Abstract
Glial disturbances are highly implicated in the pathophysiology of schizophrenia and may be linked with glutamatergic dysregulation. Myo-inositol (mI), a putative marker of glial cells, and choline (Cho), representative of membrane turnover, are both present in larger concentrations within glial cells than in neurons, and their elevation is often interpreted to reflect glial activation. Proton magnetic resonance spectroscopy (1H-MRS) allows for the evaluation of mI, Cho, glutamate, glutamate + glutamine (Glx), and N-acetylaspartate (NAA). A collective investigation of these measures in antipsychotic-naive patients experiencing their first nonaffective episode of psychosis (FEP) can improve the understanding of glial dysfunction and its implications in the early stages of schizophrenia. 3-Tesla 1H-MRS (echo time = 35ms) was performed in 60 antipsychotic-naive patients with FEP and 60 age- and sex-matched healthy controls. mI, Cho, glutamate, Glx, and NAA were estimated using LCModel and corrected for cerebrospinal fluid composition within the voxel. mI, Cho, and glutamate were elevated in the FEP group. After correction for multiple comparisons, mI positively correlated with grandiosity. The relationships between mI and glutamate, and Cho and glutamate, were more positive in the FEP group. These findings are suggestive of glial activation in the absence of neuronal loss and may thereby provide support for the presence of a neuroinflammatory process within the early stages of schizophrenia. Dysregulation of glial function might result in the disruption of glutamatergic neurotransmission, which may influence positive symptomatology in patients with FEP.
Key words: MRS, schizophrenia, neuroinflammation, glutamatergic, positive symptoms, astrocyte
Introduction
Glial disturbances are highly implicated in the pathophysiology of schizophrenia.1,2 Myo-inositol (mI) and choline-containing compounds (Cho) act as markers of glial cells and membrane metabolism, respectively.3–6 Both mI and Cho are present in higher concentrations within glial cells than in neurons7–9 and have been investigated in patients with schizophrenia using proton magnetic resonance spectroscopy (1H-MRS).10–12 Elevated levels of these neurometabolites have been proposed to reflect glial activation and have been observed in several neuroinflammatory disorders.4
Astrocytes (a subtype of glial cells) contribute to the regulation of glutamatergic neurotransmission.13,14 Glutamatergic dysregulation is thought to be involved in the schizophrenia disease process15–18 and has been evaluated in patients with schizophrenia using 1H-MRS through the measurement of glutamate, glutamine, and glutamate + glutamine (Glx).19–23 1H-MRS has also been used to ascertain levels of N-acetylaspartate (NAA), which serves as an index of neuronal integrity.24,25
Existing 1H-MRS literature is heterogeneous in terms of voxel placement, stage of illness, and medication status; antipsychotic treatment has been suggested to influence the assessment of mI, Cho, glutamatergic markers, and NAA levels.22,26–28 Our group has previously investigated neurometabolic differences in antipsychotic-naive patients experiencing their first nonaffective episode of psychosis (FEP) within the right associative striatum, an area rich in dopamine afferents and dopamine D2 receptors, which is involved in the pathophysiology of schizophrenia29,30 and is often included in the quantification of in vivo occupancy studies of antipsychotics.31,32 Our first study found higher Cho and glutamate levels in the FEP group in comparison to controls.20 We replicated these findings in a longitudinal study, in which we also found elevated baseline mI and Glx levels in the FEP group.33
In the present study, we used a larger sample to compare mI, Cho, glutamate, Glx, and NAA levels in the associative striatum between antipsychotic-naive patients with FEP and a group of age- and sex-matched healthy controls. We also explored the associations between neurometabolite levels and clinical measures, as well as the relationships amongst levels of neurometabolites. We hypothesized that neurometabolites predominantly present in glial cells would be elevated in the FEP group along with levels of glutamatergic compounds, in accordance with our previous findings. Our additional hypotheses were exploratory. We hypothesized that dysregulated neurometabolite levels would correlate with clinical symptom severity. Also, we hypothesized that abnormal levels of glial neurometabolites would be linked with disrupted glutamatergic levels in the patient group, such that the relationships between these measures would differ from healthy controls. The assessment of unmedicated patients is vital towards characterizing the pathophysiology of schizophrenia in that the confounding effects of medication are eliminated.34 To the best of our knowledge, this is the largest sample to date of antipsychotic-naive patients with FEP in which 1H-MRS was performed.
Methods
Participants
This study received approval from the Ethics and Scientific Committees of the National Institute of Neurology and Neurosurgery of Mexico (INNN). Individuals were included after providing informed written consent, which was obtained from both parents for participants under 18 years old. Participants did not receive a stipend.
Sixty-four patients were recruited during their FEP from inpatient or outpatient services at the INNN between 2008 and 2013. The Structured Clinical Interview for DSM-IV was utilized to determine inclusion. Patients met inclusion criteria if they were antipsychotic naive; all but 3 patients had less than 2 years of psychotic symptoms. Exclusion criteria included a concomitant medical or neurological illness, current substance abuse or history of substance dependence (excluding nicotine), comorbidity with other Axis I disorders, a high risk for suicide, and psychomotor agitation. Sixty-three age- and sex-matched healthy controls were also enrolled and assessed in the same manner as the patients. Controls with a history of psychiatric illness or a family history of psychosis were excluded.
Each participant was screened for drugs of abuse, including cannabis, cocaine, heroin, opioids, and benzodiazepines at the time of inclusion and 1 hour prior to the magnetic resonance imaging (MRI) scan. The current sample included a subset of participants (FEP: n = 35; controls: n = 35) previously reported upon20,33; additional subjects were added to increase statistical power.
Clinical Assessment
Patients’ psychopathology was assessed by research psychiatrists (C.d.l.F.-S., F.R.-M., P.L.-O.) using the Positive and Negative Syndrome Scale (PANSS).35
Magnetic Resonance Studies
Participants were scanned at the INNN in a 3T GE whole-body scanner (Signa Excite HDxt; GE Healthcare) with a high-resolution 8-channel head coil. The participant’s head was positioned along the canthomeatal line and immobilized using a forehead strap. Each participant was scanned using a T1-weighted spoiled gradient-echo 3-dimensional axial acquisition (SPGR, echo time [TE] = 5.7ms, repetition time [TR] = 13.4ms, inversion time = 450ms, flip angle = 20°, field of view = 25.6cm, ≥256 × ≥256 matrix, slice thickness ≤ 1.2mm), oriented above and parallel to the anterior-posterior commissure line. These T1-weighted SPGR images were reformatted to sagittal and coronal views and were subsequently used for 1H-MRS voxel localization.
1H-MRS spectra were obtained using point-resolved spectroscopy (PRESS, TE = 35ms, TR = 2000ms, spectral width = 5000 Hz, 4096 data points used, 128 water-suppressed, and 16 water-unsuppressed averages) centered on the right dorsal-caudate nucleus in volume elements (voxels) of 8ml (2×2 × 2cm). The lower end of the dorsal-caudate voxel (associative striatum) was located 3mm dorsal to the anterior commissure to include maximum gray matter (GM) and with a dorsal extension (thickness) of 2cm. Voxel placement is identified in supplementary figure 1. During the acquisition, 1H-MRS spectra were shimmed to achieve a full-width at half maximum (FWHM) of 12 Hz or less, measured on the unsuppressed water signal from the voxel. Spectra with larger FWHM were excluded from ensuing analyses.23
1H-MRS Data Analysis
All water-suppressed spectra were analyzed using LCModel version 6.3-0E.36 Spectra were normalized to the unsuppressed water signal, allowing for neurometabolite quantification, expressed in institutional units. A standard basis set of metabolites, delineated within the supplementary material, was used for analysis. In this study, Cho is the sum of glycerophosphocholine + phosphocholine, NAA is the sum of NAA + N-acetylaspartylglutamate, and creatine-containing compounds (Cr) is the sum of creatine + phosphocreatine. One analyzed spectrum is included in supplementary figure 2.
Spectra with %SD values of 20% or greater for neurometabolites of interest were considered poor quality and excluded from subsequent analyses.37,38 Four patients and 3 controls were excluded due to either rejection by LCModel analysis or a FWHM greater than 12 Hz, resulting in the inclusion of 60 patients and 60 healthy controls; of the 7 participants/spectra removed in total, 1 was previously reported upon. Glutamine was not analyzed because of poor spectra fitting. To control for correlations introduced by the LCModel fitting procedure, the triangular table of correlation coefficients was used. All reported metabolite correlations did not show strong negative pairwise correlations in the triangular table: no correlational coefficients were less than −0.5.38
T1-weighted MRI scans used for voxel localization were segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) using Statistical Parametric Mapping 8 (SPM8, Wellcome Department of Imaging Neurosciences, University College London, UK). The size and location of each area were extracted from the spectra file headers to calculate the percentage of GM, WM, and CSF content within the voxel using an in-house software, allowing for the correction of the CSF fraction of the spectroscopic values.20
Statistical Analysis
Statistical analyses were performed using SPSS Statistics version 20 (IBM Corporation). Demographic and clinical characteristics, Cramer-Rao lower bounds (CRLBs), FWHM values, signal-to-noise ratios, GM, WM, and CSF percentages, and GM/(GM + WM) were compared between groups using independent-sample t tests. Frequency data were analyzed using χ2 or Fisher’s exact tests. Neurometabolite levels were compared between groups using analyses of variance. To check for confounders, tobacco use, GM content, GM/(GM + WM), and age were each investigated as covariates. Outliers were defined as greater than 3 times the interquartile range and were removed in a neurometabolite-specific manner; 2 mI outliers, 1 glutamate outlier, and 1 Cr outlier were removed. Due to a priori hypotheses, neurometabolite level group comparisons were conducted with a significance level of P < .05.
Pearson correlations were performed to investigate the association between PANSS subscale total scores and neurometabolite levels that differed significantly between groups. If any correlation reached an uncorrected P < .05, Pearson correlations between the neurometabolite and items within the specific PANSS subscale were also examined. All investigations were corrected for multiple comparisons and a statistical threshold of P < .05 ÷ n was used, where n = # of comparisons (n = 16; 3 neurometabolites with 3 subscale total scores and 1 neurometabolite with 7 subscale items).
The relationships amongst levels of neurometabolites that differed significantly between groups were assessed using Pearson correlations. A statistical threshold of P < .05 ÷ n was used, where n = # of comparisons (n = 6; 3 neurometabolites investigated separately for each group).
Group differences in correlational coefficients were evaluated by converting correlational coefficients with Fisher’s transformation (Equation 1) and comparing them using Fisher’s z test (Equation 2), which allowed for z score acquisition. Here, comparisons were conducted with a significance level of P < .05.
| (1) |
| (2) |
Above, r represents the sample correlational coefficient, r′ is the transformed value of r, n indicates sample size, and z refers to z score.
Results
Demographic and Clinical Characteristics
Participants’ demographic and clinical characteristics are reported in supplementary table 1. Patients’ DSM-IV diagnoses were: brief psychotic disorder (n = 14), schizophreniform disorder (n = 21), and schizophrenia (n = 25). Education years were higher in the control group (t(118) = 6.40, P < .001), while tobacco use was greater in the FEP group (χ2 = 5.21, P = .039). Age, sex, handedness, and cannabis use did not differ between groups. The FEP group had a mean duration of untreated psychosis of 33.03±52.70 weeks, and mean PANSS positive, negative, and general psychopathology subscale total scores of 24.13±4.97, 24.33±5.66, and 48.75±8.38, respectively.
Neurometabolite Levels
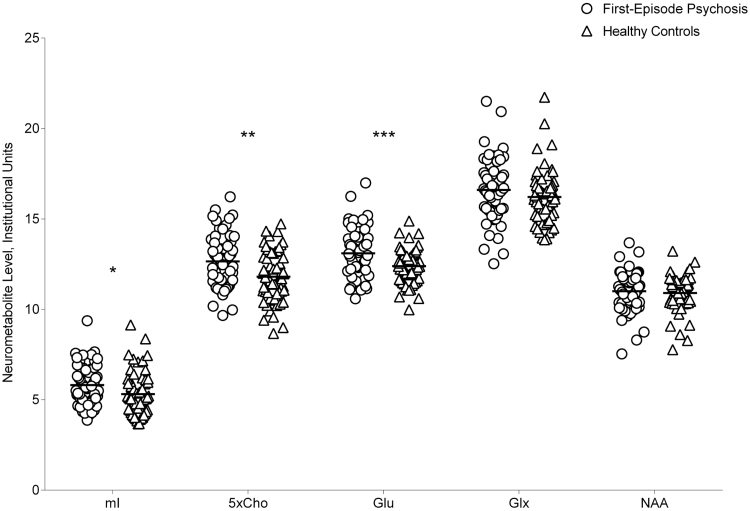
Neurometabolite levels are reported in table 1 and displayed in figure 1. mI, Cho, and glutamate levels were higher in the FEP group (F(1,116) = 5.66, P = .019; F(1,118) = 10.66, P = .001; F(1,117) = 11.63, P < .001, respectively). Glx and NAA levels did not differ between groups (F(1,118) = 1.84, P = .18; F(1,118) = 0.29, P = .59, respectively). Results were unaffected when tobacco use, GM content, GM/(GM + WM), and age were included as covariates.
Table 1.
Neurometabolite Levels in Patients With First-Episode Psychosis and Healthy Controls
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| mI | Cho | Glu | Glx | NAA | Cr | |
| FEP group | 5.81 (1.10)* | 2.53 (0.29)** | 13.10 (1.31)*** | 16.61 (1.69) | 11.00 (1.08) | 8.61 (0.74) |
| HC group | 5.31 (1.19) | 2.36 (0.28) | 12.39 (0.95) | 16.21 (1.50) | 10.90 (0.96) | 8.49 (0.75) |
Note: Cho, choline-containing compounds; Cr, creatine-containing compounds; FEP, first-episode psychosis; Glu, glutamate; Glx, glutamate + glutamine; HC, healthy control; mI, myo-inositol; NAA, N-acetylaspartate.
*P < .05.
**P < .01.
***P < .001.
Fig. 1.
Neurometabolite levels in patients with first-episode psychosis and healthy controls. Cho, choline-containing compounds; Glu, glutamate; Glx, glutamate + glutamine; mI, myo-inositol; NAA, N-acetylaspartate; *P < .05; **P < .01; ***P < .001.
Relationships With Clinical Measures
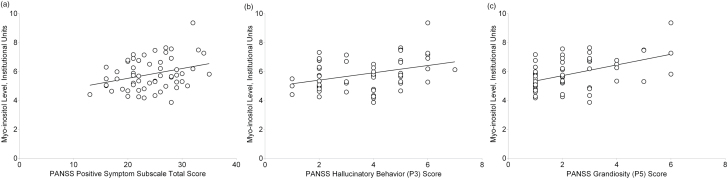
The relationships between neurometabolites and PANSS subscale total scores are presented in table 2. After correction for multiple comparisons, mI levels were positively correlated at a trend-level significance with PANSS positive total score (r(57) = .31, P-uncorrected = .017) (figure 2a) and PANSS item P3 (Hallucinatory Behavior) score (r(57) = .37, P-uncorrected = .004) (figure 2b). mI levels were also positively correlated with PANSS item P5 (Grandiosity) score (r(57) = .49, P-uncorrected < .001) (figure 2c). mI levels were not related to PANSS negative or general psychopathology total scores. Removing a potential outlier (mI > 8) did not alter findings. Cho and glutamate levels were not related to any PANSS subscale total scores. Including tobacco use, GM content, GM/(GM + WM), and age as covariates did not affect results.
Table 2.
Relationships Between Neurometabolite Levels and PANSS Subscale Total Scores
| Correlational Coefficient (r) | |||
|---|---|---|---|
| Variable | mI | Cho | Glu |
| PANSS subscale | |||
| Positive | r(57) = .31, P = .017* | r(58) = .11, P = .40 | r(58) = −.05, P = .71 |
| Negative | r(57) = −.04, P = .78 | r(58) = .03, P = .82 | r(58) = −.01, P = .91 |
| General psychopathology | r(57) = .08, P = .56 | r(58) = .06, P = .65 | r(58) = .001, P = .99 |
Note: Cho, choline-containing compounds; Glu, glutamate; mI, myo-inositol; PANSS, Positive and Negative Syndrome Scale.
*P-uncorrected < .05.
Fig. 2.
Relationships between myo-inositol levels and positive symptom subscale total (a), hallucinatory behavior (b), and grandiosity (c) scores. PANSS, Positive and Negative Syndrome Scale.
Relationships Between Neurometabolites
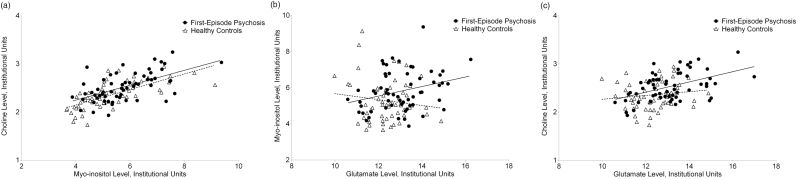
mI levels positively correlated with Cho levels in both groups (FEP: r(57) = .58, P-uncorrected < .001; control: r(57) = .69, P-uncorrected < .001) (figure 3a). mI levels positively correlated with glutamate levels in the FEP group only (FEP: r(57) = .29, P-uncorrected = .024; control: r(56) = −.13, P-uncorrected = .32) (figure 3b), though this relationship did not survive correction for multiple comparisons. Cho levels positively correlated with glutamate in the FEP group only (FEP: r(58) = .48, P-uncorrected < .001; control: r(57) = .13, P-uncorrected = .32) (figure 3c). Results were unaffected by the addition of tobacco use, GM content, GM/(GM + WM), and age as covariates.
Fig. 3.
Relationships between levels of myo-inositol and choline (a), glutamate and myo-inositol (b), and glutamate and choline (c) in patients with first-episode psychosis and healthy controls.
r-to-Z transformations identified that the relationships between levels of mI and glutamate, and Cho and glutamate, were more positive in the FEP group (Z = 2.26, P = .024; Z = 2.08, P = .038, respectively). The relationships between levels of mI and Cho were not different between groups (Z = .98, P = .33).
CRLB, FWHM, Signal-to-Noise Ratios, and Tissue Heterogeneity
CRLBs, FWHM values, signal-to-noise ratios, GM, WM, and CSF percentages, and GM/(GM + WM) did not differ between groups (supplementary tables 2 and 3).
Discussion
The present study investigated neurometabolite levels in the associative striatum within the largest sample of antipsychotic-naive patients with FEP to date and a group of age- and sex-matched healthy controls. We found elevations in mI, Cho, and glutamate levels in the FEP group. Additionally, mI levels positively correlated with grandiosity, while positive symptom total score and hallucinatory behavior positively correlated with mI levels at a trend-level significance. Lastly, the correlations between levels of mI and glutamate, and Cho and glutamate, were more positive in the FEP group.
We previously reported increases in mI and Cho levels within the associative striatum of antipsychotic-naive patients with FEP.20,33 Though most 1H-MRS studies have found unaltered levels of mI and Cho,10–12,39 others have reported deviations in these neurometabolites within several brain regions.40–44 To the best of our knowledge, no previous 1H-MRS study has observed statistical differences in mI levels within the striatum of patients with schizophrenia. However, in terms of Cho, one study found increased levels in the caudate nucleus of antipsychotic-naive patients with schizophrenia,45 while others have reported increases in the basal ganglia, encompassing caudate and lenticular nucleus regions, within medicated patients.46,47
Both mI and Cho are present in greater concentrations within glial cells than in neurons.7–9 The strong correlation between mI and Cho levels in both groups suggests that these neurometabolites are linked, although these results must be interpreted with caution due to the potential for spurious correlations.48 Elevated levels of mI and Cho are often interpreted as glial activation, which is commonly associated with a neuroinflammatory response4; accordingly, mI and Cho levels are elevated in several neuroinflammatory disorders.4,49–52 Recently, Chiappelli et al reported that mI levels within frontal WM were negatively correlated with fractional anisotropy of WM in both patients with schizophrenia and in healthy controls53; in support of the link between mI and neuroinflammation, this finding was interpreted by the authors as evidence for a general effect of inflammation on WM microstructure. Further, previous literature has suggested that schizophrenia may have a neuroinflammatory component and that antipsychotics may have anti-inflammatory effects.54–56 Additionally, anti-inflammatory agents might have beneficial effects on symptomatology as adjunctive therapies.57,58 In our study, the concomitant elevation of mI and Cho levels in the patient group may provide an 1H-MRS finding in support of early neuroinflammation that either accompanies or precedes the FEP in schizophrenia.
Furthermore, consistent with our previous reports,20,33 we found elevated glutamate levels in the FEP group. Notably, this result was also identified in the subjects not included in previous reports (F(1,48) = 13.53, P < .001; Cohen’s d = 1.05). This finding is in accordance with previous 1H-MRS literature suggesting increased levels of glutamatergic markers in antipsychotic-naive patients with schizophrenia, minimally treated patients with schizophrenia, and individuals at ultra-high risk for psychosis who later transitioned to psychosis21–23,59–61—levels that may subsequently normalize to or decrease below those of healthy controls following antipsychotic treatment.22,23,27,62–64 While some previous 1H-MRS studies investigating the basal ganglia (including lenticular nucleus, putamen, and substantia nigra regions) in patients with schizophrenia have failed to find differences in glutamatergic markers,44,65 our findings are comparable to those of Goto et al, who reported increased basal ganglia Glx in patients with first-episode schizophrenia66; however, it is important to distinguish between glutamate and Glx levels in this comparison, especially since only the former was found to be elevated in our study and the latter is a composite measure of both glutamate and glutamine levels.
The present study was also supplemented by the investigation of the relationships between levels of glutamate and levels of mI and Cho. Studies localizing mI and Cho to glial cells specifically found elevated concentrations of these neurometabolites within astrocytes.7–9 Typically, synaptic glutamate is taken up by astrocytes and converted to glutamine.13,14 Thus, the parallel increase of mI and Cho levels in patients with FEP may support a mechanism wherein astrocytic function is abnormally altered in response to a pathological process and glutamatergic neurotransmission is consequently disturbed, as suggested by our observation of increased glutamate levels. This notion is reinforced by our findings of positive correlations between levels of mI and glutamate, and Cho and glutamate, in the FEP group only, and the fact that the correlational coefficients of these relationships were more positive in the FEP group. Previous studies have observed increases in S100B, a marker for astrocyte function, in patients with schizophrenia during acute psychosis stages, in addition to concomitant increases in mI levels, supporting the notion that astrocytic activation with associated mI elevation may exist in schizophrenia.67 Likewise, elevated Cho levels have been interpreted to represent increased astrocytic turnover of glutamatergic compounds.19
Though the exact mechanism by which astrocytic dysfunction might lead to glutamatergic dysregulation has not been characterized, evidence in patients with schizophrenia has suggested a role for astrocytic overproduction of kynurenic acid, an endogenous N-methyl-d-aspartate receptor (NMDAR) antagonist.68,69 Given that the administration of exogenous NMDAR antagonists leads to increased levels of glutamatergic compounds,70,71 elevated kynurenic acid may connect the aforementioned phenomena.72–74 Additionally, astrocyte dysfunction might disturb glutamate transporter function, preventing the reuptake of extracellular glutamate75–78 and thereby contributing to dysregulated glutamatergic neurotransmission.
The relationships between mI levels and positive symptom total score, hallucinatory behavior, and grandiosity also warrant discussion. Though 2 of these correlations did not retain significance after correction for multiple comparisons, our results provide some suggestion that mI levels may be linked with positive symptomatology, reinforcing the notion that the group difference in mI levels is related to illness pathophysiology. We believe this is the first study to suggest that mI might be related to positive symptomatology, while associations with other symptom domains have been observed. Our group previously reported trend-level reductions in mI levels following clinically effective antipsychotic treatment (PANSS total score reduction of at least 30%) of antipsychotic-naive patients with FEP.33 In medicated patients with schizophrenia, Homan et al found a negative relationship between mI levels in Broca’s area and total PANSS scores.79 Furthermore, Chiappelli et al found a negative correlation between trait depressive symptoms and anterior cingulate cortex mI levels in patients with schizophrenia spectrum disorders and in healthy controls.80 The authors also observed lower mI levels in patients with at least one major depressive episode, suggesting that mI may be a biomarker of depressive symptoms in this patient population. In the present study, we propose that within the associative striatum, mI is related to positive symptomatology through astrocytic dysregulation of glutamatergic neurotransmission.81 However, despite the elevation in glutamate levels within the FEP group, glutamate was unexpectedly not associated with symptomatology. Thus, the exact mechanism connecting increased mI levels and positive symptoms remains elusive and necessitates further investigation.
In terms of NAA levels, we failed to find group differences, contrasting previous 1H-MRS studies that report reductions.10,82 Our finding suggests preserved neuronal integrity in the associative striatum at an early stage of schizophrenia. We posit that neuronal loss occurs later in the illness, resulting from either glutamate-mediated excitotoxicity or the advancement and chronicity of neuroinflammation and glial activation.4,83 These processes would align with literature suggesting progressive NAA reductions in schizophrenia.12
One methodological consideration is that neurometabolite concentrations were referenced relative to water. While mI, Cho, and glutamate levels were elevated in the FEP group, it deserves emphasis that Glx and NAA levels did not differ between groups. Thus, even though neurometabolite concentrations were corrected for CSF, decreased water content likely did not drive group differences, as further evidenced by the similar voxel CSF content between groups. Notably, when referenced to Cr levels, which importantly did not differ between groups (F(1,117) = 0.70, P = .41),10 results did not differ for analyses concerning group differences in neurometabolite levels (mI: F(1,116) = 4.45, P = .037; Cho: F(1,118) = 6.06, P = .015; glutamate: F(1,118) = 4.82, P = .030; Glx: F(1,118) = 0.02, P = .90; NAA: F(1,118) = 0.40, P = .53; supplementary table 4) and their relationships with clinical symptoms (supplementary table 5), whereas findings related to the relationships among neurometabolite levels were not identical (supplementary table 6).
Our study is not without limitations. First, the functions assigned to neurometabolites do not comprehensively delineate their physiological roles. Particularly, the involvement of mI and Cho extend beyond glial cells and neuroinflammation to include an extensive range of other structural and signaling functions. Second, the wide age range in our sample is a source of biographic inhomogeneity. While age has been shown to influence neurometabolite levels,41,42,53,84,85 it was not presently a primary focus, though analyses concerning age are included in supplementary table 7. Of note, in the full sample, mI levels were positively correlated with age at a trend-level significance (r(116) = .17, P = .066), a finding consistent with past work.53 Third, 1H-MRS cannot distinguish between extracellular and intracellular measurements and does not directly assess neurotransmission. Fourth, using a TE of 35ms at 3T renders glutamate and glutamine difficult to distinguish; thus, the glutamate peak may be contaminated by glutamine. This was evidenced by the correlational coefficients between glutamate and glutamine in the triangular table, which were close to or less than −0.5. Fifth, at a TE of 35ms, Glx levels may contaminate the NAA peak.86 Sixth, not all patients with FEP progress to schizophrenia, affecting generalizability, although 73% of the FEP group (44 patients) received a follow-up diagnosis of schizophrenia. Seventh, only the right associative striatum was studied to reduce imaging time in patients with active psychosis. However, previous 1H-MRS studies did not observe laterality differences in levels of mI, Cho, or glutamatergic markers in patients with schizophrenia.42, 87 Eighth, the group difference in nicotine smoking presents an important limitation. Ninth, chemical shift artifacts were not specifically addressed, though it is noteworthy that with PRESS at 3T, they could potentially account for a reduced water signal in the patient group. Tenth, simulated macromolecular resonances may not be representative of the true macromolecular spectrum. Finally, since all neurometabolite levels are relative to water, spurious correlations may exist between pairs of neurometabolites. However, similar to the reasoning provided by Kraguljac et al,88 our hypothesis was initially formulated in terms of ratios and primarily concerns a difference in correlations between patients and controls, which we were able to assess using r-to-Z transformations.
Taken together, our findings are suggestive of glial activation in the absence of neuronal loss and may thereby provide support for the presence of a neuroinflammatory process within the early stages of schizophrenia. Astrocytic dysfunction might disrupt glutamatergic neurotransmission, which may subsequently influence positive symptomatology in patients with FEP and may have an excitotoxic effect in later stages of the illness. Considering that approximately 20% to 35% of patients have unremitting positive symptoms following antipsychotic treatment,89,90 the development of a fuller picture of schizophrenia and its neurochemical underpinnings is vital towards understanding the pathophysiology of the illness and improving treatment interventions. Future research should continue to investigate neuroinflammation and glial abnormalities in schizophrenia, as well as their impact on glutamatergic neurotransmission.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) (182279 to C.d.l.F.-S., A.G.-G.); the CONACyT Scholarship (F.R.-M., P.L.-O.); the Sistema Nacional de Investigadores (C.d.l.F.-S., A.G.-G.); the Canadian Institute of Health Research (CIHR) (MOP-114989 to A.G.-G.) and the Canada Graduate Scholarship (E.P.).
Supplementary Material
Acknowledgments
We thank Dr Stephen Provencher for his invaluable technical support. C.d.l.F.-S. has received support from the United States National Institute of Health (US-NIH), CONACyT, the Instituto de Ciencia y Tecnología del DF (ICyTDF), Janssen, AstraZeneca, and Eli Lilly. A.G.-G. has received support from US-NIH, CIHR, the Ontario Mental Health Foundation, CONACyT, ICyTDF, the Brain & Behavior Research Foundation (Formerly NARSAD), the Ontario Ministry of Health and Long-Term Care, the Ontario Ministry of Research and Innovation Early Research Award, and Janssen. All other authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophr Res. 2015;161:4–18. [DOI] [PubMed] [Google Scholar]
- 2. Goudriaan A, de Leeuw C, Ripke S, et al. Specific glial functions contribute to schizophrenia susceptibility. Schizophr Bull. 2014;40:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bustillo JR. Use of proton magnetic resonance spectroscopy in the treatment of psychiatric disorders: a critical update. Dialogues Clin Neurosci. 2013;15:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol. 2013;8:576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malhi GS, Valenzuela M, Wen W, Sachdev P. Magnetic resonance spectroscopy and its applications in psychiatry. Aust N Z J Psychiatry. 2002;36:31–43. [DOI] [PubMed] [Google Scholar]
- 6. Vance AL, Velakoulis D, Maruff P, Wood SJ, Desmond P, Pantelis C. Magnetic resonance spectroscopy and schizophrenia: what have we learnt? Aust N Z J Psychiatry. 2000;34:14–25. [DOI] [PubMed] [Google Scholar]
- 7. Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. [DOI] [PubMed] [Google Scholar]
- 8. Griffin JL, Bollard M, Nicholson JK, Bhakoo K. Spectral profiles of cultured neuronal and glial cells derived from HRMAS (1)H NMR spectroscopy. NMR Biomed. 2002;15:375–384. [DOI] [PubMed] [Google Scholar]
- 9. Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraguljac NV, Reid M, White D, et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders–focus on magnetic resonance spectroscopy (MRS) studies. Hum Psychopharmacol. 2005;20:309–326. [DOI] [PubMed] [Google Scholar]
- 12. Schwerk A, Alves FD, Pouwels PJ, van Amelsvoort T. Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. J Neurochem. 2014;128:1–87. [DOI] [PubMed] [Google Scholar]
- 13. Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50:291–301. [DOI] [PubMed] [Google Scholar]
- 14. Xia M, Abazyan S, Jouroukhin Y, Pletnikov M. Behavioral sequelae of astrocyte dysfunction: focus on animal models of schizophrenia. Schizophr Res. 2014; doi:10.1016/j.schres.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. [DOI] [PubMed] [Google Scholar]
- 16. Poels EM, Kegeles LS, Kantrowitz JT, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry. 2014;19:20–29. [DOI] [PubMed] [Google Scholar]
- 17. Stone JM. Imaging the glutamate system in humans: relevance to drug discovery for schizophrenia. Curr Pharm Des. 2009;15:2594–2602. [DOI] [PubMed] [Google Scholar]
- 18. Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol. 2002;42:165–179. [DOI] [PubMed] [Google Scholar]
- 19. Bustillo JR, Chen H, Jones T, et al. Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry. 2014;71:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. [DOI] [PubMed] [Google Scholar]
- 23. Wijtenburg SA, Yang S, Fischer BA, Rowland LM. In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: application to schizophrenia. Neurosci Biobehav Rev. 2015;51:276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyerhoff DJ, MacKay S, Bachman L, et al. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43:509–515. [DOI] [PubMed] [Google Scholar]
- 25. Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. [DOI] [PubMed] [Google Scholar]
- 26. Gan JL, Cheng ZX, Duan HF, Yang JM, Zhu XQ, Gao CY. Atypical antipsychotic drug treatment for 6 months restores N-acetylaspartate in left prefrontal cortex and left thalamus of first-episode patients with early onset schizophrenia: A magnetic resonance spectroscopy study. Psychiatry Res. 2014;223:23–27. [DOI] [PubMed] [Google Scholar]
- 27. Théberge J, Al-Semaan Y, Williamson PC, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–2233. [DOI] [PubMed] [Google Scholar]
- 28. Wood SJ, Berger GE, Wellard RM, et al. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophr Res. 2008;102:163–170. [DOI] [PubMed] [Google Scholar]
- 29. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 30. Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–239. [DOI] [PubMed] [Google Scholar]
- 31. Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45:71–76. [DOI] [PubMed] [Google Scholar]
- 32. Graff-Guerrero A, Willeit M, Ginovart N, et al. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA psychiatry 2013;70:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carpenter WT, Jr, Schooler NR, Kane JM. The rationale and ethics of medication-free research in schizophrenia. Arch Gen Psychiatry. 1997;54:401–407. [DOI] [PubMed] [Google Scholar]
- 35. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 36. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. [DOI] [PubMed] [Google Scholar]
- 37. Jiru F, Skoch A, Klose U, Grodd W, Hajek M. Error images for spectroscopic imaging by LCModel using Cramer-Rao bounds. MAGMA. 2006;19:1–14. [DOI] [PubMed] [Google Scholar]
- 38. Provencher SW. LCModel & LCMgui User’s Manual. 2012. [Google Scholar]
- 39. Bustillo JR, Rowland LM, Jung R, et al. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33:2456–2466. [DOI] [PubMed] [Google Scholar]
- 40. Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophr Res. 2001;52:87–99. [DOI] [PubMed] [Google Scholar]
- 41. Bustillo JR, Chen H, Gasparovic C, et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 Tesla. Biol Psychiatry. 2011;69:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. [DOI] [PubMed] [Google Scholar]
- 44. Tayoshi S, Sumitani S, Taniguchi K, et al. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS). Schizophr Res. 2009;108:69–77. [DOI] [PubMed] [Google Scholar]
- 45. Bustillo JR, Rowland LM, Lauriello J, et al. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry. 2002;159:130–133. [DOI] [PubMed] [Google Scholar]
- 46. Fujimoto T, Nakano T, Takano T, et al. Proton magnetic resonance spectroscopy of basal ganglia in chronic schizophrenia. Biol Psychiatry. 1996;40:14–18. [DOI] [PubMed] [Google Scholar]
- 47. Shioiri T, Hamakawa H, Kato T, et al. Proton magnetic resonance spectroscopy of the basal ganglia in patients with schizophrenia: a preliminary report. Schizophr Res. 1996;22:19–26. [DOI] [PubMed] [Google Scholar]
- 48. Maddock R. The problem of spurious correlations between pairs of brain metabolite values measured in the same voxel with magnetic resonance spectroscopy. JAMA Psychiatry. 2014;71:338–339. [DOI] [PubMed] [Google Scholar]
- 49. Chang L, Jiang C, Cunningham E, et al. Effects of APOE e4, age, and HIV on glial metabolites and cognitive deficits. Neurology. 2014;82:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harezlak J, Buchthal S, Taylor M, et al. ; HIV Neuroimaging Consortium. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50:190–195. [DOI] [PubMed] [Google Scholar]
- 52. Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–437. [DOI] [PubMed] [Google Scholar]
- 53. Chiappelli J, Hong LE, Wijtenburg SA, et al. Alterations in frontal white matter neurochemistry and microstructure in schizophrenia: implications for neuroinflammation. Transl Psychiatry. 2015;5:e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert Rev Neurother. 2007;7:789–796. [DOI] [PubMed] [Google Scholar]
- 55. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tourjman V, Kouassi É, Koué MÈ, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151:43–47. [DOI] [PubMed] [Google Scholar]
- 57. Nitta M, Kishimoto T, Müller N, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39:1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–965. [DOI] [PubMed] [Google Scholar]
- 60. de la Fuente-Sandoval C, Leon-Ortiz P, Azcarraga M, Favila R, Stephano S, Graff-Guerrero A. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol. 2013;16:471–475. [DOI] [PubMed] [Google Scholar]
- 61. Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. [DOI] [PubMed] [Google Scholar]
- 62. Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37:2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Poels EM, Kegeles LS, Kantrowitz JT, et al. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reid MA, Kraguljac NV, Avsar KB, White DM, den Hollander JA, Lahti AC. Proton magnetic resonance spectroscopy of the substantia nigra in schizophrenia. Schizophr Res. 2013;147:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goto N, Yoshimura R, Kakeda S, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rothermundt M, Ahn JN, Jörgens S. S100B in schizophrenia: an update. Gen Physiol Biophys. 2009;28 Spec No Focus:F76–F81. [PubMed] [Google Scholar]
- 68. Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. [DOI] [PubMed] [Google Scholar]
- 71. Stone JM, Dietrich C, Edden R, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Muller N, Schwarz M. Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res. 2006;10:131–148. [DOI] [PubMed] [Google Scholar]
- 73. Müller N. Inflammation and the glutamate system in schizophrenia: implications for therapeutic targets and drug development. Expert Opin Ther Targets. 2008;12:1497–1507. [DOI] [PubMed] [Google Scholar]
- 74. Steiner J, Bogerts B, Sarnyai Z, et al. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13:482–492. [DOI] [PubMed] [Google Scholar]
- 75. Aparicio-Legarza MI, Cutts AJ, Davis B, Reynolds GP. Deficits of [3H]D-aspartate binding to glutamate uptake sites in striatal and accumbens tissue in patients with schizophrenia. Neurosci Lett. 1997;232:13–16. [DOI] [PubMed] [Google Scholar]
- 76. Lauriat TL, McInnes LA. EAAT2 regulation and splicing: relevance to psychiatric and neurological disorders. Mol Psychiatry. 2007;12:1065–1078. [DOI] [PubMed] [Google Scholar]
- 77. Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res. 2013;144:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Simpson MD, Slater P, Royston MC, Deakin JF. Regionally selective deficits in uptake sites for glutamate and gamma-aminobutyric acid in the basal ganglia in schizophrenia. Psychiatry Res. 1992;42:273–282. [DOI] [PubMed] [Google Scholar]
- 79. Homan P, Vermathen P, Van Swam C, et al. Magnetic resonance spectroscopy investigations of functionally defined language areas in schizophrenia patients with and without auditory hallucinations. Neuroimage. 2014;94:23–32. [DOI] [PubMed] [Google Scholar]
- 80. Chiappelli J, Rowland LM, Wijtenburg SA, et al. Evaluation of Myo-Inositol as a Potential Biomarker for Depression in Schizophrenia. Neuropsychopharmacology. 2015;40:2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hugdahl K, Craven AR, Nygård M, et al. Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: a (1)H MRS study. Schizophr Res. 2015;161:252–260. [DOI] [PubMed] [Google Scholar]
- 82. Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia–a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. [DOI] [PubMed] [Google Scholar]
- 83. Plitman E, Nakajima S, de la Fuente-Sandoval C, et al. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol. 2014;24:1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rowland LM, Krause BW, Wijtenburg SA, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2015; doi:10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Clementi V, Tonon C, Lodi R, Malucelli E, Barbiroli B, Iotti S. Assessment of glutamate and glutamine contribution to in vivo N-acetylaspartate quantification in human brain by (1)H-magnetic resonance spectroscopy. Magn Reson Med. 2005;54:1333–1339. [DOI] [PubMed] [Google Scholar]
- 87. de la Fuente-Sandoval C, Favila R, Alvarado P, et al. Glutamate increase in the associative striatum in schizophrenia: a longitudinal magnetic resonance spectroscopy preliminary study. Gaceta medica de Mexico 2009;145:109–113. [PubMed] [Google Scholar]
- 88. Kraguljac NV, Cutter GR, Morgan C, Lahti AC. In reply. JAMA Psychiatry. 2014;71:339–340. [DOI] [PubMed] [Google Scholar]
- 89. Lindenmayer JP. Treatment refractory schizophrenia. Psychiatr Q. 2000;71:373–384. [DOI] [PubMed] [Google Scholar]
- 90. Suzuki T, Remington G, Mulsant BH, et al. Treatment resistant schizophrenia and response to antipsychotics: a review. Schizophr Res. 2011;133:54–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.