Abstract
Reinforcement learning deficits have been associated with schizophrenia (SZ). However, the pathophysiology that gives rise to these abnormalities remains unclear. To address this question, SZ patients (N = 58) and controls (CN; N = 36) completed a probabilistic reversal-learning paradigm during functional magnetic resonance imaging scanning. During the task, participants choose between 2 stimuli. Initially, 1 stimulus was frequently rewarded (80%); the other was infrequently rewarded (20%). The reward contingencies reversed periodically because the participant learned the more rewarded stimulus. The results indicated that SZ patients achieved fewer reversals than CN, and demonstrated decreased winstay-loseshift decision-making behavior. On loseshift compared to winstay trials, SZ patients showed reduced Blood Oxygen Level Dependent activation compared to CN in a network of brain regions widely associated with cognitive control, and striatal regions. Importantly, relationships between group membership and behavior were mediated by alterations in the activity of cognitive control regions, but not striatum. These findings indicate an important role for the cognitive control network in mediating the use and updating of value representations in SZ. Such results provide biological targets for further inquiry because researchers attempt to better characterize decision-making neural circuitry in SZ as a means to discover new pathways for interventions.
Key words: schizophrenia, cognitive control, fMRI, negative symptoms, reversal learning, reward processing, reinforcement learning
Introduction
Negative symptoms, such as abnormalities in motivation and goal-oriented decision-making, are an integral aspect of schizophrenia (SZ).1–3 However, the etiology of these deficits remains unclear. One recent theory suggests that abnormalities of motivation and goal-oriented decision-making in SZ patients may arise due to maladaptive reward-learning.4 Gold et al postulates that SZ patients have difficulties creating mental representations of value for various outcomes of a decision, modifying these representations, and utilizing these representations to drive behavior.4 The current article explores this framework by examining neurophysiological predictors of value representation in SZ patients.
One paradigm thought to measure updating of value representations is the probabilistic reversal learning (PRL) task.5 In this paradigm, individuals choose between 2 stimuli (1 commonly and 1 rarely rewarded). Once the participant learns the more frequently rewarded stimulus, the reward contingencies reverse, and participants must modify their value representations through feedback. Waltz and Gold6 found that SZ patients achieved fewer reversals than controls (CN) using a PRL task, consistent with a deficit in value updating.
One potential explanation for reversal learning deficits in SZ is a blunted striatal response to reward anticipation/receipt. The striatum’s critical role in reward anticipation and in the calculation of prediction errors makes it a likely target for the source of such deficits. For example, Schlagenhauf et al7 found evidence for impaired ventral striatal prediction error responses in unmedicated SZ patients. Several other research groups have also reported blunted striatal activation in SZ patients during reward.7–13 However, this literature is mixed.14–16
Recently, researchers have examined how other brain networks may work with striatal regions to produce reversal learning deficits.17 For example, Waltz et al17 found differences in activation of default mode and executive control network regions using a PRL task. In addition, Schlagenhauf et al7 found that some SZ patients showed reduced ventral lateral prefrontal activation in a contrast of informative punishments to informative-rewards. Thus, while the striatum appears integral to valuation, understanding the role of other neural circuits (eg, the fronto-parietal network), and the integration of these networks with striatal regions may be critical to understanding reward-learning impairments.17,18
One network that has been broadly implicated in value representations is the cognitive control network (CCN).19 CCN regions such as the dorsolateral prefrontal cortex (dlPFC) have been implicated in the retrieval, maintenance, and implementation of value representations. In addition, the anterior cingulate cortex (ACC) is postulated to evaluate conflict between existing stimulus-response representations and the updating value representations.20 Dorsal parietal cortex regions (DPC) have been implicated in value representation and switching.21 Importantly, SZ patients have structural (dlPFC22, 23, ACC24, DPC25), and functional deficits (dlPFC26–28, ACC29, 30, PC31, 32) in these regions. However, the relationship of the CCN to value representations in SZ has not been tested.
Thus, the current experiment examines the neural correlates of performance on a PRL task in SZ using functional magnetic resonance imaging (fMRI) to understand whether deficits in CCN activation contribute to reversal learning impairments. We built on previous reports in several ways.7,17 First, we recruited a large sample to illuminate effects of all brain networks involved in reversal learning impairments. This sample size also allowed us to examine the relationship between individual differences in brain activity and task behavior to determine which networks may be most strongly related to behavior, a question not addressed in prior reports. We also conducted mediation analyses to test hypotheses about alternative causal paths of these relationships (ie, do Blood Oxygen Level Dependent [BOLD] abnormalities “lead” to behavioral deficits between groups or the reverse?). Second, we coded trials as a function of immediate feedback and behavior (eg, winstay-loseshift), as well as, coding the final reversal error trials allowing us to measure the neurophysiological correlates of putative value updating.5
We hypothesized that if reversal learning deficits reflected, at least in part, less stable value representations, SZ patients should show: (1) both abnormal winstay and loseshift decision-making behavior (ie, a “shiftier” pattern of responses) and (2) decreases in behavior at both the initial acquisition and reversal stages of the PRL task. In addition, we hypothesized SZ patients would have decreased BOLD activity in CCN in addition to striatal regions during trials that putatively required value updating. Third, we hypothesized that BOLD activations during these trials would be correlated with task performance, and that mediation analyses would support a plausible causal path of BOLD activations leading to task behavior deficits between groups. Finally, we predicted that impairments in behavior and imaging from conditions that putatively assess value updating would be correlated with the severity of negative symptoms, given previous work suggesting that anhedonic and amotivational symptoms may be associated with both cognitive control and reward processing deficits.33
Materials and Methods
Participants
Participants were 58 individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for SZ or schizoaffective disorder (N = 12), and 40 CN, with no personal or family history of psychosis, from the Saint Louis community. Six SZ were unmedicated. Exclusion criteria included (1) DSM-IV diagnosis of substance abuse or dependence in the past 6 months; (2) DSM-IV diagnosis of a mood disorder in the past year; (3) changes in medication dosage 2 weeks prior to consent; (4) past head injury with documented neurological sequelae and/or loss of consciousness; (5) pregnancy; (6) mental retardation; and (7) MRI contraindications. We did not exclude for current/previous anxiety, personality disorders, or smoking status. One SZ patient and 4 CNs were excluded due to excessive movement during scanning, yielding a final sample size of 57 SZ and 36 CN. These sample sizes were chosen to provide at approximately 75% power to detect a medium effect size for group differences and individual difference relationships for both behavioral and neuroimaging analyses. The Washington University Institutional Review Board approved the study. Participants provided written informed consent in accordance with Washington University’s Human Subject Committee’s criteria.
Clinical Assessment
Diagnoses were determined by the Structured Clinical Interview for DSM-IV-TR (time to repetition).34 Symptoms were assessed using the Scales for the Assessment of Positive Symptoms35 and Negative Symptoms,36 and Brief Negative Symptom Scale.37 Avolition and anhedonia were also assessed using the following self-report measures: The Revised Chapman Physical and Social Anhedonia Scales,38,39 the Temporal Experience of Pleasure Scale,40 the Snaith-Hamilton Pleasure Scale,41 and the Apathy Scale.42 The Specific Levels of Functioning scale43 was administered to assess functional status. All participants passed a drug screen and Breathalyzer.
PRL Task
Participants performed a PRL task5 during fMRI (figure S1). Two abstract visual patterns were presented simultaneously for 2500ms. Subjects were instructed to guess which pattern was most likely to yield reward (by pressing 1 of 2 buttons on a pad placed on their midsection), and stick with their response. They were told that occasionally the reward contingencies would reverse and the alternative stimulus would be associated with a high probability of reward. Participants were instructed that the task objective would be to maximize correct responses. They were given feedback (correct or incorrect) lasting 1500ms. The inter-stimulus interval was 1000ms–5000ms. The task consisted of 8 runs of 60 trials. Each run consisted of an initial acquisition where the initial values for each choice were learned. When 8 of the previous 10 trials were answered correctly the reinforcement contingencies reversed. Probabilistic negative feedback was implemented such that a correct response for each trial was followed by negative feedback 20% of the time. All subjects practiced the task prior to scanning. Participants won bonus money for increased task accuracy.
Image Acquisition and Processing
Images were acquired on a Siemens 3 Tesla Tim Trio system with a 12-channel head coil. Structural images were collected using a sagittal magnetization-prepared rapid acquisition gradient echo sequence (TR = 2.4s, TE = 3.16ms, inversion time 1s, flip = 8 degrees, 176 slices, 1mm3 voxels). Functional images were collected during 8 runs of 221 frames using a gradient echo echo-planar sequence (TR = 2000ms, TE = 27ms, flip = 77 degrees, 35 slices). Functional runs acquired axial images parallel to the anterior-posterior commissure plane with 4mm3 isotopic voxels. The MR data were normalized across runs by scaling the whole-brain signal intensity to a fixed value (mode of 1000), and removing the linear slope on a voxel-by-voxel basis to counteract the effects of drift. The data were then aligned to correct for head motion using 6 parameter rigid body rotation and translational correct algorithms.44–46 Images were then resampled into 3mm voxels, registered into Talairach space47 using 12-parameter affine transformations, and spatially smoothed with a 6-mm FWHM Gaussian filter. Data analysis was performed using in-house developed software (FIDL analysis package, http://www.nil.wustl.edu/labs/fidl/index.html 48).
Behavioral Data
Individual trials were coded using 2 schemes. First, trials were separated based on whether the decision on the trial was correct, an error, a probabilistic error (ie, correct choice that received negative feedback), or a final error (the last error preceding a task reversal).5 Second, trials were coded depending on the valence of the feedback (ie, won or lost) and choice of the same or opposite stimulus on the following trial (ie, stay or shift). Independent samples t-tests were conducted to examine group differences in 5 behavioral variables using these coding schemes: (1) Number of final errors. Final errors are of interest because they are thought to, putatively, index value updating; (2) Percentage of winstay trials as a function of the total number of wins, as a measure of positive feedback responsivity; (3) Percentage of loseshift trials as a function of the total number of loses, as a measure of negative feedback responsivity; (4) Number of errors; and (5) The number of instances where a probabilistic error was followed by a correct response (PE_COR), as a measure of the robustness of the participant’s internal representation of the correct choice. The initial acquisition phase of each run was also analyzed to determine how many trials the participant needed to learn the reward contingencies and on how many runs the initial acquisition was achieved.
fMRI
fMRI data were analyzed using 2 General Linear Models (GLM), with statistical parametric mapping canonical assumed hemodynamic response shapes. The first GLM included estimates for trials coded as winstay and loseshift. The second GLM included estimates for final error and error trials, and only included subjects who achieved more than 8 final errors (CN = 21; SZ = 21). Importantly, this contrast was chosen to highlight the neural processes most associated with value updating. We compared the groups using independent samples t-tests. Whole-brain analyses were corrected for multiple comparisons using a z-value of 2.5 and a cluster size of 35 voxels, as determined by Monte Carlo simulations to provide a whole-brain false positive rate of P < .05.49,50 Regions demonstrating significant effects in the Whole-brain analysis for the loseshift-winstay contrast were used in correlation and mediation analyses.
Correlation/Mediation Analyses
Biological, behavioral, and external variables were correlated in order to discern brain-behavior and individual difference relationships. Mediation analyses were conducted using the SPSS PROCESS toolbox51 in order to gain initial insight on whether biological or behavioral abnormalities were driving group differences. For these analyses we employed bootstrapping methods with 1000 sample iterations; 95% confidence intervals from these analyses are reported.
Results
The groups did not significantly differ in age, gender, ethnicity, or parental education. The SZ group self-reported increased levels of anhedonia, decreased levels of social and occupational functioning, and personal education compared with CN (table 1).
Table 1.
Participant Demographic, Clinical, Self-Report Measures, and Task Behavior
| Healthy Controls | Individuals with Schizophrenia | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P value | |
| Demographics | |||||
| Age (y) | 36.6 | 9.2 | 37.0 | 8.6 | .8 |
| Sex (% male) | 52.8 | 66.7 | .2 | ||
| Ethnicity (% non-Cauasian) | 66.7 | 61.4 | .6 | ||
| Personal Education (y) | 14.2 | 2.1 | 13.0 | 2.2 | .01 |
| Parental Education (y) | 12.8 | 1.5 | 12.9 | 3.2 | .9 |
| Medication status | |||||
| Atypical antipsychotics (%) | NA | 75.4 | |||
| Typical antipsychotics (%) | NA | 5.3 | |||
| Typical and atypical (%) | NA | 7.0 | |||
| Not Medicated (%) | NA | 12.3 | |||
| Clinical ratings (Item averages) | |||||
| Positive (SAPS) | NA | 0.7 | 0.7 | ||
| Disorganization (SAPS) | NA | 0.4 | 0.4 | ||
| Negative (SANS) | NA | 1.2 | 0.6 | ||
| Negative (BNSS) | NA | 1.4 | 0.8 | ||
| Self-Report Measures | |||||
| Beck Depression Inventory | 3.0 | 4.8 | 10.6 | 10.6 | <.001 |
| Social anhedonia | 8.8 | 6.4 | 16.3 | 8.0 | <.001 |
| Physical anhedonia | 11.2 | 6.2 | 18.5 | 9.1 | <.001 |
| Snaith Hamilton Pleasure | 52.4 | 3.1 | 48.1 | 8.4 | <.001 |
| TEPS consummatory pleasure | 37.8 | 5.8 | 34.1 | 8.0 | .01 |
| TEPS anticipatory pleasure | 49.2 | 4.9 | 44.8 | 8.9 | .003 |
| Apathy scale | 22.2 | 3.1 | 26.1 | 6.6 | <.001 |
| Functioning | |||||
| SLOF (personal) | 4.7 | 0.4 | 4.3 | 0.6 | <.001 |
| SLOF (informant) | NA | NA | 4.1 | 0.6 | |
| Task Behavior | |||||
| Reversals | 13 | 9.1 | 8.4 | 7.8 | .02 |
| Winstay_Ratio | 0.8 | 0.2 | 0.7 | 0.2 | .035 |
| Loseshift_Ratio | 0.4 | 0.1 | 0.5 | 0.1 | .07 |
| PE_COR | 43.0 | 16.8 | 36.8 | 15.0 | .07 |
| ERROR | 133.1 | 48.6 | 149.5 | 44.5 | .02 |
| IA_Trial | 23.5 | 15.2 | 31.7 | 15.0 | .02 |
| IA_Run | 6.6 | 2.4 | 5.7 | 2.5 | .1 |
Note: SAPS, Schedule for the Assessment of Positive Symptoms; SANS, Schedule for the Assessment of Negative Symptoms; BNSS, Brief Negative Symptwom Scale; TEPS, Temporal Experiences of Pleasure Scale; SLOF, Specific Level of Functioning Scale. IA_Trial: Average number of initial acquisition trials; IA_Run: Runs where initial acquisition was achieved; PE_COR: percentage of probabilistic errors followed by a correct response.
Task Behavior
SZ patients required more trials to learn the reward contingencies, achieved fewer reversals, and showed less responsivity to positive feedback than CN. The SZ group also showed a trend level decrease in accuracy and in our measure of the robustness of an internal representation of the correct choice (PE_COR). Finally, SZ patients showed a trend toward more responsivity to negative feedback compared with CN. Thus, SZ patients showed shiftier responding to positive and negative feedback.
Imaging Results
Loseshift-Winstay.
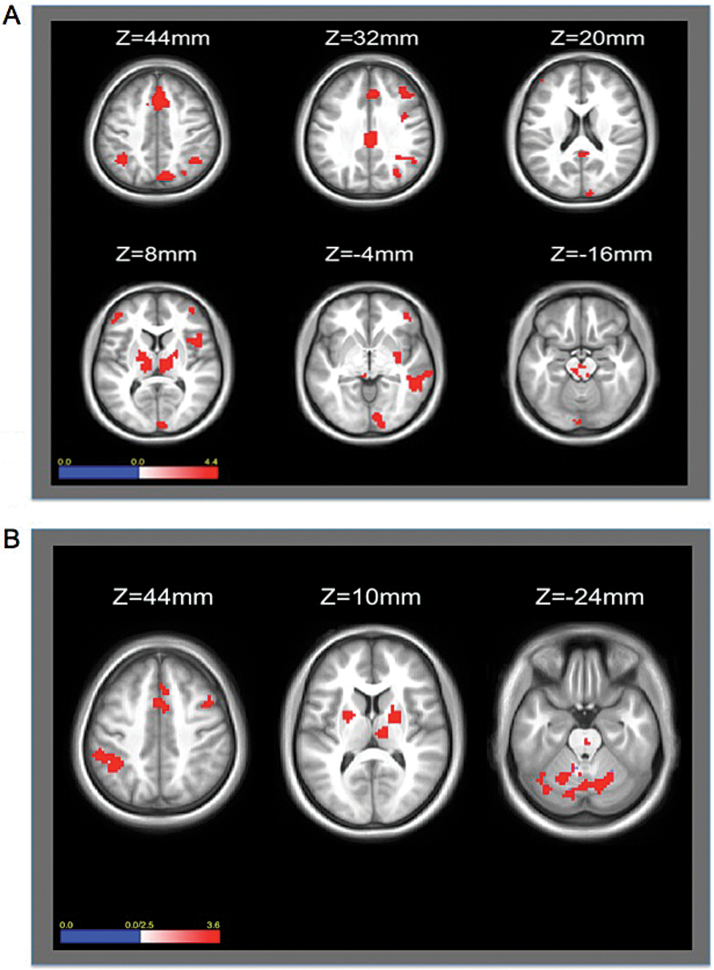
A whole-brain independent samples t-test comparing CN and SZ gropus revealed 32 Region of Interests (ROIs) that showed significantly greater BOLD signal change for the loseshift-winstay contrast for the CN compared with SZ group (figure 1A and table 2). These areas included CCN and striatal regions, but also temporal, occipital, and the cerebellar regions. When comparing activation within each group separately, CN showed significantly greater activity during lose-shift than win-stay trials for all ROIs except for 2 in the posterior cingulate cortex and right putamen (table 2). However, SZ patients only showed significantly greater activity during lose-shift compared with win-stay trials in 7 frontal-parietal regions, and showed significantly less activation for lose-shift compared with win-stay trials in 7 regions including temporal, occipital, cingulate, and striatal ROIs.
Fig. 1.
Group difference (CN-SZ) for Loseshift-Winstay (A) and Final Error-Error Contrasts (B) A: Greater activation for CN compared with SZ during loseshift compared with winstay trials in the anterior cingulate, ventromedial pre-frontal cortex, dorsolateral prefrontal cortext, parietal lobe, and cerebellum. B: Greater activity for CN compared with SZ in the anterior cingulate, posterior parietal cortex, and striatum.
Table 2.
Loseshift-Winstay Contrast
| ROI Location | BA | Talairach | Loseshift-Winstay | Loseshift | Winstay | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | Region Size (mm3) | CN-SZ | CN | SZ | CN-SZ | CN-SZ | ||
| Frontal | ||||||||||
| Inferior Frontal | 41 | 44 | 2 | 68 | 4.02 | 4.46 | 1.24 | 3.66 | −0.18 | |
| Medial Frontal | 6 | 4 | 33 | 37 | 127 | 3.60 | 4.28 | 1.14 | 4.75 | 2.27 |
| Middle Frontal | 10 | −43 | 45 | 11 | 68 | 4.00 | 4.38 | 0.69 | 4.16 | −0.25 |
| Middle Frontal | 9 | 39 | 34 | 36 | 57 | 3.68 | 5.27 | 2.92 | 4.94 | 3.43 |
| Precentral | 44 | 45 | 12 | 11 | 85 | 3.81 | 4.96 | 2.66 | 4.27 | 1.86 |
| Superior Frontal | 6 | 19 | 17 | 54 | 51 | 3.57 | 3.97 | 0.06 | 3.72 | 1.28 |
| Middle Frontal | 6 | 38 | 3 | 37 | 37 | 3.42 | 4.87 | 1.52 | 5.17 | 3.04 |
| Superior Frontal | 8 | 3 | 23 | 52 | 194 | 3.81 | 5.40 | 3.69 | 5.69 | 3.26 |
| Parietal | ||||||||||
| Inferior Parietal | 40 | 35 | −51 | 38 | 58 | 3.36 | 4.35 | 3.49 | 4.54 | 2.37 |
| Inferior Parietal | 40 | −39 | −52 | 45 | 61 | 3.31 | 4.80 | 3.35 | 4.51 | 2.25 |
| Inferior Parietal | 40 | 53 | −49 | 50 | 29 | 3.33 | 4.89 | 2.38 | 3.42 | 0.93 |
| Precuneus | 7 | 12 | −73 | 45 | 100 | 3.67 | 4.13 | 1.34 | 4.81 | 2.45 |
| Precuneus | 19 | 30 | −70 | 34 | 52 | 3.36 | 4.00 | 1.40 | 5.77 | 4.51 |
| Superior Parietal | 7 | 39 | −62 | 53 | 41 | 3.54 | 4.70 | 2.89 | 3.31 | 0.47 |
| Supramarginal | 40 | 50 | −53 | 31 | 26 | 3.13 | 3.61 | 1.12 | 3.53 | 1.60 |
| Anterior Cingulate | ||||||||||
| Anterior Cingulate | 23 | 3 | −27 | 32 | 91 | 3.29 | 4.10 | 0.67 | 4.82 | 2.42 |
| Posterior Cingulate | ||||||||||
| Posterior Cingulate | 23 | 4 | −45 | 22 | 33 | 3.13 | 1.77 | −2.74 | 4.48 | 2.32 |
| Temporal | ||||||||||
| Inferior Temporal | 20 | 51 | −56 | −11 | 12 | 3.34 | 2.19 | −2.39 | 4.67 | 1.94 |
| Middle Temporal | 21 | 64 | −27 | −6 | 28 | 3.99 | 3.65 | −1.13 | 3.58 | 0.71 |
| Right Temporal | 37 | 52 | −41 | −4 | 78 | 4.11 | 3.67 | −0.80 | 4.62 | 1.14 |
| Occipital | ||||||||||
| Lingual Gyrus | 18 | −3 | −91 | −12 | 41 | 3.54 | 2.84 | −1.86 | 2.70 | 0.61 |
| Lingual Gyrus | 17 | 11 | −89 | −1 | 65 | 3.26 | 2.47 | −1.62 | 4.48 | 2.80 |
| Middle Occipital | 18 | 8 | −96 | 15 | 54 | 3.68 | 2.30 | −2.82 | 3.45 | 1.07 |
| Thalamus | ||||||||||
| Medial Dorsal | — | 11 | −18 | 10 | 101 | 3.75 | 3.53 | −0.38 | 6.00 | 2.75 |
| Ventral Lateral | — | −16 | −14 | 9 | 104 | 3.28 | 3.18 | −0.29 | 5.73 | 3.17 |
| Striatum | ||||||||||
| Putamen | — | 27 | −7 | 2 | 51 | 3.55 | 0.93 | −4.52 | 4.71 | 1.92 |
| Putamen | — | 30 | −24 | 0 | 9 | 3.18 | 1.18 | −3.54 | 4.33 | 2.09 |
| Midbrain | ||||||||||
| Red Nucleus | — | 0 | −25 | −12 | 53 | 3.63 | 3.09 | −1.19 | 3.51 | −0.01 |
| Cerebellum | ||||||||||
| Left Cerebellum | — | −36 | −62 | −49 | 111 | 4.62 | 4.17 | −0.34 | 5.20 | 1.51 |
| Posterior Lobe | — | −6 | −78 | −34 | 87 | 4.05 | 3.78 | 0.06 | 4.36 | 1.07 |
| Posterior Lobe | — | −24 | −71 | −33 | 91 | 3.67 | 3.93 | 0.68 | 4.49 | 2.32 |
| Posterior Lobe | — | 5 | −79 | −21 | 17 | 3.22 | 2.25 | −1.92 | 1.39 | −1.39 |
Note: Bold font indicates significance (P < .05); BA, Brodmann Area.
Final Error-Error.
Thirty-one ROIs showed significantly greater BOLD signal change for CN compared with SZ for final error compared with error trials (figure 1B), including dorsolateral prefrontal, parietal, anterior cingulate, thalamic, striatal, and cerebellar regions. When comparing activation within each group separately, CN showed significantly greater activity for all 31 ROIs. However, SZ patients only showed significance for 1 cingulate ROI.
Brain/Behavior Relationships
We conducted correlations between BOLD contrasts and behavioral variables, partialling out diagnosis. To minimize the number of correlations with brain regions from the loseshift-winstay contrast, we grouped the ROIs showing group differences into 8 summary scores reflecting their anatomical location: cingulate, frontal, parietal, cerebellum, striatum, occipital, temporal, thalamus. The pattern of correlations with behavior revealed dramatic differences across anatomical regions. ROIs in parietal, cingulate, and frontal regions significantly correlated with the majority of the behavioral variables (table 3). figure S2 shows scatterplots demonstrating the association between the cingulate, frontal, and parietal ROIs with the number of final errors. In contrast, cerebellar, striatal, occipital, temporal, and thalamic ROIs did not show significant correlations with behavior. Correlations of each individual loseshift-winstay ROI with task behavior are reported in table S2.
Table 3.
Correlations (Behavior and Loseshift-Winstay Contrast)
| Brain Region | Final Error | PE_COR | Winstay | Loseshift | ERROR | IA_Trial | IA_Run |
|---|---|---|---|---|---|---|---|
| Cingulate | 0.311 | 0.346 | 0.277 | −0.178 | −0.302 | −0.258 | 0.224 |
| Frontal | 0.523 | 0.549 | 0.458 | −0.190 | −0.534 | −0.409 | 0.348 |
| Parietal | 0.585 | 0.607 | 0.470 | −0.325 | −0.525 | −0.423 | 0.373 |
| Cerebellum | 0.195 | 0.239 | 0.15 | −0.142 | −0.188 | −0.188 | 0.156 |
| Striatum | 0.165 | 0.235 | 0.179 | −0.041 | −0.201 | −0.142 | 0.114 |
| Occipital | 0.074 | 0.126 | 0.039 | −0.094 | −0.079 | −0.042 | 0.015 |
| Temporal | 0.227 | 0.198 | 0.238 | 0.043 | −0.252 | −0.256 | 0.227 |
| Thalamus | 0.177 | 0.223 | 0.145 | −0.099 | −0.178 | −0.149 | 0.102 |
Note: Bold font indicates significance after correction for multiple comparisons (P < .007)
Correlations between task behavior variables and olanzapine equivalents were calculated.52 We found no significant relationships between task behavior and dose. Correlations between BOLD activity in the winstay-loseshift contrast and dose in the frontal (P = .03) and cingulate (P = .04) regions were significant. However, these differences were not significant after correcting for multiple comparisons, although the sample size was limited. Findings also did not vary as a function of anti-psychotic class. Largely, findings did not vary for behavioral or imaging variables as a function of smoking status, however, patients who smoked did show significantly greater responsivity to positive feedback (winstay_ratio).
In contrast to the loseshift-winstay analyses, there were no significant correlations between activity in the ROIs identified in the Final Error analyses and the 7 behavioral indices (table S3).
Mediation Analyses
Mediation analyses were conducted using the SPSS PROCESS toolbox51 to observe whether group differences in task behavior and biological variables occur because (1) Biological abnormalities lead participants to perform poorly, or (2) Individuals perform poorly and this behavioral difference causes changes in biological variables. Table 4a shows the results of mediation analyzing the effect of diagnosis on task behavior with BOLD activity during the loseshift-winstay contrast as the mediator. The activity in the frontal lobe, parietal lobe, and cingulate cortex completely mediated the relationship between diagnosis and behavior. Importantly, when the analysis was reversed so that each task behavior measure was entered as a mediator of the relationship between each brain region and group, task behavior only partially mediated the relationship between group and BOLD activation (table 4b).
Table 4a.
Mediation Analysis Task Behavior and Diagnostic Group With Brain Activity (Loseshift-Winstay Contrast) as a Mediator
| Cingulate | Frontal | Parietal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct Effect | Indirect Effect | Direct Effect | Indirect Effect | Direct Effect | Indirect Effect | |||||||
| LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | |
| Final Error | −6.29 | 0.86 | −4.14 | −0.67 | −3.01 | 3.96 | −7.57 | −3.25 | −3.36 | 2.91 | −7.05 | −2.44 |
| PE_COR | −8.72 | 4.71 | −7.90 | −1.11 | −2.35 | 10.62 | −15.0 | −6.43 | −3.20 | 8.47 | −14.24 | −4.87 |
| Winstay | −0.12 | 0.02 | −0.07 | −0.011 | −0.06 | 0.08 | −0.15 | −0.06 | −0.08 | 0.057 | −0.12 | −0.04 |
| Loseshift | — | — | — | — | — | — | — | — | −0.03 | 0.06 | 0.01 | 0.07 |
| ERROR | −14.62 | 25.50 | 2.28 | 22.58 | −32.64 | 5.95 | 18.67 | 44.13 | −24.61 | 12.16 | 13.17 | 36.46 |
| IA_Trials | — | — | — | — | −5.96 | 7.64 | 4.44 | 11.43 | −4.08 | 8.90 | 3.17 | 9.78 |
| IA_Runs | — | — | — | — | −0.14 | −0.07 | −0.03 | 0.00 | −1.06 | 1.04 | −1.48 | −0.45 |
Table 4b.
Mediation Analysis Brain Activation (Loseshift-Winstay Contrast) and Diagnostic Group With Task Behavior as a Mediator
| Cingulate | Frontal | Parietal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct Effect | Indirect Effect | Direct Effect | Indirect Effect | Direct Effect | Indirect Effect | |||||||
| LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | LLCI | ULCI | |
| Final Error | −0.11 | −0.02 | −0.05 | −0.01 | −0.12 | −0.05 | −0.06 | -0.01 | −0.11 | −0.03 | −0.07 | −0.01 |
| PE_COR | −0.12 | −0.03 | −0.05 | −0.001 | −0.13 | −0.06 | −0.05 | 0.00 | −0.12 | -0.04 | −0.06 | 0.00 |
| Winstay_Ratio | −0.12 | −0.025 | −0.04 | 0.00 | −0.13 | −0.06 | −0.04 | −0.01 | −0.12 | −0.03 | -0.05 | −0.01 |
| Loseshift_Ratio | — | — | — | — | — | — | — | — | −0.13 | -0.04 | −0.04 | 0.00 |
| ERROR | −0.12 | −0.03 | −0.04 | 0.00 | −0.13 | −0.06 | −0.04 | 0.00 | −0.12 | −0.04 | −0.05 | 0.00 |
| IA Trials | — | — | — | — | −0.13 | −0.06 | −0.04 | −0.01 | −0.12 | −0.03 | −0.05 | −0.01 |
| IA Runs | — | — | — | — | −0.96 | 1.28 | −1.64 | −0.55 | −0.13 | −0.04 | −0.03 | 0.01 |
Note: LLCI, Lower level of CI; ULCI, Upper level of CI. Bold font indicates significance (ie, CI does not include zero). Both the direct effect and indirect effect CIs indicate the CI of the direct and indirect effects with the mediator in the model.
Correlations With Negative Symptoms and Function
Neither average item symptom scores nor self-report measures robustly correlated with task behavior or brain activity during the loseshift-winstay contrast (table S4).
Discussion
The goal of the current experiment was to examine the neural correlates of reversal learning abnormalities in SZ. Consistent with our hypotheses, SZ patients showed decreased task performance compared with CN. These results are consistent with a previous report by Waltz and Gold.6 The results also supported the hypothesis that CN would show greater BOLD activation compared with SZ patients in multiple neural networks, including CCN and striatal regions, during trials that putatively required value updating. However, only CCN regions showed a significant relationship to behavior. Importantly, the relationship between diagnosis and task behavior was also fully mediated by BOLD activation in CCN regions during loseshift-winstay trials. This evidence is consistent with the hypothesis that CCN abnormalities are contributing to the differences in behavior observed between groups. Importantly, we also saw loseshift-winstay BOLD activation differences in the cerebellum, which is consistent with previous literature, which suggests cerebellar activity might be modulated during high conflict situations.53 Our finding suggests a possible deficit in this modulation for SZ patients. Finally, the hypothesis that task behavior and BOLD activation would be related to symptoms and functioning in SZ patients was not supported, including no relationship between measures of anticipatory pleasure and biological/behavioral variables which may have been expected given previous reports. Each of these findings is discussed in detail below.
Much of the research regarding reward-learning in SZ has focused on decreased striatal activation during reward anticipation/receipt. Our analysis also showed that SZ patients had significantly decreased BOLD activation compared with CN in some striatal brain regions (ie, putamen). These results are consistent with previous reports.10–13 However, striatal activity failed to show a relationship to behavior, instead activity in the CCN showed a relationship to behavior. Further, behavioral performance in SZ patients was consistent with a deficit in developing adequate representations of the correct response, and could be interpreted as a deficit in error-monitoring where SZ patients fail to utilize error feedback to drive future decision-making. Specifically, SZ patients took longer to learn the initial rule, demonstrated less efficient winstay-loseshift behavior, and achieved fewer reversals. This is consistent with the hypothesis that deficits of value representation in SZ may result, in part, from abnormalities in selecting information relevant for goal representations, an integral function of the CCN. Importantly, the CCN has been associated with many different processes leaving the specificity of the current result questionable (ie, does the current study illustrate a specific reversal learning abnormality, or a more general cognitive control deficit?). Unfortunately, the current experimental design cannot easily separate these issues.
Our results replicate and extend findings of several previous reports.6,7,17 The behavioral deficits are consistent with previous reports illustrating decreased task performance and greater tendency to shift responses for SZ patients. However, we found, inconsistently with Waltz et al that SZ patients tended to shift more for positive and negative feedback.17 This further supports the role of unstable value representations in SZ during reversal learning. In contrast to Schlagenhauf et al,7 we did not find evidence for altered ventral striatal or Ventral Medial Prefrontal Cortex activation in SZ patients, though we did find altered dorsal striatal activation. Frameworks examining the dissoluble roles of the ventral and dorsal striatum suggest the ventral striatum plays a role in more passive forms of appetitive learning and the dorsal striatum plays a role in more action-contingent learning.54 Our current findings suggest that patient deficits primarily emerge when maintaining reward information for more effective action selection. However, we did not compute model-based prediction error analyses as Schlagenhauf et al did, nor did we use a ventral striatal ROI.7 mportantly, the current study extends the findings of Waltz et al17 by quantifying a relationship between task behavior and the CCN. Our findings are also consistent with Collins et al,55 who illustrated that working-memory deficits made a significant contribution to impairments on a reinforcement-learning task in SZ, with little evidence for alterations in the basic stimulus-response component of reinforcement-learning.
Together this evidence suggests that the integration of multiple neural networks may be essential to understanding value updating in SZ. To test this hypothesis, future studies need to include connectivity analyses to delineate how these networks interact/or fail to interact to produce these deficits. Recent theories have postulated that “task control networks” (including fronto-parietal regions) and “valuation networks” (including striatal regions) show increased levels of functional connectivity during motivational contexts.56,57 Under this framework, one would hypothesize that if value-updating deficits are due to a failure to integrate information between the CCN and reward processing regions, studies utilizing connectivity analyses would find that SZ patients fail to show such an increase in connectivity during rewarding vs nonrewarding contexts.
Limitations
Although we saw differential BOLD activation to final error trials, we did not demonstrate a relationship between behavior and BOLD activation during final error trials. The failure to see a relationship with behavior may have been due to the small number of final error trials per subject. Second, we were unable to replicate an association between reversal learning deficits and negative symptoms.6 However, previously reported associations were trend level, suggesting a weak relationship between these variables.6 Future studies may want to consider utilizing multiple methods (eg, ecological momentary assessment) to assess symptoms that may provide more sensitive indicators of relationships to brain function. Third, we did not use a model-based approach to the analysis of the fMRI data. However, many model-based imaging analyses suffer from poor model fits in the patient group leading to either a reduction in the number of participants or potential confounds in interpretation.7 Given that 1 of our goals was to maximize power for whole brain and correlational analyses, we choose to use a more straightforward approach to imaging analysis that allowed us to maximize sample size. Finally, the vast majority of patients were on anti-psychotic medications, which could have affected reward-related neural responses. However, correlations between task behavior and olanzapine equivalents were nonsignificant, and correlations between BOLD activation in the loseshift-winstay contrast were nonsignificant after multiple comparison correction.
Summary
The current study provides evidence for deficits in multiple neural networks associated with value representation in SZ, including the CCN that was correlated with behavior, suggesting that understanding this network may prove critical to delineating the etiology of value representation deficits. This finding is consistent with multiple reports showing that cognitive control deficits contribute to a wide range of cognitive and affective dysfunction in SZ.58 Future studies involving connectivity analyses will be necessary to further understand how the CCN interacts with other brain regions, such as striatum, to produce decision-making abnormalities. Such investigations will aid in understanding the etiology of these deficits and provide biological targets for further inquiry as researchers attempt to better characterize this decision-making neural circuitry in order to discover new pathways for treatment interventions.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Research was supported by the National Institute of Medical Health grant (MH066031).
Supplementary Material
Acknowledgments
We thank our staff and the participants who gave their time for this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kéri S, Kelemen O, Szekeres G, et al. Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia. Psychol Med. 2000;30:149–155. [DOI] [PubMed] [Google Scholar]
- 2. Bleuler E. Dementia Praecox or the Group of Schizophrenias. Zinkin J, Trans. New York, NY: International UniversitiesPress; 1950. [Google Scholar]
- 3. Kraepelin E. Dementia Praecox and Paraphrenia. Huntington, NY: Krieger Publishing Company; 1971. [Google Scholar]
- 4. Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22:4563–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schlagenhauf F, Huys QJ, Deserno L, et al. Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage. 2014;89:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl). 2006;187:222–228. [DOI] [PubMed] [Google Scholar]
- 9. Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. [DOI] [PubMed] [Google Scholar]
- 10. Gradin VB, Kumar P, Waiter G, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011:awr059. [DOI] [PubMed] [Google Scholar]
- 11. Koch K, Schachtzabel C, Wagner G, et al. Altered activation in association with reward-related trial-and-error learning in patients with schizophrenia. Neuroimage. 2010;50:223–232. [DOI] [PubMed] [Google Scholar]
- 12. Murray G, Corlett P, Clark L, et al. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol Psychiatry. 2007;13:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schlagenhauf F, Sterzer P, Schmack K, et al. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol Psychiatry. 2009;65:1032–1039. [DOI] [PubMed] [Google Scholar]
- 14. Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7:e35622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon JJ, Biller A, Walther S, et al. Neural correlates of reward processing in schizophrenia—relationship to apathy and depression. Schizophr Res. 2010;118:154–161. [DOI] [PubMed] [Google Scholar]
- 16. Waltz JA, Schweitzer JB, Ross TJ, et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waltz JA, Kasanova Z, Ross TJ, et al. The roles of reward, default, and executive control networks in set-shifting impairments in schizophrenia. PloS One. 2013;8:e57257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Linke J, Kirsch P, King AV, et al. Motivational orientation modulates the neural response to reward. Neuroimage. 2010;49:2618–2625. [DOI] [PubMed] [Google Scholar]
- 19. Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. [DOI] [PubMed] [Google Scholar]
- 20. Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371–1383. [DOI] [PubMed] [Google Scholar]
- 21. Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006;16:475–486. [DOI] [PubMed] [Google Scholar]
- 22. Rüsch N, Spoletini I, Wilke M, et al. Prefrontal–thalamic–cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 2007;93:79–89. [DOI] [PubMed] [Google Scholar]
- 23. Selemon L, Rajkowska G. Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr Mol Med. 2003;3:427–436. [DOI] [PubMed] [Google Scholar]
- 24. Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. [DOI] [PubMed] [Google Scholar]
- 25. Yildiz M, Borgwardt SJ, Berger GE. Parietal lobes in schizophrenia: do they matter? Schizophr Res Treatment. 2011;2011:581686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barch DM, Csernansky JG, Conturo T, Snyder AZ. Working and long-term memory deficits in schizophrenia: is there a common prefrontal mechanism? J Abnorm Psychol. 2002;111:478. [DOI] [PubMed] [Google Scholar]
- 27. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Semkovska M, Bédard M-A, Godbout L, Limoge F, Stip E. Assessment of executive dysfunction during activities of daily living in schizophrenia. Schizophr Res. 2004;69:289–300. [DOI] [PubMed] [Google Scholar]
- 29. Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. [DOI] [PubMed] [Google Scholar]
- 30. Morris SE, Heerey EA, Gold JM, Holroyd CB. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr Res. 2008;99:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paulus MP, Frank L, Brown GG, Braff DL. Schizophrenia subjects show intact success-related neural activation but impaired uncertainty processing during decision-making. Neuropsychopharmacology. 2003;28:795–806. [DOI] [PubMed] [Google Scholar]
- 32. Paulus MP, Hozack NE, Zauscher BE, et al. Parietal dysfunction is associated with increased outcome-related decision-making in schizophrenia patients. Biol Psychiatry. 2002;51:995–1004. [DOI] [PubMed] [Google Scholar]
- 33. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40:S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/p. 2/2001 Revision). New York: Biometrics Research Department, New York State Psychiatric Institute; 2001. [Google Scholar]
- 35. Andreasen NC. Scale for the Assessment of Positive Symptoms:(SAPS). Iowa City: University of Iowa; 1984. [Google Scholar]
- 36. Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: University of Iowa; 1983. [Google Scholar]
- 37. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374. [DOI] [PubMed] [Google Scholar]
- 39. Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J Abnorm Psychol 1978;87:399. [DOI] [PubMed] [Google Scholar]
- 40. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Snaith R, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. [DOI] [PubMed] [Google Scholar]
- 42. Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4:134–139. [DOI] [PubMed] [Google Scholar]
- 43. Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. 1983;19:9–21. [DOI] [PubMed] [Google Scholar]
- 44. Friston K, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum Brain Mapp. 1994;1:153–171. [Google Scholar]
- 45. Snyder A. Difference image versus ratio image error function forms in PET-PET realignment. In: Myers R, Cunningham V, Bailey D, Jones T, eds. Quantification of Brain Function Using PET. San Diego, CA: Academic Press; 1996:131–137. [Google Scholar]
- 46. Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992;16:620–633. [DOI] [PubMed] [Google Scholar]
- 47. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 48. Ollinger J, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI: I. The method. Neuroimage. 2001;13:210–217. [DOI] [PubMed] [Google Scholar]
- 49. Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. [DOI] [PubMed] [Google Scholar]
- 50. McAvoy M, Ollinger J, Buckner R. Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage. 2001;13:198. [Google Scholar]
- 51. Hayes AF. PROCESS: A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling [White paper]. 2012. http://www.afhayes.com/public/process2012.pdf. [Google Scholar]
- 52. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Psychopharmacology. 2014;12:235–243. [DOI] [PubMed] [Google Scholar]
- 53. Egner T, Hirsch J. The neural correlates and functional integration of cognitive control in a Stroop task. Neuroimage. 2005;24:539–547. [DOI] [PubMed] [Google Scholar]
- 54. O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. [DOI] [PubMed] [Google Scholar]
- 55. Collins AGE, Brown JK, Gold JM, Waltz JA, Frank MJ. Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci. 2014;34:13747–13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Braver TS, Krug MK, Chiew KS, et al. Mechanisms of motivation–cognition interaction: challenges and opportunities. Cogn Affect Behav Neurosci. 2014;14:443–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kinnison J, Padmala S, Choi J-M, Pessoa L. Network analysis reveals increased integration during emotional and motivational processing. J Neurosci. 2012;32:8361–8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.