Abstract
Introduction. Schizophrenia is associated with deficits in face and affect recognition, which contribute to broader social functioning deficits. The present aim was to conduct a meta-analysis of early face processing in schizophrenia, as indexed by the P100 event-related potential component. Methods. Twelve studies (n = 328 patients with schizophrenia, n = 330 healthy controls) of the P100 component during face processing were evaluated by calculating Cohen’s d for each study and overall weighted mean effect size (ES). In additional exploratory analyses, moderating influences of method and design were investigated, and the P100 component during face processing was evaluated based on valence: 5 studies (n = 225 patients, n = 225 controls) included neutral stimuli, 5 studies (n = 225 patients, n = 225 controls) included happy stimuli, and 4 studies (n = 209 patients, n = 209 controls) included fearful stimuli. Results. The amplitude of the P100 to face stimuli was smaller in patients relative to controls (ES = .41, P < .01). Methodological or design differences did not account for heterogeneity in ES. When split by valence, results indicate smaller P100 in patients relative to control subjects in response to neutral (ES = .32, P < .001) and happy (ES = .21, P < .05) stimuli, whereas there was no difference in response to fearful faces (ES = .09, P > .05). Discussion. The results indicate that P100 amplitude in response to faces is smaller in patients with schizophrenia, showing that socially relevant visual processing deficits begin earlier in processing than previously suggested. Additionally, the exploratory analyses suggest emotional specificity in these deficits. Ramifications for our understanding of face processing deficits and treatment development are discussed.
Key words: P100 component, emotion, ERP
Schizophrenia is a disorder that affects 1% of the population, and is characterized by social deficits that contribute to the marked impairment and related disability.1 Face processing is a key aspect of these social deficits, and has been the focus of many studies of schizophrenia.2 In order to assess the underlying mechanisms, many studies have examined the neural correlates of face and affect processing deficits in this population. In this regard, the use of electroencephalography (EEG) to examine event-related potentials (ERP) in response to faces is a particularly powerful investigative methodology as the high temporal resolution can give insight into underlying mechanisms and differential stages of processing. The majority of ERP studies of face processing in schizophrenia have focused on the N170 component, which is reflective of holistic processing of objects of expertise, such as faces.3 However, deficits may stem from earlier visual processing stages, such as those indexed by the P100 component, and to date, there has been no systematic review focusing on this component. Furthermore, while some of the related studies have shown significant differences between patients with schizophrenia relative to healthy controls in several key components that are associated with the processing of faces,4–8 findings reported in the critical early processing stages have been mostly inconsistent.9,10 The present review examines whether there are discrepancies in early visual processing, as indexed by the P100 ERP component, between patients with schizophrenia and healthy controls and aims to determine if differences in methodology and emotional valence of facial stimuli used between studies contribute to discrepant findings in this area.
A recent meta-analysis was conducted to examine discrepancies in amplitude between patients with schizophrenia and healthy controls for 2 event-related potentials that have been associated with face processing;11 the N170, a negative deflection occurring approximately 170ms after stimulus onset, and the N250, a negative deflection occurring approximately 250ms after stimulus onset. The amplitude of the N170 component at lateral electrode sites has been shown to be larger for human faces relative to nonhuman faces or novel categories that are not objects of visual expertise12 and is thought to reflect basic structural encoding.13,14 The N250 has been associated with face15 and object16 familiarity. In an analysis of 21 (N170: mean weighted effect size (ES) =.64) and 6 (N250: ES = .49) studies of face processing, McCleery and colleagues11 observed that both of these components were shown to differ between patients and healthy controls, giving clear evidence of discrepancies related to face processing in patients.
Although McCleery and colleagues11 demonstrates that there are facial processing discrepancies between patients and controls, the meta-analysis was limited to the N170 and N250 components. This approach provides an important perspective of the psychophysiological correlates of facial processing, but to date, our understanding of the body of literature focusing on the integral early sensory processing function remains very limited. As decreased activation in brain regions associated with early visual processing of faces, such as the occipital and fusiform gyri, has been shown for patients with schizophrenia relative to healthy controls,17 the earliest stages of face processing are important to consider. Indeed, it has been shown that meaningful face-specific categorization begins at around 100ms after stimulus onset, as indexed by the P100 component.18,19 Originally thought to reflect only basic visual processing,20 and early visual attention,21–23 an emerging literature suggests that the P100 is modulated during face processing. Specifically, studies have shown greater P100 amplitude during visual processing of faces relative to nonface objects,19,24,25 and P100 modulation by emotional expression of faces.5,26–31 For example, Pourtois and colleagues29 found enhanced P100 amplitude to fearful faces in the general population, and Kolassa and Miltner31 found enhanced P100 to angry and happy faces relative to neutral in both people with social anxiety and healthy controls. Therefore, the P100 component may provide important information about underlying mechanisms in the deficits in face and affect recognition in psychiatric disorders such as schizophrenia. Given the broader literature which suggests that patients with schizophrenia exhibit deficits in processing specific types of faces,32 and that underlying abnormalities appear to be present at several stages in affective recognition,33 synthesizing the available P100 literature is an important first step in both understanding core deficits and initiating targeted treatment approaches.
As noted, several individual studies have shown deficits in early visual processing in patients with schizophrenia, as indexed by the P100 component.4–8 In fact, 1 study has found discrepancies in P100 amplitude between individuals at-risk for schizophrenia and healthy controls, suggesting that these early processing deficits may be indicative of vulnerability to psychosis.34 However, these results have been variable, as many studies have found no differences in P100 amplitude between patients and controls.9,10,35–42 Methodological differences, such as the use of emotional vs nonemotional stimuli, may contribute to these discrepancies in the literature. It is important to address whether social processing deficits in schizophrenia, specifically face and affect processing, begin from atypicalities in early perceptual processes. Furthermore, understanding how methodological or sampling differences (eg, method of P100 extraction, inclusion of emotional faces) may contribute to discrepant findings is a vital step for moving forward. The current quantitative review aims to examine (1) whether the overall mean ES for the P100 in response to face stimuli show differential activation for patients with schizophrenia relative to healthy controls and (2) whether there are any potential moderators of this effect (eg, examining mean vs peak P100 amplitude, use of emotional vs nonemotional stimuli). Further, in a set of exploratory analyses we aimed to examine independent emotional expressions, to see whether the overall mean ES for P100 in response to specific emotions (happiness, fear, and neutral) show differential activation for patients relative to healthy controls.
Methods
Eligibility Criteria
Inclusion criteria for the analysis were as follows: (1) the study included a sample of patients meeting criteria for schizophrenia (other disorders, including schizoaffective disorder, were not included) and a healthy control group, (2) the study stimuli included faces, (3) EEG was recorded while participants viewed faces, (4) statistics were reported that allowed calculation of the P100 component, defined as a positive deflection peaking 50–170ms post stimulus onset in the occipital region,43 for both the patient and control group, and (5) the manuscript was written in English and published in a peer-reviewed journal. The cutoff date for the literature search was December 1, 2014.
Study Selection
The literature search was conducted using PubMed and Google Scholar with the following search terms: Schiz* AND P100 OR P1 AND fac*, which resulted in 375 articles. These articles were examined for eligibility and the citations were cross-referenced. Fifteen of the articles met eligibility criteria. Three articles did not provide sufficient information to calculate ES and attempts to obtain the data from the corresponding authors were unsuccessful.36,38,41 Thus, 12 (n = 328 patients, n = 330 controls) full-text articles were included in the current analyses. For the study characteristics, see table 1. For the mean characteristics of the patient samples in each study, see table 2.
Table 1.
Study Characteristics
| Study | n SZ:Ctrl | Face Stimuli | Expressions | Stimuli Color | Task | ERP |
|---|---|---|---|---|---|---|
| Brennan et al4 | 108:108 | NR | Angry, disgust, fearful, happy, neutral, sad | NR | No response required | Peak |
| Caharel et al5 | 18:18 | Self-face, 1 familiar face, 2 unknown faces | Disgust, happy, neutral | Grey scale | Respond to familiarity | Peak |
| Campanella et al6 | 14:7 | Ekman and Friesen | Happy, fearful, neutral, sad | NR | Respond to deviant face | Peak |
| Herrmann et al10 | 24:28 | 12 different faces | Neutral | Grey scale | Silently categorize face or building | Peak |
| Jetha et al37 | 40:39 | Ekman and Friesen | Angry, happy, fearful, neutral | Grey scale | No response required | Peak |
| Jung et al39 | 23:24 | Chaelee face | Fearful, happy, neutral | Color | Respond to emotional face | Peak |
| Lee et al40 | 38:38 | Chaelee face | Fearful, happy, neutral | NR | Respond to happy or fearful face | Peak |
| Müller et al7 | 15:15 | Facial emotions for brain activation inventory | Fearful, happy, neutral | Color | Rate expression | Mean |
| Obayashi et al35 | 16:23 | JACFEE and JACNeuF | Angry, happy, fearful, neutral | Grey scale | Respond to image of shoes | Peak |
| Thoma et al8 | 14:15 | NimStim | Happy, fearful, neutral, sad | Grey scale | Respond to congruency of emotional valence (body/face) | Peak |
| Turetsky et al42 | 16:16 | Penn facial emotion stimuli | Happy, neutral, somewhat sad, very happy, very sad | Grey scale | No response required (NR) | Mean |
| Wynn et al9 | 26:27 | Ekman and Friesen | Afraid, angry, ashamed, happy, sad, surprised | Grey scale | Classification (gender, emotion, or building) | Mean |
Note: ERP, Event-Related Potentials; Ctrl, Healthy Controls; NR, Not Reported; SZ, Schizophrenia Patients; JACFEE, Japanese and Caucasian Facial Expressions of Emotion; JACNeuF, Japanese and Caucasian Neutral Faces.
Table 2.
Mean Characteristics of Patient Samples
| Study | Age | Gender M:F | Clinical Status | Symptom Ratings | Illness Duration (y) | Medication Dosing (mg) |
|---|---|---|---|---|---|---|
| Brennan et al4 | 20.7 (2.9) | 70:38 | Outpatient | PANSS total: 61.14 (18.25) | 26.73 (55.34) (wk) | 390.31 (193.58) CPZ equiv |
| Caharel et al5 | 37.7 (8.29) | 10:8 | Inpatient and outpatient | NR | 13.6 (9.97) | 363.9 (297.44) CPZ equiv |
| Campanella et al6 | 47.7 (11.9) | 9:5 | Inpatient | PANSS total: 88 (15) for low; 115 (17) for high | NR | 9.01 (10.3) haloperidol equiv |
| Herrmann et al10 | 32.3 (10) | 19:5 | NR | PANSS total: 25.3 (7.1) | NR | 666 (430) CPZ equiv |
| Jetha et al37 | 42.4 (6.4) | 28:12 | Outpatient | PANSS total: 26 (5.5) | 22.63 (5.9) | NR |
| Jung et al39 | 32.2 (10.1) | 12:11 | NR | PANSS total: 81.8 (25.8) | 5.2 (4.9) | NR |
| Lee et al40 | 30.2 (10.3) | 16:22 | NR | PANSS 83.5 (22.8) | 4.3 (3.3) | 398 (103) M; 387 (120) F |
| Müller et al7 | 35.1 (9.26) | 11:4 | Outpatient | SAPS total: 29.80 (21.6); SANS total: 37.8 (13.3) | 14.33 (9.12) | NR |
| Obayashi et al35 | 32.9 (10) | 16:0 | NR | SAPS total: 7.6 SANS total: 12.5 (SDs NR) | 9.9 (7.3) | 487 (319) CPZ equiv |
| Thoma et al8 | 34.1 (11.2) | 8:6 | Inpatient and outpatient | NR | NR | 619.4 (500.5) CPZ equiv |
| Turetsky et al42 | 30.5 (6) | 12:4 | Outpatient | SAPS total: 1.4 (0.7) M; 0.3 (0.3) F; SANS total: 1.8 (0.7) M; 0.9 (0.3) F | 7.5 (5.5) M; 10.5 (8.3) F | NR |
| Wynn et al9 | 43.9 (10.2) | 21:5 | Outpatient | BPRS total 44.3 (8.9) | NR | NR |
Note: BPRS, Brief Psychiatric Rating Scale; CPZ equiv, chlorpromazine equivalent; F, Female; M, Male; NR, Not Reported; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms.
Analyses
The primary variable of interest was the amplitude of the P100 ERP component in response to face stimuli for patients with schizophrenia compared with healthy control subjects (although definitions of the P100 differed by study, all studies included a positive deflection within the range of 50–170ms poststimulus onset). For each study, pooled ES (Cohen’s d) was calculated to define the differences in P100 amplitude for the patient and control groups. Cohen’s d was defined as the difference between group amplitudes divided by pooled within-group SD of both groups.44 For the primary meta-analysis, each independent study was represented by 1 ES. When studies reported nonindependent results (eg, separated by hemisphere, multiple patient groups), conservative methods were used to calculate an independent standardized ES for each study.45,46 Specifically, 1 study reported results based on 1 control sample against multiple patient samples (patients high on symptom ratings, n = 7, and low on symptom ratings, n = 7),6 so separate ES were calculated for each patient group and the weighted mean was used for analysis.45 Most studies reported P100 data at the average of occipital electrodes. To avoid duplication of analyses, when studies reported the amplitude separately by hemisphere,4,8,37,40 we entered the mean between the hemispheres.45,46 The standardized ES were analyzed using random effects meta-analysis. For comparison of moderator variables in the primary meta-analysis, the Q test on heterogeneity between groups was used. Publication bias (the increased probability of statistically significant results to be published) was assessed using Egger’s test as well as the graphical funnel plot method. An asymmetrical funnel plot would suggest publication bias due to smaller negative studies not appearing in the literature.47 In all analyses, a positive ES is indicative of greater amplitude in healthy controls relative to patients.
Results
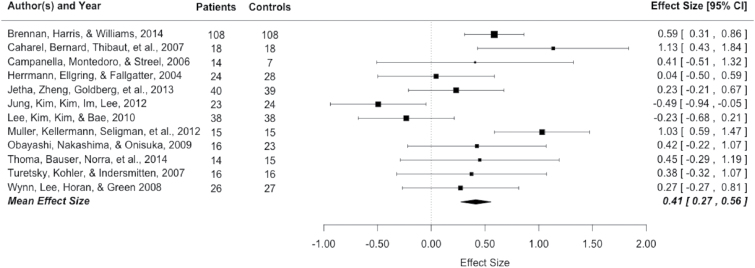
This analysis is based on 328 patients and 330 healthy controls over 12 studies. The mean weighted ES of the 12 studies was of small to medium magnitude (ES = .41, SE = .07, 95% CI: .27, .56) with the P100 amplitude of patients being smaller than that of healthy controls (figure 1). This weighted mean ES differed significantly from zero (z = 5.72, P ≤ .01). The distribution of the ES indicated heterogeneity (Q 11 = 28.04, P ≤ .01), therefore the dispersion of ES is greater than expected from sampling error. The funnel plot was symmetrical and Egger’s regression test of funnel plot asymmetry was not significant (intercept = −.17, SE = 1.8, P = .93, CI: −1.52, 2.68), which does not suggest publication bias.
Fig. 1.
P100 Effect Sizes and forest plot.
Moderating Variables
Moderating variables were tested using a mixed-effects model which showed that the ratio of males to females in the patient sample, average age of patients, average medication dosage, nor average illness duration were significant moderators (all P > .4). The Q statistic indicated that the distribution of P100 ES departed from homogeneity. Therefore, we conducted follow-up analyses to examine whether specific methodological differences impacted the results. Method of P100 extraction (mean or peak) did not account for significant heterogeneity in ES [ESpeak (n = 9) = .35, SE = .08, ESmean (n = 3) = .65, SE = .16, Q between1 = 1.16, P > .2]. Additionally, task (passive or active response) did not account for significant heterogeneity in ES [ESpass (n = 4) = .41, SE = .1, ESact (n = 8) = .33, SE = .1, Q between1 = 0.36, P > .5]. Finally, differences in design that involved the inclusion of emotional faces (rather than only using neutral faces) did not account for significant heterogeneity in ES [ESemo (n = 10) = .39, SE = .09, ESnon (n = 2) = .46, SE = .12, Q between1 = 0.01, P > .9]. However, only 2 articles did not involve the inclusion of emotional faces. For the other 10 articles, the standardized mean ES is reflective of the average of different emotional expressions. As emotion-specific deficits have been found in schizophrenia,48–50 it is important to examine whether deficits in this processing stage differ by the valence of face stimuli. Thus, further analyses were conducted to assess the impact of specific emotions when information was available. A small but sufficient amount of studies reported independent information for happy,4,37,39,40,42 fearful,4,37,39,40 and neutral4,10,37,39,42 stimuli.
Valence of Face Stimuli
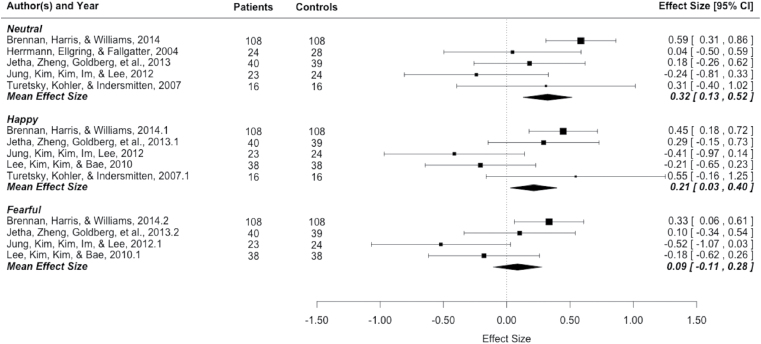
As previously stated, we were interested in conducting exploratory analyses to examine whether differences in P100 amplitude between patients and controls were dependent upon the emotional valence of the presented faces. Therefore, standardized mean ES were calculated independently for happy, neutral, and fearful faces when information was provided to do so. To prevent duplication of analyses or reporting on nonindependent samples, 3 separate small meta-analyses were conducted. The results are as follows and can be seen in figure 2.
Fig. 2.
P100 Effect Sizes and forest plot by meta-analyses based on emotional valence.
Neutral.
The analysis of neutral faces is based on 225 patients and 225 healthy controls over 5 studies.4,10,37,39,42 The mean weighted ES was of medium magnitude (ES = .32, SE = .09, 95% CI: .13, .52) and differed significantly from zero (z = 3.72, P ≤ .001). P100 amplitude while viewing neutral faces was greater for healthy controls relative to patients.
Happy.
The analysis of happy faces is based on 225 patients and 225 healthy controls over 5 studies.4,37,39,40,42 The mean weighted ES was of small magnitude (ES = .21, SE = .09, 95% CI: .03, .40), and differed significantly from zero (z = 2.27, P ≤ .05). P100 amplitude was greater for controls relative to patients when viewing happy faces.
Fearful.
The analysis of fearful faces is based on 209 patients and 209 healthy controls over 4 studies.4,37,39,40 The mean weighted ES was of small magnitude (ES = .09, SE = .1, 95% CI: −.11, .28) and did not differ significantly from zero (z = 0.87, P > .05), therefore there was no difference in P100 amplitude in response to viewing fearful faces for patients relative to controls.
Discussion
Efficient early perceptual encoding of faces is an important step for later recognition. The current review is the first comprehensive study to suggest that deficits in early visual processing are present in schizophrenia. More specifically, the results of the current meta-analysis show that there are consistent deficits in early visual processing in patients with schizophrenia, as indexed by the P100 component. This suggests that deficits in face processing in patients may begin earlier in processing than previously suggested and that an early sensory processing deficit may precede higher-order processing deficits as indexed by the N170 component. Importantly, our moderator analyses suggest that these perceptual deficits may depend on facial expression.
Much previous research has shown that patients with schizophrenia exhibit deficits in processing faces and facial affect,32 which has been associated with social functioning deficits.51,52 The present analysis also provides some evidence to suggest that early perceptual processing deficits are specific to neutral faces and faces portraying certain emotional expressions. In our 3 exploratory meta-analyses, it was found that patients show lower P100 amplitude to faces exhibiting neutral or happy expressions, but there is no difference between patients and controls for fearful expressions.
Our findings of emotional specificity in the processing deficits found in patients with schizophrenia are consistent with previous literature.48–50 For example, studies have found differential neural patterns of activation for negative vs neutral or positive facial expressions, suggesting that deficits in face and affect processing may be unique to specific emotions. Gur and colleagues53 demonstrated differential limbic region activation for patients with schizophrenia relative to controls when viewing angry and fearful faces. Differential amygdalar responses to happy but not to fearful expressions have also been demonstrated in patients with schizophrenia,54 although other studies have shown reduced amygdalar responses to fearful expressions.55,56 Importantly, Holt and colleagues57 found reduced hippocampal habituation to fearful faces in patients relative to controls. However, no differenc`es in activation to fearful faces between patients and controls were found in initial responses. Specifically, during 80-s blocks, initial hippocampal activation (blood oxygenation level-dependent [BOLD] signal during the first 40 s of repeated presentation) did not differ between patients and controls, whereas later activation (BOLD signal during last 40 s) decreased for controls but did not decrease for patients. The authors concluded that deficits in fearful face processing in patients with schizophrenia are not associated with a reduction in initial activation. Together with the current analysis, these findings suggest that deficits in fearful face processing in schizophrenia are due to later processing stages. Initial stages of face perception may only be impaired in patients when viewing neutral or positive valence faces, and may indeed be intact when viewing negative valence faces.
The primary limitation of the literature in this area relates to the small number of studies that report on the P100 component, and the smaller number of studies that report based on independent emotional expressions. As social processing deficits are robust in schizophrenia, it is important to examine the early perceptual processes associated with these deficits. As evidenced by our exploratory analysis, the literature would benefit from a detailed exploration of the deficits in recognition and processing of specific emotions, and the underlying neural impairments associated with these. Additionally, our analyses on specific emotions were limited to happy, fearful, and neutral faces. This was due to an underrepresentation of other emotional stimuli (eg, angry, sad, surprised) in the literature. The increased use of multimodal approaches, as well as a greater representation of multiple emotional categories, would aid in our understanding of the detrimental social processing deficits in schizophrenia.
As noted previously, the P100 component has been associated with basic visual processing20,58 and spatial attention,21–23 and has only recently been shown to be modulated by factors such as emotional expression in face processing. This is important in that the findings of this review may extend beyond face processing and reflect deficits in basic visual encoding in schizophrenia. Indeed, studies have shown deficits in P100 amplitude in schizophrenia when encoding nonface stimuli, such as fragmented images59,60 and simple visual stimuli differing in luminance and contrast.61 It has been suggested that early sensory processing are associated with early dorsal, rather than ventral, visual stream processing,62 and that these deficits may be associated with poor working memory performance.63 In fact, the P100 component has been successfully (80%–90% accuracy) used as a classifier for schizophrenia while patients and controls viewed basic visual images.64 It has been suggested that these deficits are a function of impairments in visual attention in patients relative to healthy controls65 and/or general deficits in early-stage visual processing.61 As all of the studies and stimuli types used in the fearful analysis were also included in the neutral and happy analyses, the differences between groups based on emotion are unlikely to be the result of differences in lower-level visual cues, such as contrast or color. However, it has been suggested that negative emotions are less discriminable66 and this is exacerbated in patients due to generalized perceptual deficits.67 The current findings may also be indicative of a decrease in visual attention to faces in general in patients with schizophrenia. Indeed, there has been evidence of an attentional advantage of emotional relative to neutral stimuli in healthy controls but not in patients.68 As our moderator analyses show the largest difference between patients and healthy controls when viewing neutral faces, it is possible that patients initially process all faces, regardless of valence, in a manner similar to the processing of fearful faces in healthy controls. Future research is needed to examine these possibilities.
When developing potential remediations, the findings of the current analysis highlight important aspects to consider. First, face processing deficits occur earlier in visual processing than previously suggested, and therefore, social functioning remediations should focus on early stages of processing as well. Second, there may be emotional specificity of deficits in face processing in schizophrenia at different stages of processing. Given that neutral and positively valence faces are affected by early perceptual stages of processing, whereas negatively valence faces may be affected by later processing, different treatments or training strategies are necessary to improve face processing in schizophrenia. Specifically, neutral and positive expressions may benefit from basic perceptual face training paradigms, whereas a focus on higher order cognition may improve processing and recognition involving negative expressions. Indeed, improvements in emotion recognition, as demonstrated both behaviorally and with ERP responses, has been shown following cognitive interventions.69 Importantly, Training on Affect Recognition (TAR) has been successful in patients with schizophrenia.70–75 Interestingly, TAR differentially impacts certain emotions, such that training improves recognition of happiness and sadness, but not fear, anger, and disgust.73 Use of training has also been successful in increasing face recognition accuracy in children76 and adults77 with autism spectrum disorders, and training in this population has been shown to modulate the P100 but not the N170 component.78 Therefore, individuals with schizophrenia may also benefit from perceptual expertise training, but this may be limited to specific emotions.
The current analysis examined whether early perceptual deficits, as indexed by the ERP P100 component, are present in patients with schizophrenia relative to healthy controls. We have found that P100 amplitude to faces is reliably decreased in patients, suggesting that deficits in face processing in this population begin earlier than previously expected. Additionally, there is some evidence that these deficits are emotion specific. We believe this is an important step towards understanding at what stage face processing deficits occur. Future directions should aim to examine how deficits in face processing may relate to other generalized perceptual deficits, and how these deficits, as indexed by the P100, relate to later stages of processing, as indexed by components such as the N170 and N250. Finally, it would be of importance to examine whether these deficits are specific to schizophrenia or extend to other clinical populations.
Funding
National Institutes of Health (R01MH096698 to H.A.E. and T.C.); National Science Foundation (#SMA-1041755 to the Temporal Dynamics of Learning Center). National Institutes of Health (R01MH094650, R21/R33MH103231 to V.M.)
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marwick K, Hall J. Social cognition in schizophrenia: a review of face processing. Br Med Bull. 2008;88:43–58. [DOI] [PubMed] [Google Scholar]
- 3. Gauthier I, Curran T, Curby KM, Collins D. Perceptual interference supports a non-modular account of face processing. Nat Neurosci. 2003;6:428–432. [DOI] [PubMed] [Google Scholar]
- 4. Brennan AM, Harris AW, Williams LM. Neural processing of facial expressions of emotion in first onset psychosis. Psychiatry Res. 2014;219:477–485. [DOI] [PubMed] [Google Scholar]
- 5. Caharel S, Bernard C, Thibaut F, et al. The effects of familiarity and emotional expression on face processing examined by ERPs in patients with schizophrenia. Schizophr Res. 2007;95:186–196. [DOI] [PubMed] [Google Scholar]
- 6. Campanella S, Montedoro C, Streel E, Verbanck P, Rosier V. Early visual components (P100, N170) are disrupted in chronic schizophrenic patients: an event-related potentials study. Neurophysiol Clin. 2006;36:71–78. [DOI] [PubMed] [Google Scholar]
- 7. Müller VI, Kellermann TS, Seligman SC, Turetsky BI, Eickhoff SB. Modulation of affective face processing deficits in Schizophrenia by congruent emotional sounds. Soc Cogn Affect Neurosci. 2014;9:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thoma P, Soria Bauser D, Norra C, Brüne M, Juckel G, Suchan B. Do you see what I feel?–Electrophysiological correlates of emotional face and body perception in schizophrenia. Clin Neurophysiol. 2014;125:1152–1163. [DOI] [PubMed] [Google Scholar]
- 9. Wynn JK, Lee J, Horan WP, Green MF. Using event related potentials to explore stages of facial affect recognition deficits in schizophrenia. Schizophr Bull. 2008;34:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. Am J Psychiatry. 2004;161:915–917. [DOI] [PubMed] [Google Scholar]
- 11. McCleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF. Meta-analysis of face processing event-related potentials in schizophrenia. Biol Psychiatry. 2015;77:116–126. [DOI] [PubMed] [Google Scholar]
- 12. Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological Studies of Face Perception in Humans. J Cogn Neurosci. 1996;8:551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eimer M. Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clin Neurophysiol. 2000;111:694–705. [DOI] [PubMed] [Google Scholar]
- 14. Sagiv N, Bentin S. Structural encoding of human and schematic faces: holistic and part-based processes. J Cogn Neurosci. 2001;13:937–951. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka JW, Curran T, Porterfield AL, Collins D. Activation of preexisting and acquired face representations: the N250 event-related potential as an index of face familiarity. J Cogn Neurosci. 2006;18:1488–1497. [DOI] [PubMed] [Google Scholar]
- 16. Pierce LJ, Scott LS, Boddington S, Droucker D, Curran T, Tanaka JW. The n250 brain potential to personally familiar and newly learned faces and objects. Front Hum Neurosci. 2011;5:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seiferth NY, Pauly K, Kellermann T, et al. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34:477–487. [DOI] [PubMed] [Google Scholar]
- 18. Herrmann MJ, Ehlis AC, Muehlberger A, Fallgatter AJ. Source localization of early stages of face processing. Brain Topogr. 2005;18:77–85. [DOI] [PubMed] [Google Scholar]
- 19. Herrmann MJ, Ehlis AC, Ellgring H, Fallgatter AJ. Early stages (P100) of face perception in humans as measured with event-related potentials (ERPs). J Neural Transm. 2005;112:1073–1081. [DOI] [PubMed] [Google Scholar]
- 20. Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–430. [DOI] [PubMed] [Google Scholar]
- 21. Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. [DOI] [PubMed] [Google Scholar]
- 22. Hillyard SA, Mangun GR, Woldorff MG, Luck SJ. Neural systems mediating selective attention. In: Gazzaniga MS, ed. The Cognitive Neurosciences. Cambridge, MA: The MIT Press; 1995:665–681. [Google Scholar]
- 23. Di Russo F, Spinelli D. Electrophysiological evidence for an early attentional mechanism in visual processing in humans. Vision Res. 1999;39:2975–2985. [DOI] [PubMed] [Google Scholar]
- 24. Halit H, de Haan M, Johnson MH. Modulation of event-related potentials by prototypical and atypical faces. Neuroreport. 2000;11:1871–1875. [DOI] [PubMed] [Google Scholar]
- 25. Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. Neuroimage. 2002;15:353–372. [DOI] [PubMed] [Google Scholar]
- 26. Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Brain Res Cogn Brain Res. 2003;17:613–620. [DOI] [PubMed] [Google Scholar]
- 27. Eger E, Jedynak A, Iwaki T, Skrandies W. Rapid extraction of emotional expression: evidence from evoked potential fields during brief presentation of face stimuli. Neuropsychologia. 2003;41:808–817. [DOI] [PubMed] [Google Scholar]
- 28. Pizzagalli D, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: an ERP study. Neuroreport. 1999;10:2691–2698. [DOI] [PubMed] [Google Scholar]
- 29. Pourtois G, Grandjean D, Sander D, Vuilleumier P. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex. 2004;14:619–633. [DOI] [PubMed] [Google Scholar]
- 30. Williams LM, Liddell BJ, Rathjen J, et al. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp. 2004;21:64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolassa IT, Miltner WH. Psychophysiological correlates of face processing in social phobia. Brain Res. 2006;1118:130–141. [DOI] [PubMed] [Google Scholar]
- 32. Mueser KT, Doonan R, Penn DL, et al. Emotion recognition and social competence in chronic schizophrenia. J Abnorm Psychol. 1996;105:271–275. [DOI] [PubMed] [Google Scholar]
- 33. Phillips ML, David AS. Facial processing in schizophrenia and delusional misidentification: cognitive neuropsychiatric approaches. Schizophr Res. 1995;17:109–114. [DOI] [PubMed] [Google Scholar]
- 34. Wölwer W, Brinkmeyer J, Stroth S, et al. Neurophysiological correlates of impaired facial affect recognition in individuals at risk for schizophrenia. Schizophr Bull. 2012;38:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obayashi C, Nakashima T, Onitsuka T, et al. Decreased spatial frequency sensitivities for processing faces in male patients with chronic schizophrenia. Clin Neurophysiol. 2009;120:1525–1533. [DOI] [PubMed] [Google Scholar]
- 36. Bediou B, Hénaff MA, Bertrand O, et al. Impaired fronto-temporal processing of emotion in schizophrenia. Neurophysiol Clin. 2007;37:77–87. [DOI] [PubMed] [Google Scholar]
- 37. Jetha MK, Zheng X, Goldberg JO, Segalowitz SJ, Schmidt LA. Shyness and emotional face processing in schizophrenia: an ERP study. Biol Psychol. 2013;94:562–574. [DOI] [PubMed] [Google Scholar]
- 38. Johnston PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci. 2005;22:1221–1232. [DOI] [PubMed] [Google Scholar]
- 39. Jung HT, Kim DW, Kim S, Im CH, Lee SH. Reduced source activity of event-related potentials for affective facial pictures in schizophrenia patients. Schizophr Res. 2012;136:150–159. [DOI] [PubMed] [Google Scholar]
- 40. Lee SH, Kim EY, Kim S, Bae SM. Event-related potential patterns and gender effects underlying facial affect processing in schizophrenia patients. Neurosci Res. 2010;67:172–180. [DOI] [PubMed] [Google Scholar]
- 41. Magnée MJ, Kahn RS, Cahn W, Kemner C. More prolonged brain activity related to gaze cueing in schizophrenia. Clin Neurophysiol. 2011;122:506–511. [DOI] [PubMed] [Google Scholar]
- 42. Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. [DOI] [PubMed] [Google Scholar]
- 44. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York, NY: Routledge Academic; 2013. [Google Scholar]
- 45. Cumming G. Understanding the New Statistics: Effect Sizes, Confidence Intervals, and Meta-Analysis. New York, NY: Routledge; 2012. [Google Scholar]
- 46. Lipsey MW, Wilson DB. Practical Meta-Analysis (Applied Social Research Methods Series, Vol 49.). Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 47. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Evangeli M, Broks ME. Face processing in schizophrenia: parallels with the effects of amygdala damage. Cogn Neuropsychiatry. 2000;5:81–104. [DOI] [PubMed] [Google Scholar]
- 49. Mandal MK, Rai A. Responses to facial emotion and psychopathology. Psychiatry Res. 1987;20:317–323. [DOI] [PubMed] [Google Scholar]
- 50. Silver H, Shlomo N, Turner T, Gur RC. Perception of happy and sad facial expressions in chronic schizophrenia: evidence for two evaluative systems. Schizophr Res. 2002;55:171–177. [DOI] [PubMed] [Google Scholar]
- 51. Hooker C, Park S. Emotion processing and its relationship to social functioning in schizophrenia patients. Psychiatry Res. 2002;112:41–50. [DOI] [PubMed] [Google Scholar]
- 52. Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull. 2003;29:487–497. [DOI] [PubMed] [Google Scholar]
- 53. Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–1366. [DOI] [PubMed] [Google Scholar]
- 54. Kosaka H, Omori M, Murata T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res. 2002;57:87–95. [DOI] [PubMed] [Google Scholar]
- 55. Gur RE, McGrath C, Chan RM, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159:1992–1999. [DOI] [PubMed] [Google Scholar]
- 56. Hempel A, Hempel E, Schönknecht P, Stippich C, Schröder J. Impairment in basal limbic function in schizophrenia during affect recognition. Psychiatry Res. 2003;122:115–124. [DOI] [PubMed] [Google Scholar]
- 57. Holt DJ, Weiss AP, Rauch SL, et al. Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biol Psychiatry. 2005;57:1011–1019. [DOI] [PubMed] [Google Scholar]
- 58. Rossion B, Caharel S. ERP evidence for the speed of face categorization in the human brain: Disentangling the contribution of low-level visual cues from face perception. Vision Res. 2011;51:1297–1311. [DOI] [PubMed] [Google Scholar]
- 59. Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. [DOI] [PubMed] [Google Scholar]
- 60. Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12:3815–3820. [DOI] [PubMed] [Google Scholar]
- 61. Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12:3815–3820. [DOI] [PubMed] [Google Scholar]
- 63. Haenschel C, Bittner RA, Haertling F, et al. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64:1229–1240. [DOI] [PubMed] [Google Scholar]
- 64. Tanaka S, Maezawa Y, Kirino E. Classification of schizophrenia patients and healthy controls using p100 event-related potentials for visual processing. Neuropsychobiology. 2013;68:71–78. [DOI] [PubMed] [Google Scholar]
- 65. Tendolkar I, Ruhrmann S, Brockhaus-Dumke A, et al. Neural correlates of visuo-spatial attention during an antisaccade task in schizophrenia: an ERP study. Int J Neurosci. 2005;115:681–698. [DOI] [PubMed] [Google Scholar]
- 66. Johnston PJ, Katsikitis M, Carr VJ. A generalised deficit can account for problems in facial emotion recognition in schizophrenia. Biol Psychol. 2001;58:203–227. [DOI] [PubMed] [Google Scholar]
- 67. Johnston PJ, Devir H, Karayanidis F. Facial emotion processing in schizophrenia: no evidence for a deficit specific to negative emotions in a differential deficit design. Psychiatry Res. 2006;143:51–61. [DOI] [PubMed] [Google Scholar]
- 68. Strauss GP, Catalano LT, Llerena K, Gold JM. The processing of emotional stimuli during periods of limited attentional resources in schizophrenia. J Abnorm Psychol. 2013;122:492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mazza M, Lucci G, Pacitti F, et al. Could schizophrenic subjects improve their social cognition abilities only with observation and imitation of social situations? Neuropsychol Rehabil. 2010;20:675–703. [DOI] [PubMed] [Google Scholar]
- 70. Frommann N, Streit M, Wölwer W. Remediation of facial affect recognition impairments in patients with schizophrenia: a new training program. Psychiatry Res. 2003;117:281–284. [DOI] [PubMed] [Google Scholar]
- 71. Frommann N, Fleiter J, Peltzer M, Steinbring A, Wölwer W. Training of Affect Recognition (TAR) in schizophrenia: generalizability and durability of training effects. Schizophr Res. 2008;98:53–55. [Google Scholar]
- 72. Habel U, Chechko N, Pauly K, et al. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010;122:113–123. [DOI] [PubMed] [Google Scholar]
- 73. Sachs G, Winklbaur B, Jagsch R, et al. Training of affect recognition (TAR) in schizophrenia–impact on functional outcome. Schizophr Res. 2012;138:262–267. [DOI] [PubMed] [Google Scholar]
- 74. Wölwer W, Frommann N, Halfmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophr Res. 2005;80:295–303. [DOI] [PubMed] [Google Scholar]
- 75. Wölwer W, Frommann N. Social-cognitive remediation in schizophrenia: generalization of effects of the Training of Affect Recognition (TAR). Schizophr Bull. 2011;37(suppl 2):S63–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tanaka JW, Wolf JM, Klaiman C, et al. Using computerized games to teach face recognition skills to children with autism spectrum disorder: the Let’s Face It! program. J Child Psychol Psychiatry. 2010;51:944–952. [DOI] [PubMed] [Google Scholar]
- 77. Faja S, Aylward E, Bernier R, Dawson G. Becoming a face expert: a computerized face-training program for high-functioning individuals with autism spectrum disorders. Dev Neuropsychol. 2008;33:1–24. [DOI] [PubMed] [Google Scholar]
- 78. Faja S, Webb SJ, Jones E, et al. The effects of face expertise training on the behavioral performance and brain activity of adults with high functioning autism spectrum disorders. J Autism Dev Disord. 2012;42:278–293. [DOI] [PMC free article] [PubMed] [Google Scholar]