Abstract
Objectives:
This trial was conducted to test the effects of an alpha7 nicotinic receptor full agonist, TC-5619, on negative and cognitive symptoms in subjects with schizophrenia.
Methods:
In 64 sites in the United States, Russia, Ukraine, Hungary, Romania, and Serbia, 477 outpatients (18–65 years; male 62%; 55% tobacco users) with schizophrenia, treated with a new-generation antipsychotic, were randomized to 24 weeks of placebo (n = 235), TC-5619, 5mg (n = 121), or TC-5619, 50mg (n = 121), administered orally once daily. The primary efficacy measure was the Scale for the Assessment of Negative Symptoms (SANS) composite score. Key secondary measures were the Cogstate Schizophrenia Battery (CSB) composite score and the University of California San Diego Performance-Based Skills Assessment-Brief Version (UPSA-B) total score. Secondary measures included: Positive and Negative Syndrome Scale in Schizophrenia (PANSS) total and subscale scores, SANS domain scores, CSB item scores, Clinical Global Impression-Global Improvement (CGI-I) score, CGI-Severity (CGI-S) score, and Subject Global Impression-Cognition (SGI-Cog) total score.
Results:
SANS score showed no statistical benefit for TC-5619 vs placebo at week 24 (5mg, 2-tailed P = .159; 50mg, P = .689). Likewise, no scores of CSB, UPSA-B, PANSS, CGI-I, CGI-S, or SGI-Cog favored TC-5619 (P > .05). Sporadic statistical benefit favoring TC-5619 in some of these outcome measures were observed in tobacco users, but these benefits did not show concordance by dose, country, gender, or other relevant measures. TC-5619 was generally well tolerated.
Conclusion:
These results do not support a benefit of TC-5619 for negative or cognitive symptoms in schizophrenia.
Key words: schizophrenia, negative symptoms, cognition
Introduction
Schizophrenia is associated not only with positive symptoms, but also with pervasive negative symptoms, cognitive dysfunction, and mood disturbances.1 Whereas positive symptoms often respond to antipsychotic therapy, negative, and cognitive symptoms are considerably less responsive2–6 Moreover, by persisting after positive symptoms are controlled,7–12 negative and cognitive symptoms prevent many people with schizophrenia from regaining their pre-morbid function or potential, and from resuming independent lives.3,5
Although hypotheses about the pathophysiology of negative and cognitive symptoms have led to the development of various drug targets,6,13 the underlying mechanisms remain poorly understood. A decade ago, the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative was sponsored by the National Institutes of Mental Health, to promote discussion and consensus by experts from government, industry and academia about the appropriate clinical trial outcome measures to use in testing therapeutic molecules against cognitive dysfunction in schizophrenia.3,14–16 Among the drug targets proposed for cognitive dysfunction in schizophrenia2,17 were the alpha7 neuronal nicotinic receptor (NNR).18–20 A similar broad-based initiative recommended measures and trial designs for testing agents targeting negative symptoms in schizophrenia.21–23
Abundant evidence favors a role for alpha7 NNRs in treating schizophrenia. First, nonclinical studies in rodents demonstrated that alpha7 antagonists produce sensory gating deficits, which may be surrogates for certain features in schizophrenia.24 Second, genetic linkage for sensory gating deficits in schizophrenia implicated the alpha7 NNR gene (CHRNA7).25 Third, postmortem studies in schizophrenia strengthened this hypothesis by detecting reduced expression of alpha7 subunit transcripts in the hippocampus26 and thalamic reticular nucleus.27 Fourth, in early clinical trials several alpha7 NNR agonists showed benefit against surrogate electrophysiological markers of the condition,28,29 and these compounds also showed benefit in later trials that assessed negative and cognitive symptoms of schizophrenia.19,30
TC-5619 is a highly selective alpha7 NNR full agonist with a Ki at the alpha7 NNR of 1nM. It is 1000 to 10000 times less potent at other NNRs and at other receptor subtypes (eg, 5HT3, opioid).31 Nonclinical studies in transgenic rodents that exhibit features with face validity to positive (sensory gating) and negative (social isolation) symptoms showed that TC-5619 ameliorated these features; other studies in naïve animals showed that TC-5619 improved cognitive symptoms (novel object recognition).31
Two phase 1 studies of TC-5619 in healthy male volunteers showed that single doses of TC-5619 were well tolerated up to 406mg (limited by orthostatic hypotension) and that multiple doses of 204mg were well tolerated (Targacept, data on file). An exploratory phase 2 study of TC-5619 evaluated doses of 1–25mg in 185 subjects with schizophrenia who were adjunctively treated with quetiapine or risperidone, and it showed statistical benefit favoring TC-5619 vs placebo in both cognitive and negative symptoms.32
This phase 2 study was conducted in order to examine the efficacy, safety, and tolerability of TC-5619 in a larger number of subjects with schizophrenia, and to test both similar (5mg) and higher (50mg) doses than those used in the exploratory trial. The primary efficacy objective was to use the SANS to test the effect of TC-5619 on negative symptoms. The key secondary efficacy objectives were to use the Cogstate Schizophrenia Battery (CSB) and UPSA-B to test effects of TC-5619 on cognitive and functional outcomes. There were two reasons for examining negative symptoms using SANS as the primary efficacy objective in this study. First, TC-5619 produced a statistically significant benefit on negative symptoms using the SANS in the prior phase 2 study. Second, Laughren and Levin23 provided regulatory support for use of a single primary outcome measure (eg, SANS) to test negative symptoms in seeking a negative symptoms indication; whereas co-primary cognitive and functional outcome measures are needed for an indication in cognitive dsysfunction.22,23
Methods
Subjects
The study was Institutional Review Board (IRB)-reviewed, filed with the US Food and Drug Administration and registered on www.clinicaltrials.gov (NCT01488929) before study initiation, and conducted according to the Guidelines of the Declaration of Helsinki.33 All subjects met DSM-IV-TR criteria for schizophrenia as facilitated using the MINI International Neuropsychiatric Interview, and they were primarily paranoid-type (89%). Subjects were required to be outpatients and to have: stable housing; availability of an informant with significant personal contact; and stable symptoms, defined as a lack of psychiatric hospitalization for 2 months before Screening, no change in antipsychotic therapy dose for 2 months prior to Screening, and a score ≤ 4 on the PANSS items for delusions, hallucination, conceptual disorganization and unusual thought content. Clozapine, sertindole and melperone were prohibited, as was use of more than one new-generation (atypical) antipsychotic drug. Subjects were required to have: a score >20 on the PANSS negative symptoms subscale; a score <6 on the Calgary Depression Scale for Schizophrenia (CDSS); a score <12 on the Simpson-Angus Scale (SAS) of extrapyramidal symptoms; and no significant suicidality as assessed by the Columbia Suicide Severity Rating Scale (CSSRS). Subjects could have no other co-morbid Axis 1 or Axis 2 DSM-4-TR disorder nor any unstable medical condition. Subjects could not be treated with mood stabilizers, antidepressants, or anxiolytics within 1 month prior to Screening. After complete description of the study to the subjects, written informed consent was obtained.
Experimental Drug and Matching Placebo
The experimental drug was a blend of TC-5619 hydrochloride and excipients in tablets. Placebo was manufactured to match the experimental drug TC-5619 in identical-appearing tablets in size, color, and shape.
Experimental Drug Protocol and Assessments
All raters were certified to use the rating instruments based upon extensive prior trial experience with the instruments, and also by demonstration that they could achieve appropriate scores with good inter-rater reliability using video assessments. Rating scores from each visit were examined remotely by an independent vendor to ascertain any discrepant or discordant scores between visits.
During Screening, all subjects were trained to use the CSB, and they completed three assessments prior to their Baseline assessment in order to eliminate any training effect. CSB was chosen in order to reduce subject burden as compared to the MATRICS Consensus Battery, which rmeasures the same seven cognitive domains, but which requires approximately twice as long to administer as CSB. Subjects also had assessments using the PANSS, SANS, SGI-Cog (a subject-rated 7-point Likert scale assessing three items: Memory and Learning; Attention and Concentration; and Speed of Thinking), and CGI-S scales. Physical examination, vital signs, serum chemistry and hematology, urinalysis, urine drug screen for illicit drugs, and ECG were performed. Assessments were also made using the SAS, the CSSRS, and the CDSS.
At Screening all subjects who met eligibility requirements and who wished to enroll in the trial signed informed consent. On day 1 all subjects who continued to meet eligibility criteria including absence of illicit drugs were randomized in a double-blind fashion in a 1:1:2 ratio to receive either TC-5619, 5mg; TC-5619, 50mg; or placebo. Safety and efficacy assessments used the same methods used at Screening. Study drug was administered orally once daily in addition to antipsychotic therapy. In a subset of subjects on day 1, week 12, and week 24, blood samples were taken before, approximately 30min, and approximately 3 hours after study drug was administered in the clinic, and samples were processed for plasma measurements of TC-5619. ECG and vital signs measurements were obtained coincidently with each blood sample taken for TC-5619 plasma measurement.
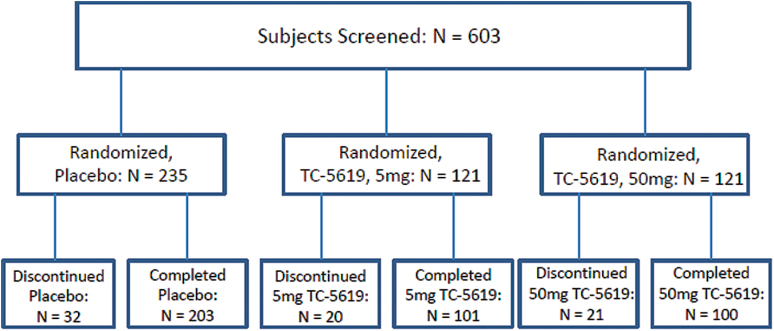
Subjects were instructed to take their daily dose of study drug each morning, at least 90min before tobacco intake (if a user) and at least 30min before taking antipsychotic therapy. Subjects returned for clinic visits at weeks 4, 8, 12, 18, and 24 during the treatment period, and at Week 26 for a follow-up visit. At each visit the same safety and efficacy assessment methods were used as at Screening and day 1. Subject compliance for TC-5619 was monitored by pill counts at each visit. Compliance with antipsychotic and other concomitant medications was assessed by subject self-report. Urinary drug screens detected illicit drug use, and urinary cotinine levels discriminated tobacco use from non-use. Disposition of subjects is shown in figure 1.
Fig. 1.
Subject disposition.
TC-5619 Plasma Levels
Sparse blood sampling from a subset of subjects (n = 277) was performed pre-dose and postdose on day 1 and weeks twelve and twenty-four to measure plasma levels. Sample analyses were performed using LC-MS/MS methods at Worldwide Clinical Trials Drug Development Solutions (Austin, TX). Plasma level means, standard deviations, medians, and ranges were summarized by cohort.
Statistical Analyses
The primary endpoint was change in SANS composite score from Baseline (day 1) to week twenty-four. Based upon parameters measured in the prior phase 2 study in which a statistically significant benefit of TC-5619 over placebo was found, this study had 90% power to detect a similar magnitude of effect on change from Baseline on the SANS at the 0.05 level using a 2-sided test. Change from Baseline was analyzed using Mixed Model Repeated Measures (MMRM) techniques with an alpha of 0.05 (two-tailed) to examine differences between the TC-5619 and placebo treatment cohorts (entire cohorts, tobacco-users and non-users combined). The MMRM model included treatment as a main factor, and SANS composite score Baseline values, time, tobacco use status, and treatment by time interaction as covariates. Because the primary outcome was assessed at two doses that could result in rejection of the null hypothesis, the graphical method was used to control for multiple comparisons. Each dose was assessed at the 0.025 level of significance. If either of these doses was significant, the alpha for this dose was recycled to the key secondaries for this dose in the order of CSB and then UPSA-B. If both CSB and UPSA-B were significant for this dose, this alpha was recycled to the other dose and SANS was tested using the recycled alpha. If SANS was significant, then CSB and UPSA-B were tested using the recycled alpha.
As the pre-specified measure of trial success, the primary efficacy analysis was performed using the modified Intent-to-Treat (mITT) population (patients with at least one postbaseline assessment) in tobacco-users and non-users combined. Secondary endpoints were examined to measure differences between TC-5619 and placebo in the entire cohorts (tobacco-users and non-users combined); in tobacco-users alone; and in non-users alone. Secondary endpoints included change from Baseline to Weeks 4, 8, 12, 18, and twenty-four in TC-5619 vs placebo in: the executive function task of the CSB (Groton Maze Learning Test); SGI-Cog; and CGI-S; and the absolute CGI-I score at week twenty-four. PANSS and its subscales were examined at Weeks 12 andtwenty-four. SANS, CSB and UPSA-B (and their subscales) were examined at Weeks 4, 8, 12, and 18. All secondary analyses were performed for the mITT and per protocol populations. The result of the mITT population was the pre-specified measure of success for secondary endpoints.
Results
Study and Subject Characteristics
The study was conducted at 64 sites in the United States, Russia, Ukraine, Hungary, Romania and Serbia. Enrollment began in December 2011 and the last subject’s last visit occurred in November 2013. A total of 477 subjects (18–65 years) were randomized (figure1; table 1). 62% of the subjects were male, and 55% were tobacco users as verified by urinary cotinine levels. Antipsychotics included: aripiprazole (11.1%); olanzapine (12.5%); paliperidone (4.9%); quetiapine (15.5%); risperidone (49.1%); and other (9.8%). 404 subjects completed the study (figure 1); the most frequent reason for discontinuation was withdrawal of consent (32 subjects)
Table 1.
Patient Features at Enrollment
| Placebo | 5mg TC-5619 | 50mg TC-5619 | ||
|---|---|---|---|---|
| Randomized subjects N (%) | 235 (49.3%) | 121 (25.4%) | 121 (25.4%) | |
| Age (Mean) | 38.6 yrs | 40.0 yrs | 38.4 yrs | |
| Gender: N (%) | Male | 141 (60%) | 81 (67%) | 75 (62%) |
| Female | 94 (40%) | 40 (33%) | 46 (38%) | |
| Race: N (%) | Asian | 1 (0.4%) | 1 (1%) | 0 |
| African American | 31 (13%) | 19 (16%) | 18 (15%) | |
| Caucasian | 196 (83%) | 95 (81%) | 101 (83%) | |
| Missing or Other | 7 (3%) | 6 (5%) | 2 (2%) | |
| Tobacco status | User | 139 (59%) | 71 (59%) | 53 (44%) |
| Tobacco non-user | 96 (41%) | 50 (41%) | 68 (56%) | |
| BMI | 26.4 | 26.4 | 27.3 | |
| Paranoid Schizophrenia: N (%) | 207 (88%) | 109 (90%) | 111 (92%) | |
| PANSS | Total Score | 77.4 | 77.3 | 76.9 |
| Negative subscale score | 25.8 | 25.9 | 25.6 | |
| Positive subscale score | 14.2 | 14.2 | 14.2 | |
| General subscale score | 37.4 | 37.2 | 37.1 |
Efficacy
Primary Efficacy Outcome Measure.
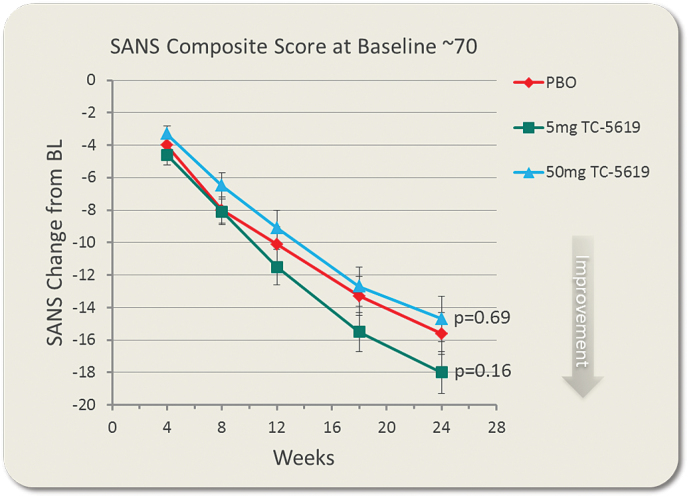
There was no statistically significant difference favoring either dose of TC-5619 over placebo in the change from baseline to week 24 in SANS composite score (TC-5619, 5mg: P = .159; 50mg: P = .689; table 2, figure 2). There was no significant difference of either dose of TC-5619 over placebo at earlier times. There was no significant difference of TC-5619 at week twenty-four or at any earlier times on any of the 5 SANS domains. Post hoc analyses showed no interactions by gender, country, or site.
Table 2.
Week 24 Results of Primary, Key Secondary and Secondary Analyses in the mITT Population
| Task | Placeboa | 5mg TC-5619a | 50mg TC-5619a |
|---|---|---|---|
| SANS composite score (baseline score ~ 70; decrease shows benefit) | −15.4 | −17.5 | −14.7 |
| −2.2 | 0.6 | ||
| P = .159 | P = .689 | ||
| CSB composite score (baseline score ~ 0; increase shows benefit) | 0.19 | 0.08 | 0.26 |
| −0.11 | 0.07 | ||
| P = .38 | P = .55 | ||
| UPSA-B total score (baseline score ~ 69; increase shows benefit) | 10.9 | 12.4 | 10.5 |
| 1.5 | −0.5 | ||
| P = .23 | P = .74 | ||
| PANSS total score (decrease shows benefit) | −11.4 | −11.7 | −10.7 |
| −0.3 | 0.7 | ||
| P = .74 | P = .47 | ||
| PANSS negative symptoms subscale score (decrease shows benefit)_ | −4.4 | −4.6 | −4.2 |
| −0.2 | 0.2 | ||
| P = .55 | P = .60 | ||
| SGI-Cog total score (decrease shows benefit) | 9.1 | 8.9 | 8.9 |
| −0.2 | −0.2 | ||
| P = .44 | P = .43 | ||
| CGI-S score (decrease shows benefit) | −0.52 | −0.54 | −0.51 |
| −0.02 | 0.01 | ||
| P = .78 | P = .91 | ||
| CGI-I score (decrease shows benefit) | 3.0 | 2.9 | 3.0 |
| 0.0 | 0.1 | ||
| P = .85 | P = .29 |
Note: aThe values in each cell by row, with the exception of SGI-Cog and CGI-I scores, are: [Least Squares Mean Change from Baseline/Mean Difference in Change from Baseline of TC-5619 versus Placebo/2-tailed P-value]. For SGI-Cog and CGI-I scores, the cell shows: [LS Mean score at Week 24/Mean Difference from Placebo/2-tailed P-value].
Fig. 2.
Change from baseline in SANS composite score by time. Shown are LS Means and Standard Errors of the Mean (SEM) at each time point.
The effect of TC-5619 on SANS composite score at week 24 appeared to show a significant improvement compared to placebo in tobacco users at one of the two doses (5mg: P = .015; 50mg: P = .155), but not in non-users. However, there was no consistency in the apparent benefit in tobacco users between countries, and either no concordance or discordance with other relevant measures (eg, PANSS negative symptoms subscale scores, CGI-I, or CGI-S).
Key Secondary Efficacy Outcome Measures.
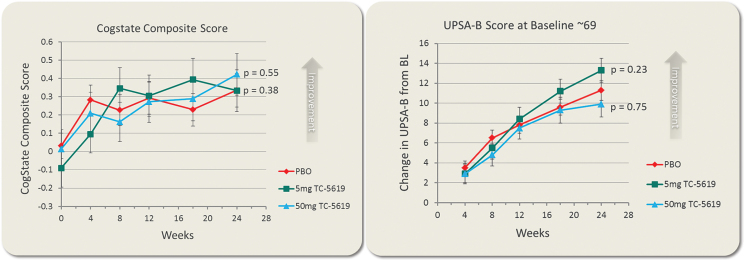
There was no statistically significant difference favoring either dose of TC-5619 over placebo in the change from Baseline to week 24 in the CSB composite score (TC-5619, 5mg: P = .384; 50mg: P = .547; table 2, figure 3A), or in the UPSA-B total score (TC-5619, 5mg: P = .230; 50mg: P = .744; table 2, figure 3B).
Fig. 3.
Change from baseline in cogstate schizophrenia battery composite score (A) and in UPSA-B total score (B). Shown are LS Means and SEM at each time point.
There was no significant difference favoring TC-5619 on CSB composite score or on UPSA-B total score at week 24 or any earlier times as a function of tobacco use. There was no significant improvement conferred by TC-5619 on any of the CSB item scores.
Secondary Efficacy Outcome Measures.
There was no statistically significant improvement favoring TC-5619 in change from baseline to week 24 in: PANSS total score (TC-5619, 5mg: P = .742; 50mg: P = .469); CGI-S (TC-5619, 5mg: P = .775; 50mg: P = .910); SGI-Cog (TC-5619, 5mg: P = .437; 50mg: P = .433); or in the absolute CGI-I score (TC-5619, 5mg: P = .851; 50mg: P = .292). There was no improvement favoring TC-5619 compared to placebo in change from Baseline in the PANSS subscale scores.
Plasma Levels of TC-5619 and Pharmacodynamic Analysis.
Exposure to TC-5619 was confirmed in >95% of subjects randomized to the 5mg or 50mg treatment arms in the study subset in whom sampling was performed (N = 277). Mean plasma concentration values showed a dose-proportional increase between the 5mg and the 50mg treatment arms. There was no statistically significant relationship between the plasma concentration of TC-5619 at any time-point with change from Baseline in the SANS composite score.
Patient Compliance.
Patient compliance with study drug was assessed by pill counts, and compliance with background antipsychotic use was assessed by self-report. Compliance was >90% for both assessments.
Safety and Tolerability
Treatment-Emergent Adverse Events (AEs).
Twenty-eight percentof subjects in the placebo cohort and thirty-four percent of subjects in the TC-5619 cohort reported AEs. All but three AEs were mild or moderate in severity. Three AE terms occurred at both a rate >2% in any cohort and also numerically greater in a TC-5619 cohort compared to placebo. These AEs were: insomnia (TC-5619, 5mg: 3.4%; TC-5619, 50mg: 2.5%; and placebo: 1.7%); increased ALT (TC-5619, 5mg: 2.6%; TC-5619, 50mg: 2.5%; and placebo: 1.7%); and orthostatic hypotension (TC-5619, 5mg: 2.6%; TC-5619, 50mg: 0.8%; and placebo: 2.1%).
Serious Adverse Events (SAEs).
Six SAEs were reported in the study, 2 in the placebo cohort (toxicity to a concomitant medication and worsening schizophrenia); 1 in the TC-5619, 5mg cohort group (worsening schizophrenia); and 3in the TC-5619, 50mg cohort (death of unknown cause in a subject found dead at home at an uncertain time after death; ruptured ovarian cyst; and death from aortic aneurysm rupture). Each SAE was considered not drug-related by the investigator.
Adverse Events Leading to Discontinuation.
Twelve AEs led to discontinuation: 7(3.0%) in the placebo cohort; 5 (4.3%) in the TC-5619, 5mg cohort; and 3 (2.5%) in the TC-5619, 50mg cohort. In the placebo cohort, the events were: toxicity to a concomitant medication; hepatic enzyme increased; autonomic nervous system imbalance; anxiety; insomnia; worsening of schizophrenia; and sleep disorder (some in more than one subject, and several in a single subject). In the TC-5619 cohorts, the events were: irritability; blood bilirubin increased; agitation; insomnia; psychotic disorder; worsening schizophrenia; ruptured ovarian cyst; ruptured aortic aneurysm; and death of unknown cause (several in a single subject).
Results of the CSSRS and CDSS.
Use of the CSSRS revealed very low levels of emergent suicidality during the trial between visits, and changes were similar in the placebo and TC-5619 cohorts. The CDSS showed a small but clinically insignificant improvement of similar magnitude within all three cohorts during the study.
Physical Examination, SAS, Vital Signs, Laboratory Analytes, and ECG.
There were no clinically meaningful changes between cohorts in physical examination, vital signs, orthostatic blood pressure changes, urine or serum laboratory measurements, and ECG interpretations and conduction intervals. There was a single-digit and similar proportion of subjects with an absolute QTcF > 450ms at any time in the 3 cohorts, and no subject had a QTcF > 500ms. SAS scores were low at Baseline (Placebo: 0.9; TC-5619, 5mg: 0.9; and TC-5619, 50mg: 1.1). Analysis of SAS scores revealed a small but clinically insignificant improvement in extrapyramidal signs that was of similar magnitude within all 3cohorts during the study (Week 24 [Placebo: 0.4; TC-5619, 5mg: 0.5; and TC-5619, 50mg: 0.6]).
Discussion
New treatments are needed for people with negative and cognitive symptoms in schizophrenia, because treatment with the currently approved antipsychotic drugs offers little benefit.6,17 Alpha7 NNRs have been characterized as viable drug targets to benefit these symptoms,13,18–20,31 and clinical trials using the alpha7 NNR agonists, DMXB and EVP-6124, have shown encouraging results in subjects with schizophrenia.19,28–30 The results of an exploratory phase 2 trial with the alpha7 NNR, TC-5619, were generally consistent with those of DMXB and EVP-6124, showing a varied benefit on scales that assessed cognitive and negative symptoms.32
In this larger phase 2 trial, however, no benefit of TC-5619 was observed in any measure of negative, cognitive, or functional outcomes. This lack of benefit was concordant across the outcome measures (eg, no benefit of either the 5mg or 50mg dose on the SANS composite and SANS domain scores, consistent with no benefit on the PANSS negative symptoms subscale score). Likewise, there was no benefit of TC-5619 on the CSB composite or item scores, and no benefit on the SGI-Cog. Finally, there was concordance in the lack of TC-5619’s benefit as assessed by clinical and functional assessment scales (UPSA-B, CGI-S, and CGI-I). The coherence of these results in a robust, well-powered phase 2 study in 477 subjects with schizophrenia is persuasive evidence that TC-5619 did not produce the encouraging benefit observed in the earlier exploratory trial.
A number of factors may underlie the conflicting efficacy findings from the earlier and current trials of TC-5619 in schizophrenia. First, the earlier trial used a 12-week treatment period and a forced titration scheme for TC-5619, beginning with 1mg and ending with 25mg; the current trial used a 24-week treatment period and a fixed dose schedule with separate treatment arms for 5mg and 50mg. It is possible that relatively low doses (eg, 1mg) are sufficient to initiate CNS processes that produce clinically observable benefits, and that higher starting doses (eg, 5mg, 50mg) do not invoke or desensitize these processes, as may a longer treatment duration. However, electrophysiological studies of TC-5619 in oocytes and HEK cells reveal: (1) no functional effects of concentrations equivalent to human doses below 1mg; and (2) progressively enhanced effects of TC-5619 without desensitization at concentrations equivalent to human doses approaching 100mg. This suggests that 50mg doses of TC-5619 should produce greater activation of the alpha 7NNR than 5mg or 1mg doses.
Second, the tobacco-using TC-5619 cohort in the earlier trial demonstrated a greater magnitude of benefit than the non-user cohort, especially against cognitive symptoms but also against negative symptoms. To explain this counterintuitive effect, we hypothesized that tobacco users who were administered TC-5619 obtained a double benefit: tobacco-derived nicotine acting on postsynaptic alpha4beta2 NNRs on hippocampal neurons to generate a procognitive effect; and TC-5619 acting presynaptically on glutamatergic and acetylcholinergic afferents to those hippocampal neurons, increasing neurotransmitter release and adding to the effect of nicotine.32 In the current trial, the mITT population was comprised of both the 55% who were tobacco users, and the 45% who were non-users; perhaps the 45% non-users blunted the overall benefit of TC-5619 in this trial. However, this factor does not explain why TC-5619 showed benefit in only a single cohort of tobacco users (5mg) in a single assessment (SANS composite score), and not in other relevant scores (eg, the PANSS negative symptom subscale score). Moreover, in this study, TC-5619 had no benefit on cognition in the tobacco-user group, even though the tobacco-user group showed the greatest cognitive benefit of TC-5619 in the earlier trial.32 Therefore, the differing methods of analyzing subjects by tobacco status was not a likely factor underlying the lack of benefit of TC-5619 in this study.
Third, the earlier trial was conducted in the United States (one-third of subjects) and India (two-thirds of subjects); whereas this study was conducted in the United States (about one-quarter of subjects), with the remaining subjects stemming from Russia and Eastern Europe. However, this differential geography does not explain the discrepant findings, because US subjects appeared to drive the majority of the benefit observed in the earlier trial; but in the current trial, US subjects showed no benefit of TC-5619.
Fourth, only risperidone or quetiapine at approved stable doses were allowed in the earlier trial; whereas newer generation antipsychotics were permitted in this trial. However, a post hoc inspection of the large subset of subjects taking risperidone or quetiapine in the current study (64.6%) showed no difference in the effect of TC-5619 compared to the subset of subjects taking other antipsychotics. Consequently, this factor does not account for the discrepancies.
A remaining possibility is that TC-5619 produces a small benefit against negative and cognitive symptoms, and that this small signal was detectable in the earlier trial with its 19 sites and potentially smaller placebo effect, but blunted in this trial by the “noise” of 64 sites and potentially larger placebo effect. However, the magnitude of placebo benefit in this 24-week trial was proportionally similar to the magnitude that would be anticipated based upon the earlier results in the 12-week trial. Hence, the current trial was not only more robust but also did now show a proportionately greater placebo effect. Therefore, its outcome (no benefit) is the more likely effect of TC-5619 in negative and cognitive symptoms of schizophrenia.
There were similar numbers of AEs, SAEs, and adverse events leading to discontinuation in the three cohorts. Two deaths occurred during this trial. In a meta-analysis of 37 studies which examined mortality in patients with schizophrenia, Saha and colleagues34 analyzed 22296 deaths. From these data, the median annualized case fatality rate (CFR) in these schizophrenic patients was 95.4 per 10000.34 This trial comprised 239 patient-years, and the application of an annualized CFR of 95.4 per 10000 to 239 patient-years generates an expected fatality of 2.3 subjects during this trial. The two deaths in this trial are compatible with this expected fatality rate in this population.
There were no clinically noteworthy differences between cohorts in physical examination, extrapyramidal signs, vital signs, laboratory measurements of serum or urine analytes, ECGs including QTcF intervals, suicidality, or depression. TC-5619 was generally safe and well tolerated in this trial.
There are a number of potential limitations of this trial. First, approximately 80% of the subjects were enrolled in Eastern Europe, with the remaining enrolled in the United States. There is a possibility that the results are confounded by differing criteria for the diagnosis of schizophrenia in the 2 geographies, as well as by differing intangible factors such as study site environment. However, no benefit of TC-5619 was observed in European vs US regions, and so this potential confound would not appear to explain the lack of benefit of TC-5619 in this trial. Another potential limitation is the source of enrolled subjects as a function of country. For example, in the United States, the majority of enrolled subjects learned about the trial from local advertisements whereas the subjects enrolled in Eastern Europe were already patients in the databases of the investigators. Again, however, this potential confound does not appear to explain the results, because there were no qualitative differences in the results from the United States vs Eastern Europe.
In conclusion, the lack of efficacy observed in this trial does not support further development of TC-5619 for schizophrenia. However, the favorable safety profile of TC-5619 in the two trials in schizophrenia and in two other trials in adult subjects with attention deficit-hyperactivity disorder (ADHD) may justify the use of TC-5619 in future trials within other therapeutic indications that have different underlying pathophysiologies (eg, Alzheimer’s Disease [AD] or prodromal AD).
Acknowledgments
The authors would like to thank: Gaston Farr for clinical operations and vendor outsourcing; Grace Li for statistical programming; Flora Meyer for data management; John Hanley for document management; Melissa Joseph for co-ordination of CMC activities; Melissa Black for administrative services; Merouane Bencherif, Pat Lippiello, Daniel Yohannes, Terry Hauser, Kristen Jordan and Greg Gatto for nonclinical discovery work; and many other Targacept staff for their support. These data were previously presented at the Schizophrenia International Research Conference (Florence, Italy in April 2014) and at the ASCP Annual Meeting (Hollywood, Florida, USA in June 2014). Dr Walling has no personal financial disclosures that are relevant to this work. He was the Principal Investigator for both the study and one of the clinical sites that conducted this study. Dr Marder has received consulting fees from Abbvie, Amgen, Genentech, Roche, Targacept, Forum, Shire, Lundbeck, Pfizer, Boeringer-Ingelheim, Jazz, and Otsuka. He has received research support from Genentech, Psychogenics, and Amgen. Dr Kane has no personal financial disclosures that are relevant to this work. Dr Fleischhacker has received research grants from Otsuka, Pfizer, Janssen, and Reckitt-Benckiser, as well as consulting honoraria from Lundbeck, Roche, Bristol-Myers Squibb, Otsuka, Richter, Janssen, Pfizer, MedAvante, Sunovion, Takeda, Endo, Targacept and Vanda. He has received speaker honoraria from Lundbeck, Janssen, Otsuka, Roche, and Takeda. He holds stock from MedAvante. Dr Keefe currently or in the past three years has received investigator-initiated research funding support from the Brain Plasticity, Inc., Department of Veteran’s Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, PsychoGenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. He currently or in the past three years has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biogen Idec, Biomarin, Boehringer-Ingelheim, Eli Lilly, EnVivo, GW Pharmaceuticals, Helicon, Lundbeck, Merck, Minerva Neurosciences, Inc., Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. Dr Keefe receives royalties from the BACS testing battery and the MATRICS Battery (BACS Symbol Coding). He is also a shareholder in NeuroCog Trials, Inc. and Sengenix. The remaining six authors are or were employed by the Sponsor of this study, Targacept, Inc., and they own shares of the common stock and/or options to purchase shares of the common stock of Targacept, Inc. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, Virginia: APA; 2013. [Google Scholar]
- 2. Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. [DOI] [PubMed] [Google Scholar]
- 3. Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. [DOI] [PubMed] [Google Scholar]
- 4. Erhart SM, Marder SR, Carpenter WT. Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull. 2006;32:234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr Scand. 2007;115:4–11. [DOI] [PubMed] [Google Scholar]
- 6. Miyamoto S, Jaskoog LF, Fleischhacker WW. Alternative psychopharmacologic targets for the treatment of schizophrenia: results from phase I and II trials. Curr Opin Psychiatry. 2013;26:158–165. [DOI] [PubMed] [Google Scholar]
- 7. Addington J, Addington D. Premorbid functioning, cognitive functioning, symptoms and outcome in schizophrenia. J Psychiatry Neurosci. 1993;18:18–23. [PMC free article] [PubMed] [Google Scholar]
- 8. O’Carroll R. Cognitive impairment in schizophrenia. Adv Psychiatric Treatment. 2000;6:161–168. [Google Scholar]
- 9. Fleischhacker W. Negative symptoms in patients with schizophrenia with special reference to the primary versus secondary distinction. Encephale. 2000;26:12–14. [PubMed] [Google Scholar]
- 10. Ojeda N, et al. Course of cognitive symptoms in schizophrenia: a review of the literature. Actas Esp Psiquiatr. 2007;35:263–270. [PubMed] [Google Scholar]
- 11. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. [DOI] [PubMed] [Google Scholar]
- 12. Reichenberg A, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bencherif M, Stachowiak MK, Kucinski AJ, Lippiello PM. Alpha7 nicotinic cholinergic neuromodulation may reconcile multiple neurotransmitter hypotheses of schizophrenia. Med Hypotheses. 2012;78:594–600. [DOI] [PubMed] [Google Scholar]
- 14. Neuchterlein KH, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. [DOI] [PubMed] [Google Scholar]
- 15. Neuchterlein KH, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 16. Kern RS, et al. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165:214–220. [DOI] [PubMed] [Google Scholar]
- 17. Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olincy A, Stevens KE. Treating schizophrenia symptoms with an alpha7 nicotinic agonist, from mice to men. Biochem Pharmacol. 2007;74:1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freedman R, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:140–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kucinski AJ, et al. Alpha7 neuronal nicotinic receptors as targets for novel therapies to treat multiple domains of schizophrenia. Curr Pharm Biotechnol. 2011;12:437–448. [DOI] [PubMed] [Google Scholar]
- 21. Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptoms assessment of schizophrenia. Schizophr Bull. 2011;37:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marder SR, Daniel DG, Alphs L, Awad AG, Keefe RSE. Methodological issues in negative symptom trials. Schizophr Bull. 2011;37:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laughren T, Levin R. Food and Drug Administration commentary on methodological issues in negative symptom trials. Schizophr Bull. 2011;37:255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. [DOI] [PubMed] [Google Scholar]
- 25. Freedman R, Coon H, Myles-Worsley M, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. [DOI] [PubMed] [Google Scholar]
- 27. Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–1597. [DOI] [PubMed] [Google Scholar]
- 28. Olincy A, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. [DOI] [PubMed] [Google Scholar]
- 29. EnVivo Pharmaceuticals, EnVivo reports positive results of its EVP-6124 clinical biomarker study in schizophrenia patients. 2009.
- 30. EnVivo Pharmaceuticals, EnVivo announces positive phase 2b clinical data in schizophrenia: EVP 6124 meets primary endpoint with statistically significant improvements in cognition and multiple secondary endpoints for improvement in function and impact on negative symptoms. 2011.
- 31. Hauser TA, Kucinski A, Jordan KG, et al. TC-5619: An alpha7 neuronal nicotinic receptor-selective agonist that demonstrates efficacy in animal models of the positive and negative symptoms and cognitive dysfunction of schizophrenia. Biochem Pharmacol. 2009;78:803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lieberman JA, Dunbar G, Segreti AD, et al. A randomized exploratory trial of an alpha7 neuronal nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacol. 2013;38:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helsinki. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Seoul, Korea: 59th WMA General Assembly; 2008.
- 34. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry. 2007;64:1123–1131. [DOI] [PubMed] [Google Scholar]