem. 6, 1277–1286 (2005). [DOI] [PubMed] [Google Scholar]
- Carvalho R. et al. The biosynthetic genes for disorazols, potent cytotoxic compounds that disrupt microtubule formation. Gene 359, 91–98 (2005). [DOI] [PubMed] [Google Scholar]
- Stevens D. C., Hari T. P. A. & Boddy C. N. The role of transcription in heterologous expression of polyketides in bacterial hosts. Nat. Prod. Rep. 30, 1391–1411 (2013). [DOI] [PubMed] [Google Scholar]
- Kang Y. et al. Knock-out and pull-out recombineering protocols for naturally transformable Burkholderia thailandensis and Burkholderia pseudomallei. Nat. Protoc. 6, 1085–1104 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. et al. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotech . 30, 440–446 (2012). [DOI] [PubMed] [Google Scholar]
- Julien B. & Fehd R. Development of a mariner-based transposon for use in Sorangium cellulosum. Appl. Environ. Microbiol. 69(10), 6299–6301 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M. et al. Critical variations of conjugational DNA transfer into secondary metabolite multiproducing Sorangium cellulosum strains So ce12 and So ce56: development of a mariner-based transposon mutagenesis system. J. Biotechnol. 107, 29–40 (2004). [DOI] [PubMed] [Google Scholar]
- Wenzel S. C. et al. Heterologous expression of a myxobacterial natural products assembly line in pseudomonads via red/ET recombineering. Chem. Biol. 12, 349–356 (2005). [DOI] [PubMed] [Google Scholar]
- Krug D. & Müller R. Secondary metabolomics: the impact of mass spectrometry-based approaches on the discovery and characterization of microbial natural products. Nat. Prod. Rep. 31, 768–783 (2014). [DOI] [PubMed] [Google Scholar]
- Moat A. G., Foster J. W. & Spector M. P. Microbial Physiology , 4th ed. (New York, Wiley-Liss, Inc. Pub) (2003). [Google Scholar]
- Trun N. & Trempy J. Fundamental bacterial genetics . (Malden, MA: Blackwell Pub.) (2004). [Google Scholar]
- Irschik H., Jansen R., Gerth K., Höfle G. & Reichenbach H. Disorazol A, an efficient inhibitor of eukaryotic organisms isolated from myxobacteria. J. Antibiot. 48, 31–35 (1995). [DOI] [PubMed] [Google Scholar]
- Hearn B. R. et al. Methanolysis products of disorazol A1. J. Nat. Prod. 69, 148–150 (2006). [DOI] [PubMed] [Google Scholar]
- Wong F. T., Jin X., Mathews I. I., Cane D. E. & Khosla C. Structure and mechanism of the trans-acting acyltransferase from the disorazol synthase. Biochemistry. 50, 6539–6548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 26, 338–362 (2009). [DOI] [PubMed] [Google Scholar]
- Till M. & Race P. R. Progress challenges and opportunities for the re-engineering of trans-AT polyketide synthases. Biotechnol. Lett. 36, 877–888 (2014). [DOI] [PubMed] [Google Scholar]
- Jensen P. R. & Hammer K. The sequence of spacers between the consensus sequences modulates the strength of prokaryotic promoters. Appl. Environ. Microbiol. 64, 82–87 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodumal S. J. et al. Total synthesis of long DNA sequences: synthesis of a contiguous 32-kb polyketide synthase gene cluster. Proc. Natl. Acad. Sci. USA 101, 15573–15578 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. D., Nietlispach D., Broadhurst R. W. & Weissman K. J. Multienzyme docking in hybrid megasynthetases. Nat. Chem. Biol. 4, 75–81 (2008). [DOI] [PubMed] [Google Scholar]
- Meiser P. & Müller R. Two functionally redundant Sfp-type 4-phosphopantetheinyl transferases differentially activate biosynthetic pathways in Myxococcus xanthus. ChemBioChem. 9, 1549–1553 (2008). [DOI] [PubMed] [Google Scholar]
- Buntin K. et al. Biosynthesis of thuggacins in myxobacteria: comparative cluster analysis reveals basis for natural product structural diversity. Chem. Biol. 17, 342–356 (2010). [DOI] [PubMed] [Google Scholar]
- Piel J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 27, 996–1047 (2010). [DOI] [PubMed] [Google Scholar]
- Dunn B. J., Watts K. R., Robbins T., Cane D. E. & Khosla C. Comparative analysis of the substrate specificity of trans- versus cis-acyltransferases of assembly line polyketide synthases. Biochemistry. 53, pp3796–3806 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopanik N. B. et al. In vivo and in vitro trans-acylation by BryP, the putative bryostatin pathway acyltransferase derived from an uncultured marine symbiont. Chem. Biol. 15, 1175–1186 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K. et al. Polyketide proofreading by an acyltransferase- like enzyme. Chem. Biol. 19, 329–339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J., Teucher M., Anastassiadis K., Skarnes W. & Stewart A. F. A recombineering pipeline to make conditional targeting constructs. Meth. Enzymol . 477, 125–144 (2010). [DOI] [PubMed] [Google Scholar]
- Meiser P., Bode H. B. & Müller R. The unique DKxanthene secondary metabolite family from the myxobacterium Myxococcus xanthus is required for developmental sporulation. Proc. Natl. Acad. Sci. USA 103, 19128–19133 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.