Abstract
Background and Objectives
There is growing interest in the clinical application of red blood cell (RBC) microparticle (MP) enumeration as they have been postulated to be effectors of coagulation and inflammation following transfusion and in sickle cell disease. No uniform approach in MP enumeration exists and a key limitation is the lack of an internal validation process. We present and validate a flow cytometric approach where an internal standard is utilized.
Materials and Methods
Glycophorin A+ Annexin V+ events were enumerated using MPs isolated from RBC units or plasma samples obtained from volunteers. A mixture of absolute counting (7Æ6 μm) and calibration beads (0Æ5, 0Æ9 and 3 μm) at a fixed ratio was added to each sample.
Results
RBC MPs were initially selected based upon a fluorescence threshold, and the 0Æ5- and 0Æ9-μm beads defined the upper and lower light scatter distribution of MPs. The ratio of 7Æ6:3-μm bead events was used as an internal standard to validate the precision of MP enumeration across samples (coefficient of variation = 2·5–7·2%) and remained constant in both platelet-rich plasma (PRP) and platelet-free plasma (PFP). RBC MP counts increased in both PRP and PFP obtained from whole blood stimulated with ionophore and increasing calcium concentrations, with PRP showing higher MP counts than PFP at every concentration studied.
Conclusion
This method is a useful strategy to detect RBC MP counts across bio-samples provided that the flow cytometer can reliably discriminate the size of the calibration beads.
Keywords: annexin V, glycophorin A, microparticles, microvesicles, packed red blood cell, red blood cell
Introduction
RBC microparticles (MPs) are shed from the membrane surface and express transmembrane proteins band 3, glycophorins, and blood group antigens but are depleted of cytoskeletal proteins such as spectrin and ankyrin [1]. Similar to MPs released from other cells, RBC MPs show loss of phospholipid asymmetry and express surface phosphatidylserine [1]. Under homoeostatic conditions, RBC MPs account for approximately 7·3% of MPs found in whole blood in contrast to the more numerous platelet-derived (38·5%) and endothelial-derived MPs (43·5%) [2]. However, under conditions of haemolysis, such as sickle cell anaemia [3], thalassaemia [4, 5], and malaria [6], the numbers of RBC MPs in circulation increase and can predominate.
Others and we have shown that RBC MPs increase in numbers with storage duration [1, 7, 8], but the fate and function of MPs following tranfusion remain unclear. Although Kupffer cells rapidly clear infused MPs in a rodent model [9], the capacity of the mononuclear phagocyte system to clear circulating MPs can be overwhelmed in humans such as that occurs in haemolytic states. It remains to be determined whether non-haemolytic states such as in the case of transfusion during trauma or critical illness are associated with increased circulating MP concentrations. Nonetheless, there is increasing recognition of the need for precise and reproducible method of MP enumeration.
Currently, there is no standardized way to enumerate RBC MPs [7]. A flow cytometric approach is generally utilized where fluorescent events, defined by glycophorin A and Annexin V staining, are analysed within a specific size range (<1 μm) based upon light scatter parameters [2, 6, 7]. However, a key limitation is the lack of a validation process where MP enumeration is compared against an internal standard. Here, we present a method using a fixed ratio of calibration beads and counting beads that can be used to validate each sample and allow for comparisons across samples. The approach can be used with any flow cytometer that is able to discriminate the different-sized populations of calibration beads.
Materials and methods
Blood samples
Adsol-preserved non-leukoreduced red cell units were obtained from the Institute of Transfusion Medicine, Central Blood Bank of Pittsburgh, Pittsburgh, PA. For select experiments, we obtained whole blood from healthy volunteers following written informed consent. The Institutional Review Board of University of Pittsburgh approved the studies.
Reagents
Glycophorin A-PE, Annexin V FITC, Mouse IgG2b, k-PE, and annexin V-binding buffer concentrate were purchased from BD Pharmingen (San Jose, CA). Flow Cytometry Absolute Counting Standard microbeads (7·6 μm) were purchased from Bangs Laboratories, Inc. (Fishers, IN). Megamix™, a blend of monodisperse fluorescent beads of three diameters (0·5, 0·9 and 3 μm), was purchased from BioCytex (Marseille, France).
Isolation and purification of red cell MPs from packed red blood cell (PRBC) units
A detailed method for isolation and purification of red cell MPs from PRBC units has been previously described [8] and is a modification of the techniques described by Westerman et al. [10] and Kriebardis et al. [11].
Flow cytometric analysis and quantification of red cell MPs
Purified MPs from PRBC units or plasma were labelled with glycophorin A-PE and annexin V FITC on ice and protected from light. The 7·6-μm absolute counting beads or a mixture of Megamix™ and absolute counting beads were added to each sample at the last step and vigorously vortexed just prior to flow cytometric analysis. Red cell MP enumeration was obtained on FACSAria flow cytometer (BD Biosciences, San Jose, CA). The flow was maintained at flow rate set at 1 with a sheath pressure of 70 psi.
Isotype control labelling of samples with mouse IgG2b-PE confirmed specificity of Glycophorin A-PE (CD235a-PE+) labelling of MPs. For red cell MP enumeration using the single counting bead method, the side scatter channel (SSC) and forward scatter channel (FSC) parameters were set to log scale. A predefined volume of the 7·6-μm absolute counting bead was added into each sample. Red cell MPs were defined as CD235a-PE+ Annexin V+ events and further discriminated by size. Calculation of MP counts was based upon the following equation: absolute number of 7·6-μm counting beads added to each sample × (number of MP events ⁄ number of 7·6-μm bead events).
Defining the parameters using a mixture of Megamix and absolute counting standard calibration beads
A mixture of Absolute Counting Standard microbead (7·6 μm) and Megamix™ calibration beads (0·5, 0·9 and 3 μm) of differing ratios was generated. RBC MPs were defined as CD235a-PE+ Annexin V+ events conforming to a light scatter distribution within the 0·5- to 0·9-μm bead range. A discrete population of CD235a-PE+ Annexin V+ events was first identified in the PE · FITC dot plot. By back gating, the position of the CD235a+ Annexin V+ population could be confirmed relative to the distribution of calibration beads in both the PE × FSC window and the SSC × FSC window.
Generation of MPs from whole blood in vitro using calcium and calcium ionophore
Whole blood was drawn from healthy volunteers and anticoagulated at 1:6 ratio of citrate phosphate dextrose (CaridianBCT, Lakewood, CO) to blood. The samples were incubated in the presence or absence of 1, 2 and 4 mM CaCl2 + 5 μM ionophore at 37°C for 2 h as previously described [12]. Following incubation, the samples were centrifuged at 100 g for 15 min producing the platelet-rich plasma (PRP) supernatant. Aliquots of the PRP were saved for analysis and the remainder was centrifuged at 500 g for 5 min producing a platelet-poor plasma (PPP) supernatant. The PPP was collected and centrifuged at 13 000 g for 3 min to obtain platelet-free plasma. For plasma samples, the following protocol was used. Each sample consisted of 1 μl mouse anti-human CD235a-PE, 5 μl Annexin V FITC and 69 μl Annexin V-binding buffer in 2% FBS into 25 μl plasma for a total volume of 100 μl. To avoid pipetting errors, a master mixture of antibody, Annexin V FITC in Annexin V-binding buffer was generated and added to individual plasma samples. Subsequently, 400 μl Annexin V-binding buffer in 2% FBS was added to the sample preparations. Immediately prior to flow cytometric analysis, 150 μl of a master bead mixture consisting of one volume of absolute counting beads (7·6 μm) to two volumes of Megamix™ was vortexed and added to each tube for a final volume of 650 μl. Acquisition for each sample was set to achieve 1000 events of the 3 μm beads.
Statistics
We performed linear regression modelling MP counts against sample input volume to determine coefficients of variation (% CV) and thereby define the range of variability of the enumeration method across experiments. Goodness-of-fit was obtained by determining the r2 value for best-fit linear regression line. Statistics were performed using GRAPHPAD PRISM5 (GraphPad software, La Jolla, CA). A P < 0·05 was considered significant.
Results
RBC MP enumeration with absolute counting bead
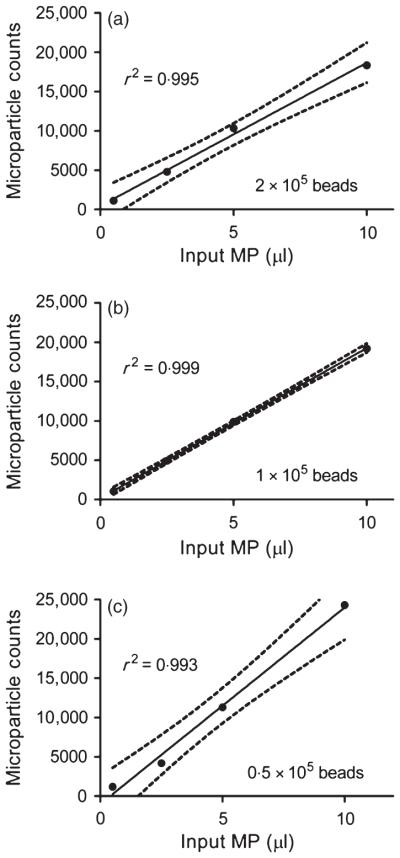
Purified, concentrated MP samples were prepared from RBC units and utilized to test the ability of 7·6-μm counting beads to enumerate across a range of MP counts (Fig. 1 a–c). Because the absolute numbers of MPs is not known a priori, increasing volumes of the MP-containing preps were assayed to predictably increase the numbers of MPs. A known amount of counting beads spiked into each sample was varied between experiments to determine the optimal numbers required for MP enumeration. Within each experiment where the numbers of counting beads was set constant, MP counts increased linearly in a highly correlative fashion (R2 = 0·99). Across experiments where the numbers of counting beads varied, the ability to predict the same number of MPs for a given volume showed an acceptable range of variability based upon the coefficient of variation (CV), particularly at lower volumes (0·5 μl, CV 6·7%; 2·5 μl, CV 8·5%; 5 μl, CV 6·8%; 10 μl, CV 15·6%). Thus, absolute counting beads allowed for enumeration of red cell MPs that are reproducible and accurate.
Fig. 1.
Red cell microparticle (MP) enumeration with varying numbers of 7·6-μm counting beads. MP preps isolated from RBC units were utilized to test the ability of absolute counting beads of 7·6 μm size to enumerate as the volume of MP-containing samples increased (a–c). A known amount of 7·6-μm counting beads spiked into each sample was varied between experiments (a: 2 × 105 counting beads), (b: 1 × 105 counting beads), (c: 0·5 × 105 counting beads). MP counts increased linearly in a highly correlative fashion (R2 = 0·99). Representative of three independent experiments is shown.
RBC MP enumeration using a set ratio of absolute counting and calibration beads
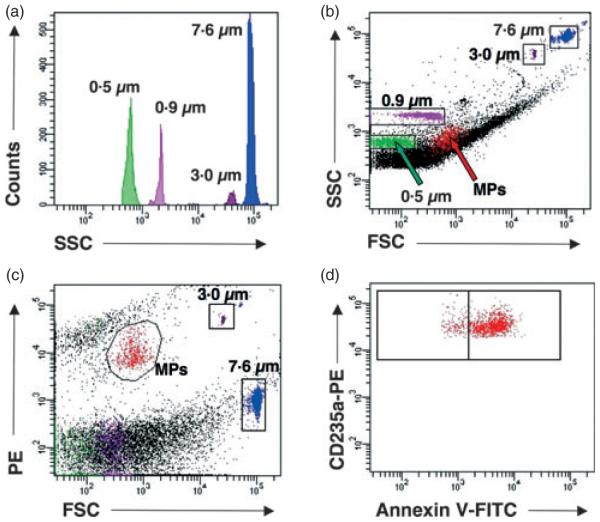
Although the counting bead allows for the enumeration of red cell MPs across samples, this method lacks internal validation. To more precisely characterize the light scatter distribution of glycophorin A+ annexin V+ MPs within each sample, a known volume of Megamix™ calibration beads containing a mixture of 0·5-, 0·9- and 3-μm-sized beads were added to a known volume of the 7·6-μm counting bead. This mixture of counting and calibration beads, which we will refer to as a ‘master’ bead mixture, yields a histogram showing distinct peaks for each sized bead without overlay (Fig. 2a). A known volume of the master mixture is added to each sample, and MPs are initially identified based upon glycophorin A+ annexin V+ events (Fig. 2d). Through back gating, we observe that RBC MPs fall within a light scatter distribution between 0·5- and 0·9- μm calibration beads in the SSC × FSC window (Fig. 2b). Regardless of the volume of MP preps tested, the 7·6- and 3-μm beads each produced a discrete cloud of events that were easily distinguished above background noise or debris independent of the parameters chosen to visualize the fluorescent signals (Fig. 2c).
Fig. 2.
Identification of RBC microparticles (MPs) based upon a fluorescent threshold and utilizing the mixture of absolute counting and calibration beads to define the light scatter parameters. Representative histogram of the mixture of absolute counting standard microbead (7·6 μm) and calibration beads (0·5, 0·9 and 3 μm) (a). Side scatter (SSC) and forward scatter (FSC) parameters of RBC MPs in relation to the 0·5-, 0·9-, 3- and 7·6-μm beads mixture spiked into the sample (b). Identification of RBC MPs through back gating of CD235a+AnnexinV+ events observed in (d) relative to the 3- and 7·6-μm beads observed in the phycoerythrin (PE) × FSC channel (c). MPs are defined as glycophorin A+ (CD235a-PE+) and annexin V FITC+ events (d).
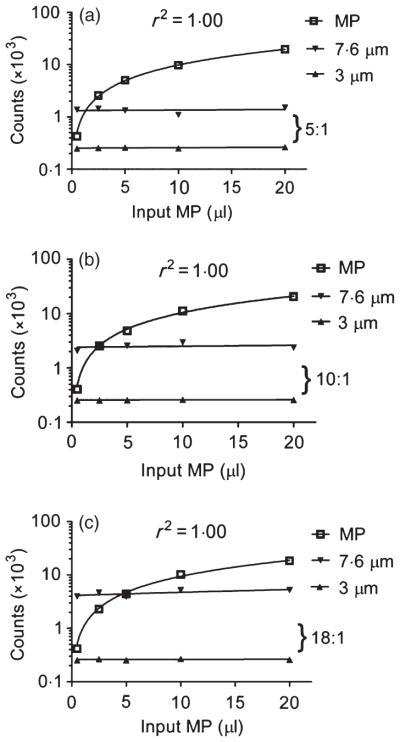
For enumeration purposes, acquisition was based upon achieving the same predefined number of 3-lm bead events in each sample because the 3 μm beads represented the fewest number of events (Fig. 2a) relative to the other calibration beads. This approach provided an additional component of standardization. We examined the ratio between the absolute counting bead (7·6 μm) and the 3-μm bead events, as this relationship should remain relatively constant across samples within a given experiment and provide an assessment of the degree of precision in our enumeration method. Different volumes of counting to calibration beads were used to generate differing ratios of 7·6:3-μm bead events (5:1, 10:1, 18:1) and assess the precision of MP enumeration (Fig. 3a–c). Across experiments where the ratio of bead events varied (5:1, 10:1, 18:1), the ability to predict the same number of RBC MPs for a given volume showed an acceptable range of variability (0·5 μl, CV 2·5%; 2·5 μl, CV 6·3%; 5 μl, CV 6·5%; 10 μl, CV 7·2%; 20 μl, CV 5·6%). Thus, a master mixture of counting and calibration beads at a set ratio enhances the accuracy of MP enumeration by providing a method to confirm the upper and lower light scatter distribution of MPs. In addition, the ratio between the 7·6-μm and the 3-μm bead events provides a method to validate the precision of enumeration across samples.
Fig. 3.
Red cell microparticle (MP) enumeration utilizing varying ratios of the counting and calibration bead mixture. RBC MP counts increased linearly (set at log scale) as the volume of MP-containing samples increased (R2 = 1·0) (a). The ratio of 7·6:3-μm bead events remained constant throughout the range of MP volumes tested for samples containing different ratios (a: 5:1), (b: 10:1), (c: 18:1).
Enumeration of RBC MPs in human plasma samples
We next assessed the ability of the master bead mixture to enumerate RBC MPs in human plasma samples. Whole blood was incubated with increasing concentrations of calcium in the presence of ionophore, and both PRP and PFP were obtained. The ratio of 7·6:3-μm beads enumerated remained relatively constant within and across PRP and PFP samples (PRP mean ratio ± SD: 10·2 ± 0·7, % CV = 6·5; PFP mean ratio ± SD: 9·3 ± 0·8, % CV = 8·4) (Fig. 4a). As a comparator, the % CV for the flow cytometer setting to achieve a predefined number of events for the 3 μm bead is 4·7% (data not shown). The numbers of RBC MPs increased in both PRP and PFP with increasing concentrations of calcium (Fig. 4b–c). PFP yielded MP counts that were approximately 3- to 8-fold less than that was obtained in PRP from the same samples (Fig. 4b–c). This is consistent with prior observations where the number of MPs obtained in the supernatant of PRBC concentrates decreased with increasing centrifugation speed and the number of centrifugation steps [7, 13]. Thus, the method of enumeration shows precision in both PRP and PFP and the ability to discriminate increases in MP counts following induction of microvesiculation across samples.
Fig. 4.

Red cell microparticle (MP) enumeration of platelet-rich plasma (PRP) and platelet-free plasma (PFP) samples. The coefficient of variation (% CV) for the ratio of 7·6:3-μm bead events in both PRP and PFP samples were obtained (a). Increases in MP counts in PRP and PFP isolated from whole blood anticoagulated with citrate phosphate dextrose and stimulated with 0, 1, 2 or 4 mM concentrations of calcium (Ca2+) in the presence of 5 μM calcium ionophore ex vivo from three subjects (S1, S2, S3) (b–c).
Discussion
The present study presents a detailed method of RBC MP enumeration using a flow cytometric approach for the purpose of analysing bio-samples. We first show the precision and reproducibility of the method using the 7·6-μm counting beads spiked into each sample. We introduce a mixture of different-sized calibration beads to a known amount of 7·6-μm counting beads at a set ratio. While the initial selection criteria is based upon a fluorescence threshold, the master bead mixture allows us to further define the relative side and forward light scatter properties of the MP events and enhance the accuracy of the enumeration process. Moreover, the estimation of the ratio of two different-sized beads provides internal validation of enumeration precision and serves as a quality measure across samples. This method should provide a useful strategy to detect red cell MP counts across bio-samples.
There is no standardized way to enumerate RBC MPs [7]. Three major sources contribute to variability in MP enumeration and these include (1) differences in how samples are processed prior to analysis [2, 7, 13], (2) different definitions of MPs applied [2, 6–8, 13] and (3) differences in flow cytometric settings and analysis of MPs [14, 15]. Ayers et al. [13] have shown that different processing methods such as increasing the time between venipuncture and centrifugation and single freeze-thaw cycle of samples increased the number of MPs, whereas a washing step, increasing the number of centrifugation steps and longterm storage of MP samples at −80°C reduced the numbers of Annexin V+ MPs. In contrast, others have shown that RBC MPs (defined as glycophorin A+ Annexin V+ events) are much less affected by temperature and storage duration in −80°C than platelet-derived or endothelial-derived MPs [2]. Our study showed that different processing methods (PRP vs. PPP) of the same sample not surprisingly alters MP counts and are consistent with the findings by Rubin et al. [7] that the number of RBC MPs was dependent upon the centrifugation protocols. Controlling for these measures, however, our method shows that accuracy and precision can be achieved across samples. Nonetheless, these and our findings confirm that strict adherence to a protocol for sample collection, processing and storage are necessary for comparison across bio-samples.
In our study, we defined red cell MPs as glycophorin A+ Annexin V+ events as previously reported [6, 8] but not universally applied [7]. Defining MPs based upon the presence of a cell-specific antigen (in the case for red cells, glycophorin A) and surface phosphatidylserine using Annexin V binding increases the specificity of the assay and is in agreement with the definition employed by the International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Subcommittees for platelet MPs [14]. A distinct population was identified using this definition and allowed us to compare the light scatter distribution of RBC MPs relative to the calibration beads of known size. Regardless of the parameters chosen to visualize the events, this method allowed us to visualize a discrete cloud of events above background noise or debris. However, it is recognized that Annexin Vneg MPs exist [7, 8, 13]. We have noted that purified MPs prepared from RBC units contained a number of events that could be characterized as either glycophorin Ahi Annexin Vneg or glycophorin Alow Annexin Vneg (data not shown). These events may reflect different populations of RBC MPs, but can prove challenging to discriminate fully from fluorescent noise. Thus, we have chosen to use glycophorin A+ Annexin V+ events as the standard for defining red cell MPs because this definition increases specificity and is easily applied.
There are two main strategies of analysing MPs by flow cytometry: (1) initial selection of MPs based upon size by defining the light scatter parameters (i.e. scatter-based threshold), and then analysing fluorescent signals that are limited to events preselected by size; (2) selection based upon fluorescent signals (i.e. fluorescence threshold) and further restricting the analysis by scatter gating [14]. The ISTH Scientific and Standardization Subcommittee proposed a strategy to enumerate platelet-derived MPs using a size-standardized window, employing a blend of calibration fluorescent beads to define the upper and lower limits prior to the analysis of samples [14]. This strategy utilized the 0·9-μm beads within the Megamix™ calibration mixture to help set the upper limit of the MP region and the 0·5-μm beads to help establish the forward scatter (FS) threshold and lower MP limit at a standardized level corresponding to the median value [14, 15].
One limitation of this approach is that the calibration strategy of predefining scatter parameters for MP analysis could be achieved reliably only with instruments that measure FS parameter with a wide solid angle (1–19°; Beckman-Coulter, Inc., Miami, FL, USA) but was inapplicable with instruments utilizing FS signal height (FSC-H) measured with a lower solid angle (1–8°; Beckton-Dickinson, Franklin Lakes, NJ) without substantial modifications [16]. In our study, we employed the latter strategy where the initial detection was based upon the identification of fluorescent signals (i.e. glycophorin A+ Annexin V+ events) but improved upon the events analysis by incorporating the same Megamix™ calibration mixture used by the ISTH in each sample to ensure that the MPs conform to the defined light scatter distribution criteria.
In summary, our study utilizes a mixture of calibration and counting beads at a predefined ratio for RBC MP enumeration that is precise but can be theoretically applied to all flow cytometers with the technical capability to discriminate discrete fluorescent signals for each size population of calibration beads. The novelty of this approach is that it incorporates the bead mixture as an internal standard that allows not only the operator to define the upper and lower limit of detection based upon the light scatter distribution profile of MPs, but also to validate enumeration precision across samples. A stock mixture of beads can be generated, the ratio confirmed, and aliquots of the mixture at this confirmed ratio utilized to assay samples. It is well recognized that there are many sources of variability in MP enumeration. Protocolizing the sample collection, processing and storage of bio-samples can minimize the pre-analytic conditions that contribute to variability. Defining red cell MPs as glycophorin A+ Annexin V+ events enhances specificity and is easily applicable. Finally, the use of the master mixture of beads in each sample helps better define the scatter parameters and minimizes variability.
Acknowledgements
JSL conceived, designed, analysed, interpreted and wrote the paper. ZX designed, acquired and analysed the data. TBO helped design, acquire and interpreted the data. JPC assisted and performed some of the experiments. MRR analysed and interpreted the data. All authors approved the submitted manuscript.
Funding
American Association for the Surgery of Trauma (AAST) ⁄ Novo Nordisk Research Scholarship Award in Hemostasis and Resuscitation (MRR); NIH HL086884, Vascular Medicine Institute Hemostasis and Vascular Biology Grant from the Institute for Transfusion Medicine (JSL).
Footnotes
Conflict of interest
All authors have no conflict of interest to declare.
References
- 1.Salzer U, Zhu R, Luten M, et al. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion. 2008;48:451–462. doi: 10.1111/j.1537-2995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 2.Shah MD, Bergeron AL, Dong JF, et al. Flow cytometric measurement of micro-particles: pitfalls and protocol modifications. Platelets. 2008;19:365–372. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- 3.Allan D, Limbrick AR, Thomas P, et al. Release of spectrin-free spicules on reoxygenation of sickled erythrocytes. Nature. 1982;295:612–613. doi: 10.1038/295612a0. [DOI] [PubMed] [Google Scholar]
- 4.Lamchiagdhase P, Nitipongwanich R, Rattanapong C, et al. Red blood cell vesicles in thalassemia. J Med Assoc Thai. 2004;87:233–238. [PubMed] [Google Scholar]
- 5.Pattanapanyasat K, Noulsri E, Fucharoen S, et al. Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom. 2004;57:23–31. doi: 10.1002/cyto.b.10064. [DOI] [PubMed] [Google Scholar]
- 6.Nantakomol D, Dondorp AM, Krudsood S, et al. Circulating red cell-derived microparticles in human malaria. J Infect Dis. 2011;203:700–706. doi: 10.1093/infdis/jiq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin O, Crettaz D, Canellini G, et al. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang. 2008;95:288–297. doi: 10.1111/j.1423-0410.2008.01101.x. [DOI] [PubMed] [Google Scholar]
- 8.Xiong Z, Cavaretta J, Qu L, et al. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51:610–621. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willekens FL, Werre JM, Kruijt JK, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 10.Westerman M, Pizzey A, Hirschman J, et al. Microvesicles in haemoglobinopathies offer insights into mechanisms of hypercoagulability, haemolysis and the effects of therapy. Br J Haematol. 2008;142:126–135. doi: 10.1111/j.1365-2141.2008.07155.x. [DOI] [PubMed] [Google Scholar]
- 11.Kriebardis AG, Antonelou MH, Stamoulis KE, et al. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 12.Allan D, Thomas P, Limbrick AR. The isolation and characterization of 60 nm vesicles (‘nanovesicles’) produced during ionophore A23187-induced budding of human erythrocytes. Biochem J. 1980;188:881–887. doi: 10.1042/bj1880881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayers L, Kohler M, Harrison P, et al. Measurement of circulating cell-derived microparticles by flow cytometry: sources of variability within the assay. Thromb Res. 2011;127:370–377. doi: 10.1016/j.thromres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix R, Robert S, Poncelet P, et al. Overcoming limitations of microparticle measurement by flow cytometry. Semin Thromb Hemost. 2010;36:807–818. doi: 10.1055/s-0030-1267034. [DOI] [PubMed] [Google Scholar]
- 15.Robert S, Poncelet P, Lacroix R, et al. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: a first step towards multicenter studies? J Thromb Haemost. 2009;7:190–197. doi: 10.1111/j.1538-7836.2008.03200.x. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix R, Robert S, Poncelet P, et al. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2010;8:2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]