Abstract
Although timing deficits are a robust finding in schizophrenia (SZ), the notion of a genuine time perception disorder in SZ is still being debated because distortions in timing might depend on neuropsychological deficits that are characteristics of the illness. Here we used meta-analytic methods to summarize the evidence of timing deficits in SZ and moderator analyses to determine whether defective timing in SZ arises from nontemporal sources or from defective time perception. PubMed Services, PsycNET, and Scopus were searched through March 2015, and all references in articles were investigated to find other relevant studies. Studies were selected if they included subjects with a primary diagnosis of SZ compared to a healthy control (HC) group and if they reported behavioral measures of duration estimation (perceptual and motor explicit timing). Data from 24 studies published from 1956 to 2015, which comprised 747 SZ individuals and 808 HC, were included. Results indicate that SZ individuals are less accurate than HC in estimating time duration across a wide range of tasks. Subgroup analyses showed that the fundamental timing deficit in SZ is independent from the length of the to-be-timed duration (automatic and cognitively controlled timing) and from methods of stimuli estimation (perceptual and motor timing). Thus, time perception per se is disturbed in SZ (not just task-specific timing processes) and this perturbation is independent from more generalized cognitive impairments. Behavioral evidence of disturbed automatic timing should be more thoroughly investigated with the aim of defining it as a cognitive phenotype for more homogeneous diagnostic subgrouping.
Key words: schizophrenia, timing, cognitive deficits, meta-analysis
“Perception of the world consists of a hierarchy of dimensions and time occupies the highest level of that hierarchy.” Navon, 1978.
Introduction
As everything exists in a temporal dimension, the capacity to capture and correctly process the temporal information embedded in relevant events is an absolute necessity for higher organisms.1 In fact, the ability to perceive, remember, and organize behavior in periods ranging from seconds to minutes (ie, interval timing) mediates several functions, from basic motor coordination2 to higher goal-directed behavior such as decision making regarding different reward sequences.3
Currently timing is a topic of intensive investigation. It is a multifaceted process that is involved whenever we make a deliberate estimate of a discrete duration of time in order to compare it with a previously memorized standard (ie, explicit timing). Alternatively, it is automatically engaged whenever sensorimotor information is temporally structured (ie, implicit timing).4 Further functional and neuroanatomical distinctions5 can be made among the processes that are recruited when estimates of duration are given in the form of perceptual discrimination (ie, perceptual timing) or in the form of a motor response (ie, motor timing). Certainly, timing is inextricably interrelated with other cognitive processes such as attention, long-term memory and working memory. On one hand, timing has been defined as a “basic unit of ability”6 that modulates other cognitive and behavioral processes. On the other hand, the cognitive ability required for the perception of incoming temporal information, its storage and retrieval from long-term memory, and “on-line” comparison with other elements in working memory necessarily relies on the above-mentioned cognitive resources.
Pathophysiological distortions in time perception have been reported in a number of different neuropsychiatric disorders. They are primarily associated with dopaminergic, fronto-striatal, and deep grey matter structural dysfunctions, such as those found in schizophrenia (SZ), attention deficit hyperactivity disorder, autism and Parkinson’s disease.6–8 However, as dopaminergic modulation in the frontostriatal circuitry supports other cognitive processes such as working memory9 and reward-based learning,10,11 which are required for accurate timing, questions still remain about whether increased timing variability in the above mentioned disorders arises from a cognitive dysfunction that is independent from defective interval timing per se.
Numerous studies that focused on SZ demonstrated that patients have reduced accuracy in estimating periods of time in the minutes-to-hours range,12,13 and in timing stimulus duration in the range of milliseconds to a few seconds.14–19 As SZ has been characterized as a deficiency in the temporal coordination of information processing in the brain,20 it has been argued that impairments of time processing could be an essential part of its pathogenesis21 and that disruption of patients’ internal clock might be associated with highly disabling symptoms like delusions or hallucinations.22 However, recent evidence23,24 challenges the notion of a genuine time perception disorder in SZ. Indeed, the internal clock of SZ patients is not faster than that of normal controls24 and SZ patients’ performance on timing tasks is impaired only in cognitively demanding trials.23 This suggests that timing disturbances in SZ are secondary to disease-related cognitive impairments and that attention25 and working memory deficits24 explain the observed time perception impairment. This issue has not, however, been clarified by empirical findings.
In this systematic review and meta-analysis we quantitatively/qualitatively analyzed explicit timing abilities in patients affected by SZ compared to matched healthy controls (HC). As the time intervals used in SZ studies range from milliseconds to hours, and make different demands on other cognitive processes, it is difficult to disentangle deficits of temporal perception from generalized impairments of attention26,27 and memory.28 To address this problem, we categorized each study according to interval duration (ie, longer or shorter than 500ms)29–31 to isolate deficits in the different mechanisms underlying the “cognitively controlled” and “automatic” timing systems.30,32 In fact, the automatic system measures time without attentional or cognitive modulation and is primarily involved in timing intervals in the subsecond range, while cognitively controlled timing is primarily based on higher level cognitive circuits that fulfill attentional and memory requirements which are recruited for longer period estimations.33 Thus, the primary aim of the present systematic review and meta-analysis was to investigate whether patients with a diagnosis of SZ are differentially impaired in the ability to produce an explicit estimate of elapsed time by automatic (primary) and cognitively controlled (secondary) processes and to determine whether timing deficits in SZ arise from cognitive impairments other than defective interval timing per se.
Materials and Methods
Literature Search and Study Selection
The paper inclusion/exclusion criteria, search strategy and primary variables assessed were defined a priori according to the guidelines proposed in the PRISMA statement.34 An extensive literature review was conducted to search for articles that investigated explicit timing abilities in SZ. PubMed Services, PsycNET (including the PsycINFO, PsycBOOKS, PsycCRITIQUES, PsycARTICLES, and PsycEXTRA databases), and Scopus were searched through March 2015, without limits concerning year of publication. The key word schizophrenia was used in combination with any of the following terms: temporal processing, temporal discrimination, time perception, temporal estimation, time estimation, internal clock, and interval timing. The reference list of identified studies was also hand-searched to obtain additional articles. Studies were considered for inclusion if they: (1) were published in English in a peer-reviewed journal; (2) included subjects with a primary diagnosis of SZ on the basis of ICD35–39 and DSM40–45 criteria; (3) reported behavioral measures of duration estimation (perceptual and motor explicit timing); and (4) compared a SZ group with a HC group. Studies were excluded if: (1) they enrolled mixed samples of SZ and SZ-spectrum subjects and data were not reported separately for the 2 groups24,46–50; (2) subjects were required to use explicit counting in order to keep track of time15,51–55; and (3) inferences about patients’ timing ability could not be drawn from the reported data.56–62
The literature search yielded 134 studies, 50 of which fulfilled the inclusion/exclusion criteria on the basis of abstracts. Full texts of the candidate studies were independently screened by 2 evaluators (V.C. and F.P.) and 25 papers were excluded (schizotypal sample: 2; no HC group: 4; mixed samples of SZ patients and SZ-spectrum subjects, data not reported separately for the 2 groups: 5; no valid measure of time perception abilities: 8; explicit counting: 6). The same evaluators further excluded 1 paper as in the candidate investigation18 the same sample of already included study was described.19 At the end of the selection process, 24 studies were accepted as eligible for meta-analysis: 11 that required time measurements in the subsecond range (ie, automatic timing),16,17,63–71 10 that reported measures of cognitively controlled timing,13,14,72–79 and 3 that evaluated both.19,80,81
A total of 747 SZ individuals and 808 HC subjects from the above mentioned studies were included in the meta-analysis. After the eligibility assessment, a data extraction sheet and a coding scheme were developed, pilot tested on 5 randomly selected studies and refined accordingly. The first author (V.C.) extracted the following data from the included studies and the last author (F.P.) checked them: (1) sample size; (2) clinical characteristics: illness duration, Positive And Negative Syndrome Scale (PANSS) score, chlorpromazine equivalent doses for typical and atypical antipsychotics; (3) Method of timing assessment: type of temporal processing engaged (automatic vs cognitively controlled timing), method of temporal stimulus estimation (motor vs perceptual timing), explicit timing task employed (temporal bisection, method of estimation, duration discrimination, duration threshold, detection of temporal irregularity, finger tapping), modality of temporal stimulus presentation (visual vs. auditory).
Table 1 displays the characteristics of the included studies.
Table 1.
Characteristics of Studies Estimating Explicit Timing Abilities in SZ
| Clinical characteristics | Timing assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Sample | Medication | CE (mg/die) | PANSS average score | Duration of Illness (years) | Task | Method | Process | Duration | Modality | Findings |
| Bolbecker et al63 | 66 SZ 73 HC | 15 typical 49 atypical antipsychotic 7 anticonvulsant 15 antidepressant 7 anticholinergic | 315 | 56.5 | N/A | Temporal bisection | P | Automatic | 300–600 ms | A | increased temporal variability in SZ |
| Bourdet et al64 | 22 SZ 14 HC | 22 typical antipsychotic | 334 | N/A | N/A | Detection of temporal irregularity | P | Automatic | 500 ms | A | SZ less accurate in detecting temporal irregularities |
| Broadhurst72 | 24 SZ 24 HC | N/A | N/A | N/A | N/A | Estimation of elapsed time | P | Controlled | 5 min | N/A | SZ overestimated interval duration (faster internal clock) |
| Carlson et al73 | 30 SZ 20 HC | N/A | N/A | N/A | N/A | Estimation production & reproduction of intervals | M/P | Controlled | 1–10 s | V | the internal clock for SZ ran faster more for interval production than for estimation |
| Carroll et al19 | 28 SZ 31 HC | 6 typical 12 atypical antipsychotic | N/A | 62 | N/A | Temporal bisection | P | Automatic 300 and 600 ms | A | increased temporal variability in SZ (perceptual alterations) | |
| Controlled 3 and 6 sec | |||||||||||
| Carroll et al65 | 32 SZ 31 HC | 7 typical 14 atypical antipsychotic 1 psychotropic medications 1 drug free 9 drug naive | N/A | 60 | N/A | Finger tapping | M | Automatic | 500 ms | A | faster tapping rates and greater tapping variability in SZ (motor-related and perceptual alterations) |
| Delevoye- Turrell et al66 | 24 SZ 22 HC | N/A | 290 | 77.1 | 16.1 | Finger tapping | M | Automatic | preferred rate | V | slower tapping rates, but normal internal clock in SZ |
| Davalos et al67 | 10 SZ 10 HC | 9 atypical antipsychotic 1 drug free | N/A | N/A | N/A | Duration discrimination | P | Automatic | 400 ms | A,V | SZ less accurate (increased error rate) particularly in estimating shorter intervals |
| Davalos et al17 | 15 SZ 16 HC | 14 atypical antipsychotic 1 drug free | N/A | N/A | N/A | Duration discrimination | P | Automatic | 400 ms | A | SZ less accurate than controls (perceptual alterations) |
| Davalos et al68 | 15 SZ 30 HC | 14 atypical antipsychotic 1 drug free | N/A | N/A | N/A | Duration discrimination | P | Automatic | 500 ms | A | greater error rates in SZ either in estimating the shorter and the longer interval |
| Davalos et al69 | 20 SZ 20 HC | 13 typical 5 atypical antipsychotic 2 drug naive | N/A | N/A | N/A | Duration discrimination | P | Automatic | 200 ms | A | greater error rates in SZ either in the easy and the difficult discrimination |
| Densen et al74 | 10 SZ 10 HC | N/A | N/A | N/A | N/A | Estimation of elapsed time | P | Controlled | 5–120 s | N/A | SZ overestimated the 5 and 30 sec. duration but not the 10 and 120 sec. duration |
| Elvevåg et al16 | 19 SZ 23 HC | 19 atypical antipsychotic 2 both 4 anticholinergic | N/A | N/A | N/A | Duration discrimination & temporal bisection | P | Automatic | 500 and 200-800 ms | A, V | SZ overestimated the 500 ms interval in the temporal discrimination task and underestimated the 800 ms interval in the temporal bisection task |
| Johnson et al13 | 40 SZ 40 HC | 40 drug free | N/A | N/A | N/A | Estimation of elapsed time | P | Controlled | 30 s | N/A | SZ overestimated an unspecified period of 30 sec and underestimated a specified period of 30 sec |
| Lee et al80 | 38 SZ 38 HC | 5 typical 33 atypical antipsychotic | 618 | N/A | 11.7 | Temporal bisection | P | Automatic 400–800 ms | A | SZ underestimated the longer intervals (800 & 2000) | |
| Controlled 1000–200 ms | |||||||||||
| Lhamon and Goldstone14 | 37 SZ 41 HC | N/A | N/A | N/A | N/A | Duration discrimination | P | Controlled | 1 s | A | SZ overestimated interval duration even after feed.-back |
| Orme et al75 | 80 SZ 117 HC | N/A | N/A | N/A | N/A | Estimation of elapsed time | P | Controlled | 30 min | N/A | SZ underestimated interval duration |
| Papageorgiou et al76 | 60 SZ 35 HC | 58 atypical 10 both 10 anticholinergic 2 drug free | N/A | N/A | N/A | Duration discrimination & | P | Controlled | 1200 ms | A | SZ had significantly more incorrect responses in the discrimination of time intervals |
| Finger tapping | 1067–1125 ms | ||||||||||
| Rutschmann77 | 7 SZ 9 HC | 7 typical antipsychotic | 507.1 | N/A | N/A | Verbal estimation & production of temporal interval | M/P | Controlled | 500–2000 ms | A | SZ underestimated interval durations in either task (slower internal clock) |
| Todd et al70 | 21 SZ 25 HC | 11 typical 10 atypical antipsychotic | 599 | N/A | 4.95 | Duration threshold task | P | Automatic | 50ms with a 10ms rise and fall | A | SZ performed equally to controls in the discrimination of filled intervals |
| Todd et al71 | 20 SZ 22 HC | 2 typical 18 atypical antipsychotic 2 drug free | 500 | N/A | 10.7 | Duration & interval threshold task | P | Automatic | 50ms (step size of 8 ms) | A | SZ showed higher discrimination thresholds than controls |
| Tracy et al78 | 19 SZ 43 HC | 16 typical 3 atypical antipsychotic | 282 | N/A | N/A | Time production; Estimation of elapsed time; Dual-task condition | P | Controlled | 15 s; 7–40 s | A | SZ less accurate than controls in the time production task; larger decline in the dual-task condition |
| Turgeon et al81 | 15 SZ 15 HC | reported for the original 20 patients group | N/A | N/A | N/A | Finger tapping | M | Automatic Controlled |
500ms 1 s |
A | SZ showed normal rhythm-production performance (well- calibrated internal clock) |
| van der Veen et al79 | 12 SZ 21 HC | 2 drug free 10 drug naive | 0 | 71 | N/A | Estimation of elapsed time | P | Controlled | 1 s | V | SZ performed at the same level as controls and used the feed-back to improve performance |
Note: SZ, schizoprenic patients; HC, healthy controls; CE, chlorpromazine equivalents; P, perceptual timing; M, motor timing; A, auditory; V, visual; N/A, not available.
Eighteen of the 24 included studies evaluated perceptual timing, in which subjects typically stated whether a stimulus duration or interstimulus interval (ISI) was shorter or longer than another13,14,16,17,19,63,64,67–72,74,75,78–80; 3 studies used a temporal bisection task,19,63,80 which required subjects to judge whether temporal stimuli were more similar to the long or to the short standard duration previously presented; 6 studies employed the method of verbal estimation of intervals13,72,74,75,78,79; 6 studies14,16,17,67–69 examined subjects’ ability to compare the duration of a probe sensory stimulus with that of a target stimulus previously stored in working or reference memory (ie, a duration discrimination task5); 2 studies70,71 adopted psychophysical procedures (ie, adaptive methods) to measure the smallest difference in duration between 2 intervals the participant could detect in 70% of the trials (ie, duration/interval threshold task); and 1 study64 examined the ability to detect temporal irregularities in a stream of standard tones. Three studies65,66,81 employed measurements of motor timing in which subjects reproduced the timed duration or ISI with a sustained, delayed, or periodic motor action.5 Specifically, the finger tapping task requires that participants first tap in time with computer-generated tones separated by a fixed intertone interval (tone-paced tapping), after which the tones are discontinued and participants are required to continue tapping at the established pace (self-paced tapping).65 Lastly, 3 studies73,76,77 adopted 2 concurrent methods such as the estimation and the production of temporal intervals or the temporal bisection and the finger-tapping tasks.76
Data Synthesis and Analysis
To quantify the degree of impaired explicit timing processing in SZ, effect sizes were calculated for the difference in scores between SZ and HC using techniques detailed in Lipsey and Wilson.82 As different assessments were used, raw scores were entered such that the direction of the effect size was negative if the performance of the SZ group was worse than that of the HC group. The effect size estimate used was Cohen’s d, which is commonly employed for continuous measures.83 When raw data were not reported, the estimate was computed from exact P, t, or F values and from binary proportions.82 If insufficient data were provided, the corresponding authors were contacted by e-mail and requested to provide details that were not included in the original publication.
After inputting the effect size for each study, meta-analytic techniques were applied to obtain a combined effect expressing the magnitude of association, weighted for sample size. Stouffer’s Z, a test of the cumulative evidence on the common null hypothesis, provided an indication of the significance of the difference in explicit timing performances between SZ and HC groups. A 95% CI was calculated based on the SE. In order to account for clinical (ie, differences associated with participants, interventions, or outcomes)84 and methodological (eg, differences in study design)84 heterogeneity among the included studies, a random effect model was used, as this includes consideration of heterogeneity in the effect estimate.84 The total heterogeneity of the effect sizes (Q) was calculated and considered significant for P < .05. A significant Q means that the observed dispersion among effects exceeds the amount that would be expected from sampling errors and suggests that other explanatory variables should be investigated. When a significant level of heterogeneity was reached, the I 2 index, an estimate of the total proportion of variance due to heterogeneity,85,86 was determined. The between studies variance (τ2), a measure of interstudy variance representing both the degree to which true effects vary across experiments and the degree to which individual studies give biased assessments,87 was also computed and used to assign weights to the included studies. Furthermore, to investigate the impact of potential effect modifiers (ie, factors that can affect the considered outcome), subgroup analyses were performed on subsets of studies stratified by: (1) type of temporal processing engaged and (2) method of temporal stimulus estimation. In the case of complex data structure, subgroup analyses split the data from all participants to make comparisons between subgroups.84 Specifically, here we: (1) compared the effect size for automatic timing with the effect size for cognitively controlled timing abilities and (2) computed a summary effect for the impact of the method of stimulus estimation (perceptual and motor) on automatic and cognitively controlled timing performance combined. Mixed effect analyses (separately for each potential modifier) were performed; here a random-effects model was used to combine studies within each subgroup and a fixed effect model to combine subgroups; τ2 was not assumed to be the same for all subgroups.88
Publication bias was examined by using a funnel plot of SEs and SDs in the mean; in the case of asymmetry, Duval and Tweedie’s Trim and Fit analysis was performed.89
All analyses were completed in Comprehensive Meta-analysis package, version 2 (http://www.meta-analysis.com).
Results
The first analysis concerned potential differences in explicit timing performance between 747 SZ and 808 HC. Table 2 reports the effect size for each included study.
Table 2.
Effect Sizes of Explicit Timing Abilities in SZ and HC
| Study Name | Sample Size | Outcome | Method of Assessment | d |
|---|---|---|---|---|
| Bolbecker et al63 | 66 patients 73 controls | Automatic | Perceptual | d = −0.5712; 95% CI = −0.903, −0.234; v = 0.029 |
| Bourdet et al64 | 22 patients 14 controls | Automatic | Perceptual | d = −1.4462; 95% CI = −2.168, −0.724; v = 0.136 |
| Broadhurst72 | 24 patients 24 controls | Controlled | Perceptual | d = −0.9278; 95% CI = −1.507, −0.347; v = 0.088 |
| Carlson et al73 | 30 patients 20 controls | Controlled | Perceptual | d = 1.087; 95% CI = 0.498, 1.676; v = 0.09 |
| Carlson et al73 | 30 patients 20 controls | Controlled | Motor | d = −1.2; 95% CI = −1.797, −0.603; v = 0.093 |
| Carroll et al19 | 28 patients 31 controls | Automatic | Perceptual | d = −0.5343; 95% CI = −1.043, −0.025; v = 0.067 |
| Carroll et al19 | 28 patients 31 controls | Controlled | Perceptual | d = −0.9326; 95% CI = −1.459, −0.405; v = 0.072 |
| Carroll et al65 | 32 patients 31 controls | Automatic | Motor | d = −0.7729; 95% CI = −1.278, −0.266; v = 0.067 |
| Delevoye-Turrell et al66 | 24 patients 22 controls | Automatic | Motor | d = −0.8966; 95% CI = −1.469, −0.306; v = 0.091 |
| Davalos et al67 | 10 patients 10 controls | Automatic | Perceptual | d = −1.393; 95% CI = −2.305, −0.481; v = 0.216 |
| Davalos et al17 | 15 patients 16 controls | Automatic | Perceptual | d = −1.6272; 95% CI = −2.406, −0.848; v = 0.158 |
| Davalos et al68 | 15 patients 30 controls | Automatic | Perceptual | d = −1.1168; 95% CI = −1.759, −0.473; v = 0.108 |
| Davalos et al69 | 20 patients 20 controls | Automatic | Perceptual | d = −1.1276; 95% CI = −1.773, −0.481; v = 0.109 |
| Densen et al74 | 10 patients 10 controls | Controlled | Perceptual | d = −0.99; 95% CI = −1.856, −0.124; v = 0.195 |
| Elvevåg et al16 | 19 patients 23 controls | Automatic | Perceptual | d = 0.537; 95% CI = 0.018, 1.056; v = 0.070 |
| Johnson et al13 | 40 patients 40 controls | Controlled | Perceptual | d = −0.845; 95% CI = −1.295, −0.395; v = 0.053 |
| Lee et al80 | 38 patients 38 controls | Automatic | Perceptual | d = −0.6603; 95% CI = −1.114, −0.206; v = 0.054 |
| Lee et al80 | 38 patients 38 controls | Controlled | Perceptual | d = −0.7327; 95% CI = −1.189, −0.275; v = 0.054 |
| Lhamon and Goldstone14 | 37 patients 41 controls | Controlled | Perceptual | d = −0.458 95% CI = −0.901, −0.015 v = 0.051 |
| Orme et al75 | 80 patients 117 controls | Controlled | Perceptual | d = −0.2989; 95% CI = −0.595, −0.001; v = 0.023 |
| Papageorgiou et al76 | 60 patients 35 controls | Controlled | Perceptual | d = −0.5175; 95% CI = −0.905, −0.129; v = 0.039 |
| Papageorgiou et al76 | 60 patients 35 controls | Controlled | Motor | d = −0.3911; 95% CI = −0.776, −0.006; v = 0.039 |
| Rutschmann77 | 7 patients 9 controls | Controlled | Perceptual | d = −0.6262; 95% CI = −1.550, 0.298; v = 0.222 |
| Rutschmann77 | 7 patients 9 controls | Controlled | Motor | d = 0.236; 95% CI = −0.670, 1.142; v = 0.213 |
| Todd et al70 | 17 patients 20 controls | Automatic | Perceptual | d = −0.6746; 95% CI = −1.316, −0.032; v = 0.107 |
| Todd et al71 | 20 patients 22 controls | Automatic | Perceptual | d = −0.918; 95% CI = −1.535, −0.301; v = 0.099 |
| Tracy et al78 | 9 patients 43 controls | Controlled | Perceptual | d = −1.1248; 95% CI = −1.856, −0.392; v = 0.140 |
| Turgeon et al81 | 15 patients 15 controls | Automatic | Motor | d = 0.0365; 95% CI = −0.649, 0.721; v = 0.122 |
| Turgeon et al81 | 15 patients 15 controls | Controlled | Motor | d = 0.0365; 95% CI = −0.649, 0.721; v = 0.122 |
| van der Veen et al79 | 12 patients 21 controls | Controlled | Perceptual | d = 0.3088; 95% CI = −0.377, 0.993; v = 0.122 |
Note: d, standardized mean difference; CI, confidence interval; v, variance.
The overall effect size for SZ vs. HC was −0.64 (95% CI = −0.82, −0.45; z = −6.63; P < .001); this implies that the mean of the observed effect was significantly different from 0.0 and suggests there was a moderate difference in timing performance such that SZ individuals performed worse than HC subjects. However, the dispersion among effects exceeded the amount expected by chance (Q[23df] = 69.62; P < .001) and the total proportion of variance between studies, due to real differences in the effect size, was high (I 2 = 66.97%) whereas the inter-study variance was negligible (τ2 = 0.14). In order to ascertain whether variations among studies was due to clinical heterogeneity, 2 mixed effects analyses were performed using: (1) type of temporal processing engaged (cognitively controlled and automatic timing) and (2) method of temporal stimulus estimation (perceptual and motor) as potential modifier. Studies were combined across subgroups of: (1) automatic timing, (2) cognitively controlled timing, (3) motor timing, and (4) perceptual timing, considering a minimum of 4 studies for each modifier.90 In the first analysis, the combined effect in the subgroup of studies88 investigating automatic timing (N = 14) was compared to the combined effect in the subgroup of studies considering cognitively controlled timing (N = 16).
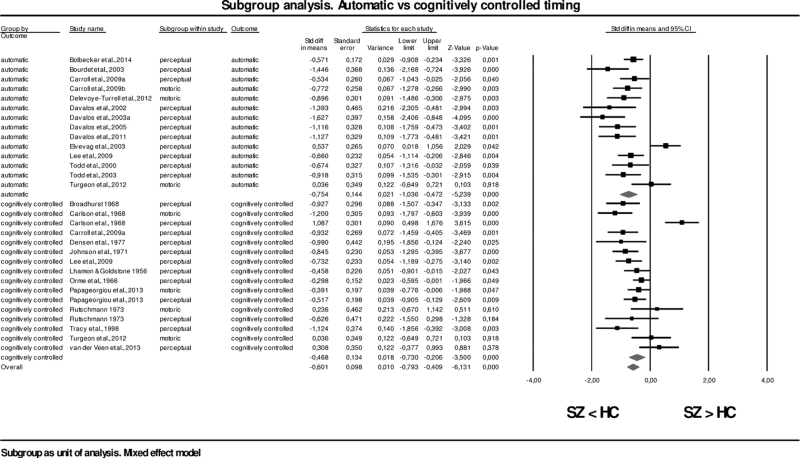
Table 3 presents the forest plot showing the combined effect sizes (and 95% CIs) for automatic and cognitively controlled timing and the overall effect size expressing the impact of the temporal process engaged (controlling for the method of temporal stimulus estimation)88 under the random effects model.
Table 3.
Forest plot of standardized mean differences of automatic vs cognitively controlled timing in SZ and HC
|
The overall effect in the automatic timing subgroup (which included 341 subjects) was −0.75 (95% CI = −1.04, −0.47; z = −5.24; P < .001) and −0.47 (95% CI = −0.73, −0.21; z = −3.50; P < .001) in the cognitively controlled timing subgroup (which included 487 subjects), showing a significant deficit in both abilities in SZ compared to HC. Conversely, no significant difference was found in the between-studies variance (Q[1df] = 2.12; P = .15), which implies that SZ patients’ difficulties in estimating periods of time did not vary when additional cognitive processes were required. Nevertheless, although heterogeneity was reduced by splitting participants’ data into subgroups, particularly in the automatic subsample (Q[13df] = 43.24; P < .001), the observed effect was still significantly different from 0.0. Similarly, a second subgroup analysis in which studies were stratified based on the method of temporal stimulus estimation employed, independently from the temporal process engaged, demonstrated that perceptual and motor timing were significantly impaired in SZ. The overall effect size for studies exploring perceptual timing (21 papers, 579 patients) was −0.64 (95% CI = −0.89, −0.39; z = −4.99; P < .001) and −0.55 (95% CI = −0.93, −0.17; z = −2.84; P < .001) for motor timing (6 papers, 168 patients), suggesting a moderate effect, while the between-studies variance was not different from 0.0 (Q[1df] = 0.14; P = .71), demonstrating that both timing subcomponents were equally impaired in the SZ subgroup. The dispersion among effects was significant in both subgroups, but naturally reduced in the motor timing sub-sample (Q[5df] = 12.94 P = .02).
Table 4 reports results of the overall meta-analysis and the 2 subgroup-analyses performed on explicit timing in SZ patients.
Table 4.
Standardized Mean Differences in Explicit Timing Abilities (a) Between SZ and HC, Stratified by: (b) Type of Temporal Processing Engaged, and (c) Method of Temporal Stimulus Estimation
| Effect Size (± 95% CI) | Test of Null (2-Tail) | Heterogeneity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number Studies | Point Estimate | Standard Error | Variance | Lower Limit | Upper Limit | Z Value | P Value | Q Value | df (Q) | P Value | I 2 | τ2 | |
| (a) Explicit timing in SZ (single study as unit of analysis, outcome averaged). Random effect model | |||||||||||||
| 24 | −0.64 | 0.10 | 0.01 | −0.82 | −0.45 | −6.63 | <.001 | 69.62 | 23.00 | <.001 | 66.97 | 0.14 | |
| (b) Explicit timing by type of temporal processing engaged (subgroup as unit of analysis). Mixed effects model | |||||||||||||
| Automatic 14 | −0.75 | 0.14 | 0.02 | −1.04 | −0.47 | −5.24 | <.001 | 43.24 | 13 | <.001 | 69.94 | 0.19 | |
| Cognitively controlled 16 | −0.47 | 0.13 | 0.02 | −0.73 | −0.21 | −3.50 | <.001 | 57.28 | 15 | <.001 | 73.81 | 0.20 | |
| Q test for the difference between subgroups | 2.12 | 1 | .15 | ||||||||||
| (c) Explicit timing by method of temporal stimulus estimation (subgroup as unit of analysis). Mixed effect model | |||||||||||||
| Motor | 6 | −0.55 | 0.19 | 0.04 | −0.93 | −0.17 | −2.84 | <.001 | 12.94 | 5 | .02 | 61.37 | 0.13 |
| perceptual | 21 | −0.64 | 0.13 | 0.02 | −0.89 | −0.39 | −4.99 | <.001 | 87.13 | 20 | <.001 | 77.04 | 0.21 |
| Q test for the difference between subgroups | 0.14 | 1 | .71 | ||||||||||
The overall predicted publication bias for automatic and cognitively controlled timing was negligible because Duval and Tweedie’s Trim and Fit analysis89 revealed no missing study, resulting in no shift of the overall mean weighted effect size (−0.56). Finally, the classic fail-safe N analysis estimated that 762 nonsignificant or missing studies were needed to render the observed meta-analytical results nonsignificant.
Discussion
For years timing disturbances have been reported in individuals with SZ on a variety of timing tasks.13,15,53,55,74,91–94 Specifically, the described timing deficits suggest an acceleration of the internal clock,95 because patients overestimate the interval duration when verbally reporting it15 or during repetitive tapping,65 and underestimate the interval duration during time production tasks.15,94 However, given the correspondences between cognition and interval timing abilities, the notion of a genuine time perception disorder in SZ is still being debated.24,25,76 To our knowledge, this is the first review and meta-analysis that has specifically addressed explicit timing in SZ patients focusing on whether disturbances in this function are due to a primary perceptive deficit or to secondary disease-related cognitive impairments in working memory, executive functioning, and attention.
Here we found that SZ individuals are less accurate than HC subjects in estimating durations ranging from milliseconds to several minutes in a wide range of tasks traditionally employed to study explicit timing. This result is interesting because neuropsychological13,15,53,55,74,91–94 and pharmacological96,97 evidence suggests that SZ patients are impaired in temporal processing. Intriguingly, also subjects without a diagnosis of SZ who are prone to visual hallucinations,98 subjects with schizotypal features99 and individuals deemed to be at high genetic risk for developing SZ (eg, one parent diagnosed with SZ)25 demonstrate alterations in time estimation resembling those observed in SZ. This suggests that a timing dysfunction might be considered an endophenotype of SZ and schizotypal personality disorder.
Relevant to the long-running debate about the legitimacy of the hypothesis that defective temporal event structuring is a key feature of SZ, several authors29,30,97 proposed the existence of 2 distinct timing mechanisms: an automatic mechanism for measuring duration in the millisecond range and a cognitively controlled mechanism, mediated by attention and drawing upon working memory, for measuring intervals in the range of seconds. Following this line of argument, we stratified papers according to interval duration. We assumed that, if disturbances in timing are secondary to disease-related cognitive impairments,100 then there should be a significant difference in the magnitude of deficits associated with different timing systems. We found that SZ patients are equally impaired in timing events in both the milliseconds and the seconds range, thus suggesting that impaired temporal processing is a pervasive, as well as a primary deficit in SZ. This finding is in line with previous evidence14,16,17,19,62,67,92,101,102 of the lack of correlation between timing abilities and working memory, attention or executive functioning capacities in SZ and HC. Although a recent meta-analysis showed that temporal processing is mediated by a distributed network that can be differentially engaged depending on task requirements,103 our results suggest that SZ patients’ timing deficits cannot be differentiated on the basis of task demands. The latter implies that time perception per se, and not merely task-specific timing processes, is disrupted in SZ. Recent pharmacological studies96,97 provide further support to the hypothesis that altered time perception is a distinctive deficit in SZ since the NMDA receptor antagonists, which are used to model the putative hypoglutamate state of schizophrenia, produce cognitive changes resembling those observed in prodromal stages of SZ, concurrently impairing timing abilities. Indeed, memantine and ketamine were used to investigate whether drug-induced perturbations in cognitive processing interfere with timing performance. Memantine was found to disrupt the timing of auditory stimuli in the seconds, but not the milliseconds range, whereas ketamine had a specific effect on accuracy in a timing task, but not in a color discrimination task matched for working memory and attentional demands. These findings suggest that timing disturbances in SZ are more pervasive and not just due to working memory deficits. Moreover, as shown in temporal bisection tasks,19 comparable bisection points between SZ and nonpsychiatric groups indicate that the higher attentional demands required for timing longer durations have no effect on temporal encoding. This suggests that the fundamental timing deficit in SZ is independent from the length of the to-be-timed duration and, consequently, from more generalized cognitive impairments.16,19 Nevertheless, it is also possible that in SZ patients (unlike normal subjects) the amount of attention allocated to stimulus processing (which is assumed to be very small for events lasting for very short intervals) is larger, thus suggesting that the observed disturbance in timing brief durations can be attributed to abnormalities in attentional processes. However, the latter hypothesis seems rather implausible considering the poor effort and generalized capacity limitations in attention allocation during the identification of visual targets commonly observed in SZ.104 Further support for the primary deficit hypothesis comes from electrophysiological studies examining mismatch negativity (MMN) responses, a component of the event-related potential elicited in response to infrequent auditory deviant stimuli in the context of recurring standard stimuli. Duration-MMN was utilized as a physiological measure of preconscious temporal processing corresponding to the preattentive neural activity that is available for the conscious perception of time during temporal discrimination tasks.105 An advantage of the MMN paradigm is the fact that it is generated in the absence of focused attention or motor responses and, therefore, is less affected by deficits in other cognitive processes.105,106 Deficits in duration-MMN (vs frequency-MMN) are a robust feature of SZ70,71,107–112 and have not been observed in other major psychiatric disorders such as major depressive disorder and bipolar disorder,107,113 suggesting that defective MMN generation could be considered a cognitive endophenotype of SZ.
The evidence of a specific weakness of SZ in a process linked to alerting the organism to a novel stimulus71 challenges the recent dimensional approach to psychiatric disturbances, which suggests the existence of a continuum ranging from SZ to bipolar disorders that shares a common biological phenotype,114–117 and instead supports Kraepelin’s traditional dichotomy between the 2 entities.118 Note that this issue has not been clarified by empirical findings. Indeed, alterations in temporal perception may represent a psychosis-related phenotype and constitute a trait dysfunction that crosses diagnostic categories; however, a recent investigation63 found increased temporal variability in bipolar patients irrespective of whether psychotic symptoms were present. Moreover, patients diagnosed with SZ show perturbed discrimination of simultaneous vs synchronous events.119 Thus, for patients to become aware of the asynchrony between 2 sensory events they have to be separated by longer delays than for controls. Again, evidence of abnormalities in the low-level temporal coding of event structure appears to be independent from memory or attention impairments.
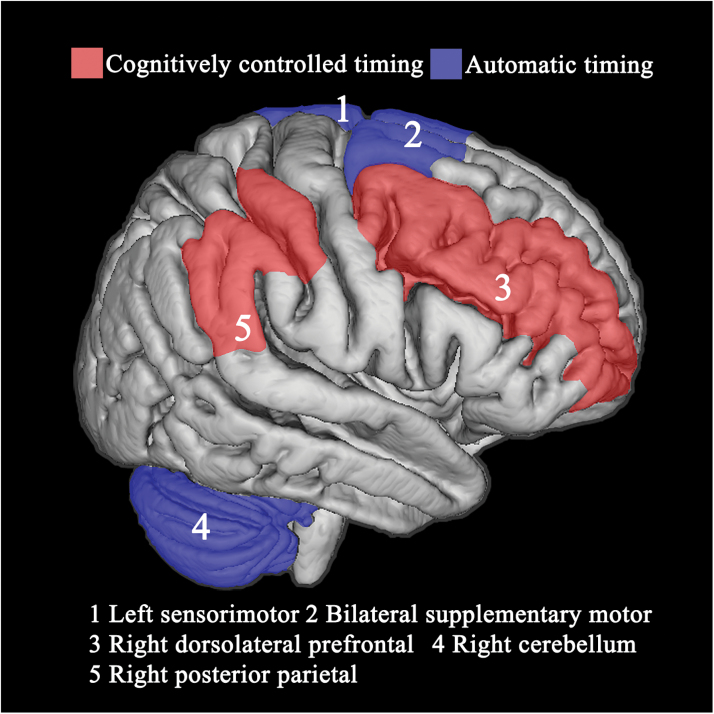
Taken together, these findings could be relevant for understanding the pathophysiology of SZ in the sense that psychopathological dimensions of delusions, hallucinations, and disorganized behavior could be conceptualized as phenomenological expressions of dysfunctional neural timing.120 Importantly, the foregoing evidence of a fundamental defect in automatic timing fits well with the influential cognitive dysmetria theory proposed by Andreasen,20 which conceptualizes SZ as a misconnection in the fluid, coordinated sequences of thought and action stemming from a dysfunction of the cortico-cerebellar-thalamic-cortical circuit, specifically of the cerebellar node, an area predominantly activated during sub-second durations processing.103 Recent results from neuroanatomical121 and imaging studies103 demonstrated that the cerebellum is a crucial node in a densely interconnected network involved in the processing of temporal information that encompasses the premotor areas in the frontal lobe, selected regions of the prefrontal and parietal cortices, as well as the subcortical regions. In particular, sub-second durations (ie, automatic timing) likely recruit subcortical structures such as the basal ganglia and the cerebellum, while supra-second durations (ie, cognitively controlled timing) involve cortical structures such as the dorsolateral prefrontal cortex and the posterior parietal cortex. However, the degree of functional specialization of each brain region is still being investigated. An intermediate hypothesis is that a large distributed system is devoted to temporal processing in different circumstances, but the processing weight of the different nodes of this system change according to task contingencies.122 Moreover, a strong link between specific timing mechanisms is conceivable, given the dense anatomical connections between the nodes of the automatic and cognitively controlled timing systems. For example, the two-way communication between the basal ganglia and cerebellum might provide on-line modulation to the feed-forward timing signals emitted by the cerebellum.123
Furthermore, as SZ is associated with widespread reductions in grey matter124 volume and disruptions in the organization of anatomical connectivity,125 abnormalities in the synchronized oscillatory activity of neurons are expected (see Uhlhaas and colleagues126 for a review). Since the oscillatory properties of cortical neurons appear to produce the internal clock ticks and to provide a distinct pattern of activity to represent durations,127 SZ deficits in time perception could arise from abnormal neural oscillations and synchrony resulting from abnormalities in several cortical areas and their connectivity.
Figure 1 depicts brain regions differentially involved in automatic and cognitively controlled timing.5,128
Fig. 1.
Depiction of brain regions associated with secondary cognitively controlled (red) and primary automatic (blue) timing systems.
Certainly, the temporal fragmentation of conscious experience and the corresponding lack of temporal organization in thoughts and behavior, probably resulting from alterations in the mechanisms that mediate the generation of coherent and coordinated activity in cortical circuits, could result in translation errors that contribute to delusional interpretations of reality.129 Indeed, patients with SZ show a genuine impairment in making simultaneity judgments130 that cannot be attributed to attentional or decisional biases. This causes defective discrimination of event structures over time, which contributes to the rupture in the sense of continuity frequently described at a clinical level. In addition, patients diagnosed with SZ show disturbances in the temporal flow of causal experience due to a greater tendency to bind together events that occur at brief intervals (ie, hyperbinding) resulting in an abnormal sense of agency and awareness of action, such as delusions of influence and megalomania.131,132 Overall, an in-depth examination of the contingent relationship between altered time processing and psychiatric symptoms could increase our understanding of the neurobiological processes that are responsible for SZ. Here the challenge is to reduce diagnostic heterogeneity by aggregating neurobiological and behavioral findings in more homogeneous diagnostic subgroups of SZ that show specific pathological distortions over time, thus providing greater insight into the underlying pathophysiology.133
Before concluding we would like to discuss several issues. First, an intrinsic characteristic of meta-analysis techniques is that they depend entirely on the included studies. We do not, however, believe than any significant bias affected the above mentioned results, particularly for studies that investigated automatic and cognitively controlled timing (number of missing studies in classic fail and safe N test: 762). Conversely, as subgroup analysis requires a minimum of 4 studies to be performed,90 we could not compare studies evaluating SZ perceptual and motor timing abilities split by type of temporal processing engaged. Indeed, several psychophysical experiments122 suggest that even in the sub-second range (ie, in the automatic timing domain) the sensorimotor processing required (perception vs production) affects timing accuracy, because temporal variability is greater in perceptual tasks than in motor timing tasks. The fact that the interaction between duration and sensorimotor processing was not explored in the population under investigation might constitute a limitation of the present meta-analysis. Furthermore, although we aggregated patients’ data on the basis of SZ diagnosis, heterogeneity of the clinical characteristics might have weakened the robustness of the present results. Indeed, except for a few studies, data on duration of illness, sample matching (ie, age, gender, educational level, and IQ), inventory scores, and medication status were unavailable, so we could not investigate the effect of further explanatory variables on the main result. Specifically, there is a large consensus on the dopaminergic modulation of several timing processes96,134 because the D2 receptor antagonist haloperidol impairs temporal sensitivity in HC,135,136 and appropriate responses to antipsychotic medication have been reported to be associated with better time perception.137,138 Moreover, although a number of investigations found that the perception of time in SZ was independent from the PANSS score,19,63,65 disturbed time estimation was significantly correlated with PANSS positive symptom scores in one study.23 Given the differential neurochemical and neural substrate of positive and negative symptoms in SZ, and the diverse effect that hyper and hypoactivity in dopamine transmission have on the internal clock speed, it is possible that the relationship between temporal sensitivity and psychotic symptoms is true only for positive symptoms. Future studies in which patients are stratified according to the deficit and nondeficit and/or positive/negative types of SZ will help clarify this issue.
However, evidence of altered time perception in people with no diagnosis of SZ25, such as SZ patients’ relatives who have no clinical symptoms or take antipsychotic drugs, strongly suggests that a dysfunction in timing can be considered a trait marker of the SZ cognitive profile. Furthermore, timing distortions were also observed in drug free patients, 13 and in pharmacological manipulation studies,96,97 implying that these alterations cannot be entirely explained by the adverse effect of antipsychotic drugs on timing tasks. Future studies should more thoroughly evaluate the relationship between timing deficits and clinical variables to reveal possible patterns of dysfunctional interaction.
In conclusion, the data that emerged from the present meta-analysis show that subjects diagnosed with SZ perform more poorly than HC in all explicit timing domains, with medium to large effect sizes. As the timing apparatus can be dissected into an automatic system and a cognitively controlled one, our meta-analytic approach provides the best-evidence synthesis for the existence of a primary timing dysfunction in SZ, because no significant differential deficit in a specific timing system is detectable. As timing has been proposed to be a “primary cognitive function” involved in several aspects of neuropsychological performance (such that variations in the efficiency of specific cognitive processes derive from changes in timing accuracy) this finding may provide deeper insight into the clinical phenotype of SZ. Moreover, as interval timing is a valuable heuristic for explaining either the nature of specific cognitive disorders or the neurobiological substrates of timing dysfunctions, it can be assumed as a model system to differentiate specific ongoing pathophysiological processes in SZ to detect more homogeneous clinical subtypes. Finally, the neuroscientific study of timing in SZ may provide relevant indications for the implementation of specific therapeutic strategies to treat the disorder, because interventions aimed at reducing temporal variability have already proved effective in conditions characterized by the same dopaminergic and frontostriatal dysfunctions as those found in SZ139.
Acknowledgment
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Balci F, Meck WH, Moore H, Brunner D. Timing deficits in aging and neuropathology. In: Woods J, Bizon A, eds. Animal Models of Human Cognitive Aging. Totowa, NJ: Humana Press; 2009:161–201. [Google Scholar]
- 2. Buonomano DV. The biology of time across different scales. Nat Chem Biol. 2007;3:594–597. [DOI] [PubMed] [Google Scholar]
- 3. Mazur JE. Fixed and variable ratios and delays: further tests of an equivalence rule. J Exp Psychol Anim Behav Process. 1986;12:116–124. [PubMed] [Google Scholar]
- 4. Piras F, Coull JT. Implicit, predictive timing draws upon the same scalar representation of time as explicit timing. PLoS One. 2011;6:e18203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18:137–144. [DOI] [PubMed] [Google Scholar]
- 6. Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135:656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to date and new methods. J Neurosci Methods. 2006;151:15–29. [DOI] [PubMed] [Google Scholar]
- 8. Wearden JH, Smith-Spark JH, Cousins R, Edelstyn NM, Cody FW, O’Boyle DJ. Stimulus timing by people with Parkinson’s disease. Brain Cogn. 2008;67:264–279. [DOI] [PubMed] [Google Scholar]
- 9. Blanchard MM, Chamberlain SR, Roiser J, Robbins TW, Müller U. Effects of two dopamine-modulating genes (DAT1 9/10 and COMT Val/Met) on n-back working memory performance in healthy volunteers. Psychol Med. 2011;41:611–618. [DOI] [PubMed] [Google Scholar]
- 10. Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. [DOI] [PubMed] [Google Scholar]
- 11. Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. [DOI] [PubMed] [Google Scholar]
- 12. Rabin AI. Time estimation of schizophrenics and non-psychotics. J Clin Psychol. 1957;13:88–90. [DOI] [PubMed] [Google Scholar]
- 13. Johnson JE, Petzel TP. Temporal orientation and time estimation in chronic schizophrenics. J Clin Psychol. 1971;27:194–196. [DOI] [PubMed] [Google Scholar]
- 14. Lhamon WT, Goldstone S. The time sense: Estimation of one second durations by schizophrenic patients. AMA Arch Neurol Psychiatry. 1956;76:625–629. [PubMed] [Google Scholar]
- 15. Tysk L. Time estimation by healthy subjects and schizophrenic patients: a methodological study. Percept Mot Skills. 1983;56:983–988. [DOI] [PubMed] [Google Scholar]
- 16. Elvevåg B, McCormack T, Gilbert A, Brown GD, Weinberger DR, Goldberg TE. Duration judgements in patients with schizophrenia. Psychol Med. 2003;33:1249–1261. [DOI] [PubMed] [Google Scholar]
- 17. Davalos DB, Kisley MA, Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain Cogn. 2003;52:295–301. [DOI] [PubMed] [Google Scholar]
- 18. Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009;70:181–190. [DOI] [PubMed] [Google Scholar]
- 20. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 21. Bonnot O, de Montalembert M, Kermarrec S, Botbol M, Walter M, Coulon N. Are impairments of time perception in schizophrenia a neglected phenomenon? J Physiol Paris. 2011;105:164–169. [DOI] [PubMed] [Google Scholar]
- 22. Vogeley K, Kupke C. Disturbances of time consciousness from a phenomenological and a neuroscientific perspective. Schizophr Bull. 2007;33:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterburs J, Nitsch AM, Miltner WHR, Straube T. Impaired representation of time in schizophrenia is linked to positive symptoms and cognitive demand. PLoS One. 2013;8:e67615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roy M, Grondin S, Roy MA. Time perception disorders are related to working memory impairment in schizophrenia. Psychiatry Res. 2012;200:159–166. [DOI] [PubMed] [Google Scholar]
- 25. Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–118. [DOI] [PubMed] [Google Scholar]
- 26. Kimble M, Lyons M, O’Donnell B, Nestor P, Niznikiewicz M, Toomey R. The effect of family status and schizotypy on electrophysiologic measures of attention and semantic processing. Biol Psychiatry. 2000;47:402–412. [DOI] [PubMed] [Google Scholar]
- 27. Nestor PG, O’Donnell BF. The mind adrift: Attentional dysregulation in schizophrenia. In: Parasuraman R, ed. The Attentive Brain. Cambridge, MA: The MIT Press; 1998:527–546. [Google Scholar]
- 28. Chen EYH, McKenna PJ. Memory dysfunctions in schizophrenia. In: Pantelis C, Nelson HE, Barnes TRE, eds. Schizophrenia: A Neuropsychological Perspective. Chichester: John Wiley & Sons; 1996:107–124. [Google Scholar]
- 29. Rammsayer TH. Are there dissociable roles of the mesostriatal and mesolimbocortical dopamine systems on temporal information processing in humans? Neuropsychobiology. 1997;35:36–45. [DOI] [PubMed] [Google Scholar]
- 30. Rammsayer TH. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52:273–286. [DOI] [PubMed] [Google Scholar]
- 31. Rammsayer TH, Hennig J, Haag A, Lange N. Effects of noradrenergic activity on temporal information processing in humans. Q J Exp Psychol B. 2001;54:247–258. [DOI] [PubMed] [Google Scholar]
- 32. Rammsayer TH. Dopaminergic and serotoninergic influence on duration discrimination and vigilance. Pharmacopsychiatry. 2004;22:39–43. [DOI] [PubMed] [Google Scholar]
- 33. Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. [DOI] [PubMed] [Google Scholar]
- 34. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89:873–880. [PubMed] [Google Scholar]
- 35. World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Ninth Revision. Geneva, Switzerland; 1975. [Google Scholar]
- 36. World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Eight Revision. Geneva, Switzerland; 1965. [Google Scholar]
- 37. World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Seventh Revision. Geneva, Switzerland; 1955. [Google Scholar]
- 38. World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Sixth Revision. Geneva, Switzerland; 1949. [Google Scholar]
- 39. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland; 1992. [Google Scholar]
- 40. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association;1994. [Google Scholar]
- 41. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association;1987. [Google Scholar]
- 42. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association;1980. [Google Scholar]
- 43. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968. [PubMed] [Google Scholar]
- 44. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association;1952. [Google Scholar]
- 45. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, revised. Washington, DC: American Psychiatric Association;2000. [Google Scholar]
- 46. Dilling CA, Rabin AI. Temporal experience in depressive states and schizophrenia. J Consult Psychol. 1967;31:604–608. [DOI] [PubMed] [Google Scholar]
- 47. Foucher JR, Lacambre M, Pham BT, Giersch A, Elliott MA. Low time resolution in schizophrenia Lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res. 2007;97:118–127. [DOI] [PubMed] [Google Scholar]
- 48. Oades RD, Wild-Wall N, Juran SA, Sachsse J, Oknina LB, Röpcke B. Auditory change detection in schizophrenia: sources of activity, related neuropsychological function and symptoms in patients with a first episode in adolescence, and patients 14 years after an adolescent illness-onset. BMC Psychiatry. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang SM, Kuo LC, Ouyang WC, Hsu HM, Ma HI. A fast-moving target in the Valpar assembly task improved unimanual and bimanual movements in patients with schizophrenia. Disabil Rehabil. 2013;35:1608–1613. [DOI] [PubMed] [Google Scholar]
- 50. Waters F, Jablensky A. Time discrimination deficits in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res. 2009;167:12–20. [DOI] [PubMed] [Google Scholar]
- 51. Ojeda N, Ortuño F, Arbizu J, et al. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp. 2002;17:116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ortuño FM, Lopez P, Ojeda N, Cervera S. Dysfunctional supplementary motor area implication during attention and time estimation tasks in schizophrenia: a PET-O15 water study. Neuroimage. 2005;24:575–579. [DOI] [PubMed] [Google Scholar]
- 53. Tysk L. Estimation of time and the subclassification of schizophrenic disorders. Percept Mot Skills. 1983;57:911–918. [DOI] [PubMed] [Google Scholar]
- 54. Tysk L. Time perception and affective disorders. Percept Mot Skills. 1984;58:455–464. [DOI] [PubMed] [Google Scholar]
- 55. Tysk L. Estimation of time by patients with positive and negative schizophrenia. Percept Mot Skills. 1990;71:826. [DOI] [PubMed] [Google Scholar]
- 56. Accornero N, Argenta G, Pistone A, Decina P, Zelazek S. Research on estimation of time in schizophrenics and oligophrenics. Acta Neurologica. 1973;28:708–713. [PubMed] [Google Scholar]
- 57. De La Garza CO, Worchel P. Time and space orientation in schizophrenics. J Abnorm Psychol. 1956;52:191–194. [DOI] [PubMed] [Google Scholar]
- 58. Jenkins SB, Winkelman AC. Inverted perception of time sequence in mental disorders. Int J Neuropsychiatry. 1966;2:122–128. [PubMed] [Google Scholar]
- 59. Lhamon WT, Goldstone S. Temporal information processing in schizophrenia. Arch Gen Psychiatry. 1973;28:44–51. [DOI] [PubMed] [Google Scholar]
- 60. Normington CJ. Time estimation in process-reactive schizophrenia. J Consult Psychol. 1967;31:222. [DOI] [PubMed] [Google Scholar]
- 61. Persić-Brida M, Brajković L. Experience of time with mental disorder. Coll Antropol. 2004;28:363–376. [PubMed] [Google Scholar]
- 62. Weinstein AD, Goldstone S, Boardman WK. The effect of recent and remote frames of reference on temporal judgments of schizophrenic patients. J Abnorm Psychol. 1958;57:241–244. [DOI] [PubMed] [Google Scholar]
- 63. Bolbecker AR, Westfall DR, Howell JM, et al. Increased timing variability in schizophrenia and bipolar disorder. PLoS One. 2014;9:e97964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bourdet C, Brochard R, Rouillon F, Drake C. Auditory temporal processing in schizophrenia: high level rather than low level deficits? Cogn Neuropsychiatry. 2003;8:89–106. [DOI] [PubMed] [Google Scholar]
- 65. Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Delevoye-Turrell Y, Wilquin H, Giersch A. A ticking clock for the production of sequential actions: where does the problem lie in schizophrenia? Schizophr Res. 2012;135:51–54. [DOI] [PubMed] [Google Scholar]
- 67. Davalos DB, Kisley MA, Ross RG. Deficits in auditory and visual temporal perception in schizophrenia. Cogn Neuropsychiatry. 2002;7:273–282. [DOI] [PubMed] [Google Scholar]
- 68. Davalos DB, Kisley MA, Freedman R. Behavioral and electrophysiological indices of temporal processing dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 2005;17:517–525. [DOI] [PubMed] [Google Scholar]
- 69. Davalos DB, Rojas DC, Tregellas JR. Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophr Res. 2011;127:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Todd J, Michie PT, Budd TW, Rock D, Jablensky AV. Auditory sensory memory in schizophrenia: inadequate trace formation? Psychiatry Res. 2000;96:99–115. [DOI] [PubMed] [Google Scholar]
- 71. Todd J, Michie PT, Jablensky AV. Association between reduced duration mismatch negativity (MMN) and raised temporal discrimination thresholds in schizophrenia. Clin Neurophysiol. 2003;114:2061–2070. [DOI] [PubMed] [Google Scholar]
- 72. Broadhurst A. Time estimation related to personality, cognitive speed and schizophrenia. Life Sci. 1969;8:69–78. [DOI] [PubMed] [Google Scholar]
- 73. Carlson VR, Feinberg I. Individual variations in time judgment and the concept of an internal clock. J Exp Psychol. 1968;77:631–640. [DOI] [PubMed] [Google Scholar]
- 74. Densen ME. Time perception and schizophrenia. Percept Mot Skills. 1977;44:436–438. [DOI] [PubMed] [Google Scholar]
- 75. Orme JE. Time estimation and the nosology of schizophrenia. Br J Psychiatry. 1966;112:37–39. [DOI] [PubMed] [Google Scholar]
- 76. Papageorgiou C, Karanasiou IS, Kapsali F, et al. Temporal processing dysfunction in schizophrenia as measured by time interval discrimination and tempo reproduction tasks. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:173–179. [DOI] [PubMed] [Google Scholar]
- 77. Rutschmann J. Time judgments by magnitude estimation and magnitude production and anxiety: a problem of comparison between normals and certain schizophrenic patients. J Psychol. 1973;85:187–223. [DOI] [PubMed] [Google Scholar]
- 78. Tracy JI, Monaco C, McMichael H, et al. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Percept Mot Skills. 1998;86:515–526. [DOI] [PubMed] [Google Scholar]
- 79. van der Veen FM, Röder CH, Smits M. Feedback processing in schizophrenia: effects of affective value and remedial action. Psychiatry Res. 2013;213:108–114. [DOI] [PubMed] [Google Scholar]
- 80. Lee KH, Bhaker RS, Mysore A, Parks RW, Birkett PB, Woodruff PW. Time perception and its neuropsychological correlates in patients with schizophrenia and in healthy volunteers. Psychiatry Res. 2009;166:174–183. [DOI] [PubMed] [Google Scholar]
- 81. Turgeon M, Giersch A, Delevoye-Turrell Y, Wing AM. Impaired predictive timing with spared time interval production in individual with schizophrenia. Psychiatry Res. 2012;197:13–18. [DOI] [PubMed] [Google Scholar]
- 82. Lipsey MW, Wilson DB. Practical Meta-analysis. Thousand Oaks, CA: SAGE Publications; 2001. [Google Scholar]
- 83. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1977. [Google Scholar]
- 84. The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org.
- 85. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 86. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. [DOI] [PubMed] [Google Scholar]
- 88. Borenstein M, Hedges L V., Higgins JPT, Rothstein HR. Introduction to Meta-analysis. West Sussex, UK: John Wiley; 2009. [Google Scholar]
- 89. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 90. Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64:1187–1197. [DOI] [PubMed] [Google Scholar]
- 91. Brown SM, Kieffaber PD, Carroll CA, et al. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn. 2005. [DOI] [PubMed] [Google Scholar]
- 92. Carroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Volz HP, Nenadic I, Gaser C, Rammsayer T, Häger F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12: 313–316. [DOI] [PubMed] [Google Scholar]
- 94. Wahl OF, Sieg D. Time estimation among schizophrenics. Percept Mot Skills. 1980;50:535–541. [DOI] [PubMed] [Google Scholar]
- 95. Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. [DOI] [PubMed] [Google Scholar]
- 96. Coull JT, Morgan H, Cambridge VC, et al. Ketamine perturbs perception of the flow of time in healthy volunteers. Psychopharmacology (Berl). 2011;218:543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rammsayer TH. Effects of pharmacologically induced changes in NMDA receptor activity on human timing and sensorimotor performance. Brain Res. 2006;1073-1074:407–416. [DOI] [PubMed] [Google Scholar]
- 98. Coy AL, Hutton SB. The influence of hallucination proneness and social threat on time perception. Cogn Neuropsychiatry. 2013;18:463–476. [DOI] [PubMed] [Google Scholar]
- 99. Lee KH, Dixon JK, Spence SA, Woodruff PW. Time perception dysfunction in psychometric schizotypy. Pers Individ Dif. 2006;40:1363–1373. [Google Scholar]
- 100. Lewis PA, Miall RC. Remembering the time: a continuous clock. Trends Cogn Sci. 2006;10:401–406. [DOI] [PubMed] [Google Scholar]
- 101. Rammsayer T. Temporal discrimination in schizophrenic and affective disorders: evidence for a dopamine-dependent internal clock. Int J Neurosci. 1990;53:111–120. [DOI] [PubMed] [Google Scholar]
- 102. Elvevåg B, Brown GD, McCormack T, Vousden JI, Goldberg TE. Identification of tone duration, line length, and letter position: an experimental approach to timing and working memory deficits in schizophrenia. J Abnorm Psychol. 2004;113:509–521. [DOI] [PubMed] [Google Scholar]
- 103. Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. [DOI] [PubMed] [Google Scholar]
- 104. Granholm E, Fish SC, Verney SP. Pupillometric measures of attentional allocation to target and mask processing on the backward masking task in schizophrenia. Psychophysiology. 2009;46:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Näätänen R, Schröger E, Karakas S, Tervaniemi M, Paavilainen P. Development of a memory trace for a complex sound in the human brain. Neuroreport. 1993;4:503–506. [DOI] [PubMed] [Google Scholar]
- 106. Näätänen R. Attention and Brain Function. London: Psychology Press, 1992. [Google Scholar]
- 107. Catts SV, Shelley AM, Ward PB, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995;152:213–219. [DOI] [PubMed] [Google Scholar]
- 108. Kasai K, Okazawa K, Nakagome K, et al. Mismatch negativity and N2b attenuation as an indicator for dysfunction of the preattentive and controlled processing for deviance detection in schizophrenia: a topographic event-related potential study. Schizophr Res. 1999;35:141–156. [DOI] [PubMed] [Google Scholar]
- 109. Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. [DOI] [PubMed] [Google Scholar]
- 110. Michie PT, Budd TW, Todd J, et al. Duration and frequency mismatch negativity in schizophrenia. Clin Neurophysiol. 2000;111:1054–1065. [DOI] [PubMed] [Google Scholar]
- 111. Todd J, Michie PT, Jablensky AV. Do loudness cues contribute to duration mismatch negativity reduction in schizophrenia? Neuroreport. 2001;12:4069–4073. [DOI] [PubMed] [Google Scholar]
- 112. Todd J, Michie PT, Schall U, Karayanidis F, Yabe H, Näätänen R. Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008;63:58–64. [DOI] [PubMed] [Google Scholar]
- 113. Umbricht D, Koller R, Schmid L, et al. How specific are deficits in mismatch negativity generation to schizophrenia? Biol Psychiatry. 2003;53:1120–1131. [DOI] [PubMed] [Google Scholar]
- 114. Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry Res. 2009;169:212–219. [DOI] [PubMed] [Google Scholar]
- 115. Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. 2009;1:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Maier W. Common risk genes for affective and schizophrenic psychoses. Eur Arch Psychiatry Clin Neurosci. 2008;258(suppl 2):37–40. [DOI] [PubMed] [Google Scholar]
- 117. Möller HJ. Bipolar disorder and schizophrenia: distinct illnesses or a continuum? J Clin Psychiatry. 2003;64(suppl 6):23–27; discussion 28. [PubMed] [Google Scholar]
- 118. Kraepelin E, Diefendorf AR. Clinical Psychiatry: A Text-Book for Students and Physicians. New York, NY: Macmillan, 1915. [Google Scholar]
- 119. Schmidt H, McFarland J, Ahmed M, McDonald C, Elliott MA. Low-level temporal coding impairments in psychosis: preliminary findings and recommendations for further studies. J Abnorm Psychol. 2011;120:476–482. [DOI] [PubMed] [Google Scholar]
- 120. Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn Sci. 2000;4:14–21. [DOI] [PubMed] [Google Scholar]
- 121. Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Merchant H, Zarco W, Prado L. Do we have a common mechanism for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. J Neurophysiol. 2008;99:939–949. [DOI] [PubMed] [Google Scholar]
- 123. Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Spoletini I, Cherubini A, Di Paola M, et al. Reduced fronto-temporal connectivity is associated with frontal gray matter density reduction and neuropsychological deficit in schizophrenia. Schizophr Res. 2009;108:57–68. [DOI] [PubMed] [Google Scholar]
- 125. Chiapponi C, Piras F, Piras F, Fagioli S, Caltagirone C, Spalletta G. Cortical grey matter and subcortical white matter brain microstructural changes in schizophrenia are localised and age independent: a case-control diffusion tensor imaging study. PLoS One. 2013;8:e75115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. [DOI] [PubMed] [Google Scholar]
- 127. Karmarkar UR, Buonomano DV. Timing in the absence of clocks: encoding time in neural network states. Neuron. 2007;53:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Giersch A, Lalanne L, Corves C, et al. Extended visual simultaneity thresholds in patients with schizophrenia. Schizophr Bull. 2009;35:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Haggard P, Martin F, Taylor-Clarke M, Jeannerod M, Franck N. Awareness of action in schizophrenia. Neuroreport. 2003;14:1081–1085. [DOI] [PubMed] [Google Scholar]
- 132. Maeda T, Takahata K, Muramatsu T, et al. Reduced sense of agency in chronic schizophrenia with predominant negative symptoms. Psychiatry Res. 2013;209:386–392. [DOI] [PubMed] [Google Scholar]
- 133. Heinrichs RW. In Search of Madness: Schizophrenia and Neuroscience. New York, NY: Oxford University Press, 2001. [Google Scholar]
- 134. Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291 . [DOI] [PubMed] [Google Scholar]
- 135. Rammsayer T, Lustnauer S. Sex differences in time perception. Percept Mot Skills. 1989;68:195–198. [DOI] [PubMed] [Google Scholar]
- 136. Rammsayer TH. On dopaminergic modulation of temporal information processing. Biol Psychol. 1993;36:209–222. [DOI] [PubMed] [Google Scholar]
- 137. Angle HV. Role of chlorpromazine in maintaining timing behavior in chronic schizophrenics. Psychopharmacologia. 1973;28:185–194. [DOI] [PubMed] [Google Scholar]
- 138. Ulferts J, Meyer-Lindenberg A, Gallhofer B. Time discrimination: a comparison between healthy controls, unmedicated schizophrenics, zotepine-treated schizophrenics and schizophrenics treated with conventional neuroleptics. Neuropsychiatrie. 1999;13:133–138. [Google Scholar]
- 139. del Olmo MF, Cudeiro J. Temporal variability of gait in Parkinson disease: effects of a rehabilitation programme based on rhythmic sound cues. Parkinsonism Relat Disord. 2005;11:25–33. [DOI] [PubMed] [Google Scholar]