Abstract
Background: There has been growing support for dysfunctions of the excitatory glutamatergic system and its implications for the psychophysiology of schizophrenia. However, previous studies reported mixed results regarding glutamate concentrations in schizophrenia with varying deviations across brain regions. Methods: We used an optimized proton magnetic resonance spectroscopy procedure to measure absolute glutamate concentrations in the left hippocampal region and the anterior cingulate cortex (ACC) in 29 medicated patients with schizophrenia and in 29 control participants without mental disorder. Results: The glutamate concentrations were significantly lower in the ACC but higher in the hippocampus of patients compared to controls. ACC and hippocampal glutamate concentrations correlated positively in patients but not in controls. ACC glutamate was weakly associated with Clinical Global Impression score and duration of illness in patients. Conclusion: Glutamate concentrations in schizophrenia deviate from controls and show associations with disease severity. A higher concentration of hippocampal glutamate in schizophrenia compared to controls is shown. The association between ACC and hippocampus glutamate concentrations in patients with schizophrenia suggests an abnormal coupling of excitatory systems compared to controls as predicted by previous glutamate models of schizophrenia.
Key words: schizophrenia, glutamate, hippocampus, anterior cingulated, MR spectroscopy, glutamatergic hypothesis of schizophrenia
Introduction
Schizophrenia research has recently turned its attention to deficits in the glutamatergic system as a potential etiological factor. Studies demonstrated that antagonist administrations of the glutamate receptor N-methyl-d-aspartate (NMDA) induced symptoms of schizophrenia in healthy subjects.1–4 In patients with schizophrenia, these drugs exacerbated both positive and negative symptoms.5–7 Furthermore, a review6 reported that enhanced NMDA receptor function reduces negative symptoms and improves cognitive functioning in medicated schizophrenic patients. The excitatory glutamatergic system is implicated in the pathophysiology of schizophrenia, developing the “glutamatergic hypothesis of schizophrenia.”7–9 Carlsson AN and Carlsson ML10 proposed that projections from the glutamate system in the cortex modulate the dopaminergic system in the substantia nigra/ventral tegmental area (VTA) and deficits in this pathway would ultimately result in psychosis and other symptoms of schizophrenia. Investigation into the neurotransmitter activity of glutamate is necessary to test this model and would support developments in pharmacotherapy of schizophrenia.
The anterior cingulate cortex (ACC) and the hippocampus are interesting targets for the assessment of glutamate because they have been associated with structural and functional changes in schizophrenia. In the ACC, studies with patients revealed abnormal activation, most commonly hypoactivation, in this region.11 Imaging studies have largely concluded that the hippocampus is smaller in patients compared to healthy subjects.12,13 Other imaging studies report abnormal hippocampal activity that could suggest abnormal function circuitry to other connected regions, including the prefrontal cortex.14,15
Within these regions, there have been efforts to investigate the excitatory glutamatergic system for abnormal activation within schizophrenia. Magnetic resonance spectroscopy (MRS) provides in vivo information on glutamate concentrations in specific areas of the brain.16 A meta-analysis found lower concentrations of glutamate in the frontal region, including the ACC, of patients with schizophrenia compared to healthy participants17 but other studies have found no differences.18,19 Within the hippocampus, there is some evidence of altered ionotropic glutamate receptor activity.20–23 However, other studies17,19 found no differences between patients and healthy participants. Recent work has demonstrated that glutamate may be elevated in the early stages of schizophrenia,24–26 in unmedicated patients19,27 and in nonremitted patients28 but unchanged or reduced below normal in chronic and medicated patients.29–31 Although the exact characterization of the glutamate abnormalities in schizophrenia has yet to be determined, this research suggests there is a relationship between the impact of the illness and glutamate concentration.
This in vivo spectroscopy study investigates the glutamate concentrations within the ACC and the hippocampus of medicated patients with schizophrenia compared to healthy control participants without schizophrenia (controls). We expect to find differences in the glutamate concentrations between the 2 groups, which would support the assumption that abnormalities in the glutamatergic system are pathophysiological characteristics of schizophrenia. Furthermore, we set out to investigate the relationship between ACC and hippocampal glutamate concentrations in patients and healthy controls.
Methods and Materials
Participants
Twenty-nine patients with schizophrenia (19 males) were compared to 29 controls (19 males) matched for gender. Demographic and additional data describing patients and controls are presented in table 1. Patients were recruited among inpatients (n = 13) and outpatients (n = 16) of the Charité Department of Psychiatry and Psychotherapy, Campus Mitte. Diagnosis was assessed with the Structured Clinical Interview for Axis I DSM-IV Disorders32 by an experienced senior consultant psychiatrist. Twenty-eight patients had been treated with risperidone at the time of the scan (17 had been treated less than 1 y, 7 longer than 1 y, and 4 longer than 5 y; mean dose 4.1±1.56mg). Three of the patients additionally received benzodiazepines. One patient was treated with haloperidole. Psychopathological symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS)33 and the Clinical Global Impression Scale (CGI).34 The duration of illness was assessed years between the first episode and the current investigation. Patients with other psychiatric Axis I disorders were excluded.
Table 1.
Demographic and Clinical Data of all Participants
| Variable | Healthy Controls (n = 29)a | Patients (n = 29)a | Statistics | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | χ2/t b | df | P | |
| Age (y) | 30.9 | 8.4 | 27.6 | 6.8 | −1.64 | 56 | .018 |
| Gender (male/female) | 19/10 | 19/10 | 0.00 | 1 | 1 | ||
| Education (y) | 12.0 | 1.6 | 11.1 | 1.5 | −2.03 | 53 | .985 |
| # Smoker/ex-smoker/nonsmoker | 13/1/14 | 21/2/4 | 7.76 | 2 | .021 | ||
| # Cigarettes smoked per day | 6.9 | 10.1 | 14.2 | 11.6 | 2.51 | 53 | .015 |
| Age at disease onset (y) | 22.9 | 3.8 | |||||
| Duration of illness (y) | 3.8 | 4.1 | |||||
| CGI score | 4.9 | 0.7 | |||||
| PANSS positive subscore | 17.8 | 6.7 | |||||
| PANSS negative subscore | 19.5 | 5.2 | |||||
| PANSS general subscore | 35.4 | 8.3 | |||||
Note: CGI, Clinical Global Impression Scale; PANSS, Positive and Negative Syndrome Scale.
a29 patients and 29 controls were included in the sample. Missing data included: 1 control and 2 patients from smokers’ data, 1 patient from disease onset, 1 patient from duration of illness, and 1 patient from CGI.
bStudent’s t for age, education, and number of cigarettes smoked per day; χ2 for gender and smoker status.
Controls were recruited through newspaper advertisements. According to personal interviews (Mini-International Neuropsychiatric Interview35), these participants were free of medical, neurological, and psychiatric disorders. Healthy participants with a family history (first degree) of Axis I disorder were excluded. In addition, exclusion criteria for all subjects were abnormalities in the magnetic resonance imaging, general medical disorders, or any clinically relevant abnormalities. The study was approved by the ethics committee of Charité University Clinic, Berlin, Germany. After complete description of the study to the subjects, informed written consent was obtained from all participants.
Magnetic Resonance Spectroscopy
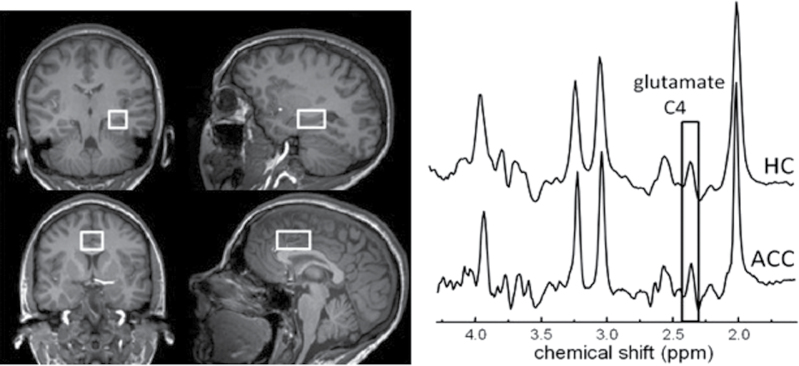
For each subject, magnetic resonance measurements were carried out on a 3-T-scanner (MEDSPEC 30/100; Bruker Biospin, Ettlingen, Germany) using a circularly polarized head coil. After automated global shim of the linear, xz, z 2, and x 2 − y2 field components, T1-weighted images were acquired using Modified Driven Equilibrium Fourier Transform (echo time [TE] = 3.8ms, repetition time [TR] = 20.53ms; 128 contiguous slices, 1.5mm thick; 1-mm inplane [x − y] resolution). Magnetic resonance spectra were acquired from 2- × 3- × 2-cm3 voxels, including the left hippocampus, and from 2.5- × 4- × 2-cm3 voxels, including the ACC (see figure 1). After manual shimming to achieve water line widths (full width at half maximum) of 7–9 Hz and 6–7 Hz for the 2 brain voxels, respectively, the radiofrequency power needed for a 90° excitation pulse was determined. Subsequently, calibration of water suppression (3 chemical-shift selective radiofrequency pulses with Gaussian modulation of 43 Hz full width at half maximum) was carried out, followed by acquisition of spectra with the PRESS (point-resolved spectroscopy) sequence using a Shinnar-LeRoux-optimized 90° pulse and Mao refocusing pulses.36 Eight subspectra of 16 phase-cycled scans were each recorded with TR = 3 seconds, yielding 128 averages, followed by acquisition of unsuppressed water spectra (n = 8). Since we focused on glutamate quantification with little interference by glutamine, an TE of 80 milliseconds was chosen as described elsewhere.37 The spectra also permit deducing concentrations of metabolites like total choline (tCho), total creatine (tCr), and N-acetylaspartate (NAA). Metabolite quantification was based on a previous procedure using an external phantom approach described by Schubert and colleagues.37 The radio frequency power p and the water signal a calculated by fitting in the time domain and corrected for relaxation effects were used for later scaling. Since we did not aim to quantify resonances downfield of tCho, 3.5–1.5 ppm was chosen as the frequency range of interest. The metabolite basis set was formed using phantom spectra from voxels in the center of spherical glass bottles containing metabolites (glutamate, glutamine: 100 mmol/l each, NAA: 50 mmol/l) in phosphate buffer, pH 7.2, at 37°C, using the procedure described for the calibration phantom but with 32 averaged scans. Before further processing, the 8 individual in vivo metabolite subspectra were corrected for eddy currents using water-unsuppressed spectra (n = 8). Spectra quantification was carried out using a program package based on a time domain-frequency domain-fitting procedure.38,39 In the present spectra, the resonances of tCho, tCr, NAA, glutamate, and glutamine were fitted by including phantom spectra for the latter 3, and imposing the following prior knowledge37: constant frequency differences for glutamate, glutamine, and NAA; equal line widths; and adjustment of signal line shape to purely Lorentzian. Although at 3 T and the chosen TE of 80 milliseconds interfering macromolecule resonances have largely decayed, the baseline was not entirely flat. Since no macromolecular prior knowledge was used, the fitting procedure routinely includes estimation of the baseline nonparametrically by regularization, as previously described in detail.38 The regularization parameters were chosen after extensive testing.40 The fitting method yields values for the uncertainties of both the metabolite amplitudes (Cramér-Rao lower bounds) and the baseline. Extensive tests yielded mean uncertainties (corresponding to Cramér-Rao lower bounds plus uncertainties from baseline modeling) for the fitting of glutamate of 13.1% for the hippocampus voxel and 10.5% for the ACC voxel. Uncertainties for NAA, tCr, and tCho were substantially lower while those for glutamine were above 30% in most cases, prohibiting their further consideration. The metabolite concentrations were calculated from their amplitudes with a formula as described by Schubert et al38.
Fig. 1.
Spectroscopic voxel positions analyzed in the present work together with typical magnetic resonance spectra. Top: hippocampus, bottom: anterior cingulate cortex.
The transverse relaxation times (in milliseconds) of metabolites were used as previously determined37 applying TEs of 50, 80, 135, 250, and 330 milliseconds (n = 3, SD in parenthesis): 194 (37) for Glu, 278 (31) for NAA, 179 (9) for tCr, and 282 (45) for tCho in the ACC; and 171 (22), 267 (15), 198 (31), and 291 (13), respectively, for the hippocampus voxel. For glutamine, the T2 values of glutamate were used. No correction for longitudinal relaxation effects was carried out. The deviation caused by the T1 effect at TR = 3 seconds and an assumed T1 of glutamate at 3 T of about 1.2 seconds41 was largely compensated by the deviation estimated for the aqueous, buffered glutamate phantoms where TR was set at 5 seconds and T1 was determined to be 1.47 seconds.
Gamma-aminobutyric acid (GABA), a major candidate interferent in the quantification of the glutamate C4 resonance, was not included in the spectrum analysis. However, our fitting procedure was largely insensitive to GABA contribution because of the fair separation of the 2 resonance signals at the chosen TE of 80 milliseconds.37 Likewise, aspartate is a compound likely to contribute to the fit in the chosen spectral region. We therefore tested the effect of including fixed aspartate amplitudes corresponding to concentrations at and above the physiological level (ie, from 1 to 3 mmol/l)42 in the fit. Fitting of spectra from the hippocampus voxels of 11 subjects gave mean increases of the predicted glutamate level of at most 0.5% at 3 mmol/l aspartate, regardless of the GABA resonance. Therefore, the present method of glutamate fitting can be considered unaffected by aspartate contribution. Segmentation into gray matter, white matter, and cerebrospinal fluid (CSF) of the T1-weighted images was performed using spm2.43 Classification of pixels was based on which spm2 tissue classification had the greatest probability. To correct the metabolite concentrations for the CSF fraction in the voxels studied, the concentration values were divided by (1 − CSF fraction).
Data Analysis
ANCOVA were used for the effect of diagnosis (schizophrenia vs no schizophrenia) on concentrations of glutamate and other metabolites in the ACC with age and gender as covariates. A second ANCOVA was performed for the metabolites in the hippocampus. Partial correlation analyses controlling for age and gender, 2-tailed, were conducted to determine the relationship of the glutamate concentrations of the participants with schizophrenia with age of disease onset, number of episodes, Chlorpromazine equivalents, PANSS scores, and CGI scores. Partial correlational analyses were also conducted to assess the relationship between the glutamate concentrations of the 2 brain regions in both samples. Missing metabolite values were due to low signal quality and not included in the final analyses (see table 2). We decided a priori to evaluate statistical significance using a 2-sided design with alpha set at .05.
Table 2.
Group Comparisons of Metabolite Concentrations in the Hippocampus and ACC
| Metabolite | Hippocampus | ACC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n = 29)a | Patients (n = 29)a | Statistics | Controls (n = 29)a | Patients (n = 29)a | Statistics | |||||
| Meanb (SD) | Meanb (SD) | F/t c | Df | P | Meanb (SD) | Meanb (SD) | F/t c | df | P | |
| Glutamate | 10.42 (1.53) | 12.10 (1.47) | 14.58 | 1 | <.001 | 15.17 (1.11) | 14.46 (1.56) | 5.56 | 1 | .022 |
| NAA | 11.43 (1.04) | 11.04 (0.80) | 5.26 | 1 | .026 | 13.23 (1.17) | 12.70 (0.99) | 5.22 | 1 | .026 |
| Choline | 2.34 (0.25) | 2.37 (0.26) | 0.027 | 1 | .869 | 2.42 (0.27) | 2.26 (0.27) | 4.01 | 1 | .048 |
| Creatine | 9.47 (0.90) | 9.85 (1.14) | 1.24 | 1 | .270 | 10.59 (0.89) | 10.17 (1.00) | 2.61 | 1 | .112 |
| Grey matter | 0.61 (0.06) | 0.63 (0.05) | 1.32 | 56 | .192 | 0.56 (0.03) | 0.57 (0.03) | 1.49 | 56 | .141 |
| White matter | 0.33 (0.06) | 0.31 (0.05) | -1.2 | 56 | .234 | 0.27 (0.04) | 0.29 (0.05) | 2.32 | 56 | .024 |
| CSF | 0.06 (0.02) | 0.06 (0.02) | -0.32 | 56 | .747 | 0.17 (0.03) | 0.14 (0.04) | -3.86 | 56 | <.001 |
Note: ACC, anterior cingulate cortex; CSF, cerebral spinal fluid.
a29 patients and 29 controls were included in the sample. Owing to insufficient spectra quality, some results are missing: 2 for Glu, 1 for tCho, and 1 for tCr from hippocampus of controls and 1 for tCr from ACC of patients.
bMean concentrations in mmol/l.
c F for Glu, NAA, tCho, andtCr. Student’s t for grey matter, white matter, and CSF.
Results
Covariates
Since there is evidence that age and gender have an effect on glutamate concentrations in the ACC and hippocampus,44 they were included as covariates in all analyses. However, the 1-way ANCOVA on glutamate levels in ACC revealed no significant effect of covariates, neither age, F(1,54) = 2.690, P = .107, nor gender, F(1,54) = 1.260, P = .267. The ANCOVA on glutamate concentration in hippocampus showed a significant effect of the covariate age, F(1,52) = 8.387, P = .006, but no significant effect of gender, F(1,52) = 0.006, P = .941. To further elucidate the influence of age, correlation analyses with glutamate were conducted for both groups separately. In controls, no significant relationship was observed between age and ACC glutamate, r(27) = −.15, P = .449, but there was a significant relationship with hippocampal glutamate, r(25) = −.39, P = .043. In the patients group, there was no significant relationships among age and ACC, r(27) = −.26, P = .182, or hippocampal glutamate, r(27) = −.36, P = .055.
Our patients with schizophrenia smoked significantly more cigarettes per day than the controls (see table 1). Nevertheless, we found no significant correlation between number of cigarettes smoked per day and the glutamate in the hippocampus, r(25) = −.21, P = .297, or the ACC, r(25) = .10, P = .630, of patients with schizophrenia, nor with the glutamate in the hippocampus, r(25) = −.08, P = .716, or the ACC, r(26) = −.3, P = .127, of controls. Furthermore, a study by Gallinat and Schubert45 found no significant association between smoking and glutamate concentrations in the ACC or hippocampus. Therefore, we did not include smoking data as a covariate.
Main Results
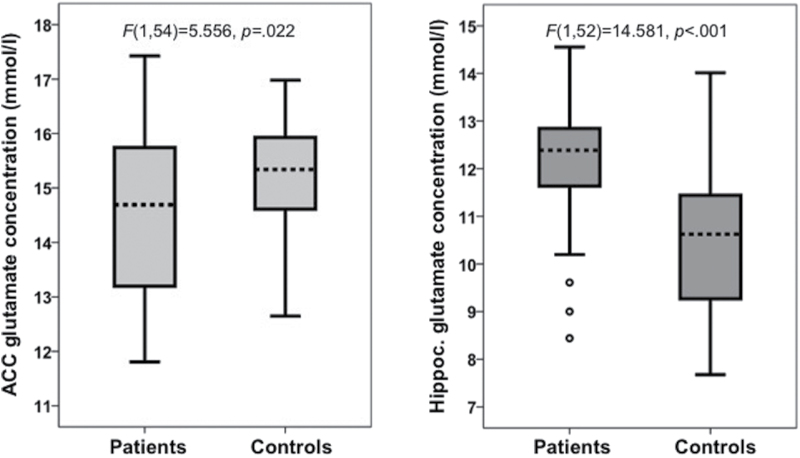
A significant main effect of diagnosis was observed for glutamate in ACC, F(1,54) = 5.556, P = .022 (figure 2). In ACC, glutamate concentration was significantly lower in participants with schizophrenia (M = 14.46, SD = 1.56 mmol/l) than in controls (M = 15.17, SD = 1.11 mmol/l). In the hippocampus, glutamate concentration in participants with schizophrenia (M = 12.1, SD = 1.47 mmol/l) was significantly higher than in controls (M = 10.41, SD = 1.53 mmol/l), reflected in a main effect of diagnosis, F(1,52) = 14.581, P < .001 (figure 2). Group comparisons for all measured metabolites are shown in table 2.
Fig. 2.
Absolute glutamate concentration in the anterior cingulate cortex (left) and hippocampus (right) in controls and patients with schizophrenia.
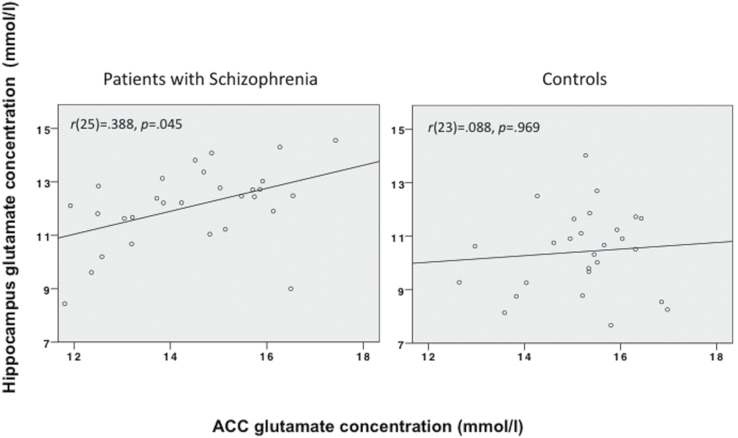
A positive correlation between the ACC glutamate concentrations and hippocampal glutamate concentrations was observed in schizophrenic patients, r(25) = .388, P = .045, but not in controls r(23) = .008, P = .969 (figure 3). A Fisher’s r-to-z transformation determined there was a significant difference between these 2 correlations (z = −5.65, P < .001). We also found a significant negative correlation between the CGI scores and the glutamate concentrations in the ACC, r(24) = −.409, P = .038, but not in the hippocampus, r(24) = −.007, P = .972. Glutamate concentrations in the ACC or the hippocampus did not correlate significantly with participants with schizophrenia’s PANSS positive scores, r(24) = .034, P = .868 and r(24) = .153, P = .457, or negative scores, r(24) = .114, P = .578 and r(24) = .137, P = .504, respectively.
Fig. 3.
Correlations between the absolute glutamate concentration in the anterior cingulate cortex and the absolute glutamate concentration in the hippocampus in controls (right) and patients with schizophrenia (left).
The duration of illness had a significant relationship with ACC glutamate, r(24) = .497, P = .010, but not in the hippocampal glutamate concentration, r(24) = .028, P = .891. There was no relationship between the age at first manifestation and both the hippocampal glutamate, r(24) = −.226, P = .268, and the ACC glutamate concentration, r(24) = −.289, P = .152. There was no significant relationship between the number of episodes and the hippocampal, r(24) = .260, P = .200, or the ACC glutamate r(24) = .210, P = .304. There was no significant relationship between the Chlorpromazine dosage equivalent and the hippocampal glutamate, r(20) = −.135, P = .549, or the ACC glutamate concentration, r(20) = −.210, P = .348. When all correlation analyses were corrected for multiple testing (Bonferroni), no association reached significance.
Discussion
Our study focused on the comparison of glutamate in 2 important brain regions in patients with schizophrenia and controls. As predicted, we found differences in glutamate levels within both the ACC and hippocampus in patients with schizophrenia compared to controls. In addition to glutamate, we quantified NAA, tCr, and tCho. For the quantification of all these metabolites, we used a spectroscopic method that detects this substance reliably and with fair selectivity over other compounds, especially glutamine.37,46 Such selectivity is hardly attainable using PRESS at 3 T and (usually the shortest possible) TE of 30 milliseconds, in which frequently only the sum of glutamate + glutamine (Glx) can be measured with acceptable precision. We avoided interference by macromolecules with our moderately long TE of 80 milliseconds, where these resonances have nearly completely decayed and their residuals are fittable by a rather smooth baseline. In contrast to other methods for glutamate using the quite reliable TE-averaging47 or rather complex editing schemes,48,49 our method is based on the PRESS sequence available at most MR scanners and thus lends itself to clinical application at a large scale.
The higher hippocampal glutamate concentration in patients with schizophrenia offers an interesting contribution to this line of research. Some MRS studies reported increased Glx levels in the hippocampus of schizophrenic patients compared to controls,50,51 or increased Glx to creatine ratio,19 while others observed no Glx differences.52,53 However, Glx is a combined measure of Glu, Gln, and GABA levels, and the isolation of glutamate levels of the hippocampus is relatively unexplored. By focusing on glutamate with 3 Tesla or higher field strength, the current investigation is one of the first to provide evidence for an abnormally high absolute concentrations of glutamate in the hippocampus.19,54,55
The glutamate concentration in the ACC of patients with schizophrenia was significantly lower than in the ACC of controls. This finding supports previous evidence of lower glutamate levels in patients in the ACC.17 There was a significant negative relationship between the ACC glutamate and the CGI, a subjective general rating of illness severity, although this correlation did not survive Bonferroni correction. This relationship is in line with previously found associations of glutamate abnormalities with measures of dysfunction such as the Life Skills Profile rating scale,56 current symptom exacerbation,57 cognitive performance,24 and negative symptomatology.28 The weak association of glutamate and CGI suggests a more general role of glutamate for the schizophrenia phenotype, apart from psychopathological ratings. Further investigation of glutamate activity and objective measures of symptoms or illness severity would make a stronger case.
These results are predicted by the model of glutamategic modulation of the dopamine system put forth by Carlsson AN and Carlsson ML.10 Deficiency in the glutamate system would decrease mesocortical dopamine activity and might affect the mesolimbic dopamine pathway.58 Thus, hypoactivity of the ACC could interrupt the modulation of the dopaminergic pathway in the VTA region, which would then in turn alter activity in the hippocampus through the mesolimbic pathway. A positive correlation between the glutamate concentration in the ACC and hippocampus was only found within the schizophrenic sample, which further suggests a systematic aberration associated with the disorder. However, these results only provide suggestive evidence for this model and would require further investigations that take into account the connectivity of these regions and data on both dopaminergic and glutamatergic activity.
Abnormalities in the NMDA receptor activity may provide an explanation for our results the concerning glutamate levels. Animal studies have demonstrated that phencyclidine or ketamine administration alters forebrain dopaminergic activity.2,59–61 In healthy human participants, administrations of ketamine have been linked with increases in glutamine62 and glutamate63 in the ACC. It is therefore necessary that future investigations should involve linking NMDA receptor function to glutamate level abnormalities.
We only observed a positive association between duration of illness and glutamate concentrations in patients’ ACC. These results differ from reports of decreased prefrontal glutamate (Glx) in chronic schizophrenic subjects.64 In a longitudinal study by Aoyama and colleagues,56 there was a significant decrease of the total glutamatergic metabolites (tGL) in the thalamus in unmedicated schizophrenic patients over the follow-up period of 80 months. The decline of glutamate-associated metabolites was interpreted as part of a disease progression, which is not the case in our glutamate data. This may be due to a relatively short duration of illness since we investigated relatively young patients at the mean age of 27.6 years. Previous research reported no change of glutamate and associated metabolites in a sample with early schizophrenia in the course of a 12-month neuroleptic treatment.24,56
We assessed other metabolites to further clarify the nature of our observed glutamate levels. A recent study65 showed 20% higher glutamine in the ACC of schizophrenia patients. Although the spectral signal used for our quantification of glutamate has been shown to be clearly dominated by glutamate, it may contain a slight contamination by glutamine.37 Additionally, the signal-to-noise ratio for glutamine in our spectra is low, leading to fitting uncertainties mostly above 30%, but we can roughly estimate the level of this compound in ACC. Since the mean values of this estimate are not statistically different between control subjects (2.17 mmol/l) and patients (2.24 mmol/l), we do not confirm the previous findings.65
A reduced NAA concentration was observed in both the hippocampus and ACC in patients compared to controls. Regardless of disease condition, the metabolite concentrations in our study were computed using their transverse relaxation times as determined in the appropriate voxels in 3 healthy volunteers. However, T2 of NAA in white matter of patients with schizophrenia has been found to be shorter by 6.4%66 and 10%67 which might account for the reduced NAA level we find in ACC as well as in hippocampus. A similar effect may be responsible for the lower tCho concentration observed in the ACC voxel in our study, since shorter T2 for tCho has been determined in a similar anterior cingulate voxel in schizophrenia patients.68 However, to our knowledge, T2 values for glutamate in schizophrenia patients have not been determined as yet. Future studies should measure transverse relaxation times of glutamate in patients as well, because, beyond allowing for appropriate correction, this might add valuable information about this metabolite.
Our study has several limitations. First, we lack of information regarding GABA activity. Carlsson’s model10 includes GABA function as an important component in the modulation of dopamine pathways. One model69 proposes that NMDA receptor activity regulates the function of GABAergic neurons, which corresponds to the inhibitory and excitatory pathways of Carlsson’s glutamate degeneration hypothesis. Accordingly, studies that examine both glutamate and GABA deviations in schizophrenia have found a similar elevation27 or reduction30 of these metabolites in patients with schizophrenia compared to controls. Data on GABA function in conjunction with glutamate function would be important in explaining the mechanisms of glutamate’s role in schizophrenia in our sample. Secondly, the present study is cross-sectional and cannot provide evidence of progression of glutamate degeneration.17 Finally, the explanatory power of the results is subject is limited since only 20% of the glutamate signal, measured by H-MRS, are from the neurotransmitter pool stored and synthesized in synaptic nerve terminal or presynaptic glia. The remaining 80% represent the general major metabolic function of glutamate in the brain.70 However, reported associations between MRS measured glutamate concentrations and cerebral function can be interpreted as argument that the signal also represents neurotransmission.46
Conclusion
This in vivo spectroscopy study revealed differences in glutamate concentration within both the ACC and hippocampus in patients with schizophrenia compared to healthy control participants without schizophrenia. The glutamate levels in the ACC region were significantly lower and the levels in the hippocampus were largely higher in patients with schizophrenia compared to controls. These results support the glutamate degeneration hypothesis of schizophrenia10 and imply that abnormalities in the glutamatergic system in these regions are associated with schizophrenia and the severity of the illness.
Funding
This work was supported by a grant of the Stanley Medical Research Institute (02T-247), NGFNplus BMBF01GS08159 and BMBF01GQ0914.
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. [DOI] [PubMed] [Google Scholar]
- 2. Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. [DOI] [PubMed] [Google Scholar]
- 3. Snyder SH. Phencyclidine. Nature. 1980;285:355–356. [DOI] [PubMed] [Google Scholar]
- 4. Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. [DOI] [PubMed] [Google Scholar]
- 5. Malhotra AK, Pinals DA, Adler CM, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. [DOI] [PubMed] [Google Scholar]
- 6. Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. [DOI] [PubMed] [Google Scholar]
- 7. Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990;16:425–432. [DOI] [PubMed] [Google Scholar]
- 8. Deutsch SI, Mastropaolo J, Schwartz BL, Rosse RB, Morihisa JM. A “glutamatergic hypothesis” of schizophrenia. Rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989;12:1–13. [PubMed] [Google Scholar]
- 9. Wachtel H, Turski L. Glutamate: a new target in schizophrenia? Trends Pharmacol Sci. 1990;11:219–220. [DOI] [PubMed] [Google Scholar]
- 10. Carlsson AN, Carlsson ML. Neurotransmitter interactions in schizophrenia-therapeutic implications. Biol Psychiatry. 1999;46:153–179. [DOI] [PubMed] [Google Scholar]
- 11. Adams R, David AS. Patterns of anterior cingulate activation in schizophrenia: a selective review. Neuropsychiatr Dis Treat. 2007;3:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarley RW, Niznikiewicz MA, Salisbury DF, et al. Cognitive dysfunction in schizophrenia: unifying basic research and clinical aspects. Eur Arch Psychiatry Clin Neurosci. 1999;249(suppl):69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. [DOI] [PubMed] [Google Scholar]
- 14. Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison PJ, Eastwood SL. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus. 2001;11:508–519. [DOI] [PubMed] [Google Scholar]
- 16. Kauppinen RA, Williams SR, Busza AL, van Bruggen N. Applications of magnetic resonance spectroscopy and diffusion-weighted imaging to the study of brain biochemistry and pathology. Trends Neurosci. 1993;16:88–95. [DOI] [PubMed] [Google Scholar]
- 17. Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stanley JA, Williamson PC, Drost DJ, et al. An in vivo proton magnetic resonance spectroscopy study of schizophrenia patients. Schizophr Bull. 1996;22:597–609. [DOI] [PubMed] [Google Scholar]
- 19. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dean B, Scarr E, Bradbury R, Copolov D. Decreased hippocampal (CA3) NMDA receptors in schizophrenia. Synapse. 1999;32:67–69. [DOI] [PubMed] [Google Scholar]
- 21. Eastwood SL, Burnet PW, Harrison PJ. GluR2 glutamate receptor subunit flip and flop isoforms are decreased in the hippocampal formation in schizophrenia: a reverse transcriptase-polymerase chain reaction (RT-PCR) study. Brain Res Mol Brain Res. 1997;44:92–98. [DOI] [PubMed] [Google Scholar]
- 22. Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39:25–32. [DOI] [PubMed] [Google Scholar]
- 23. Law AJ, Deakin JF. Asymmetrical reductions of hippocampal NMDAR1 glutamate receptor mRNA in the psychoses. Neuroreport. 2001;12:2971–2974. [DOI] [PubMed] [Google Scholar]
- 24. Bustillo JR, Rowland LM, Mullins P, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De la Fuente-Sandoval C, León-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA psychiatry. 2013;70:1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. [DOI] [PubMed] [Google Scholar]
- 28. Egerton A, Brugger S, Raffin M, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reid MA, Stoeckel LE, White DM, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. 2013;39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lutkenhoff ES, van Erp TG, Thomas MA, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. [DOI] [PubMed] [Google Scholar]
- 32. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. Washington, DC: American Psychiatric Publishing; 2012:84. [Google Scholar]
- 33. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 34. Guy W. The Clinical Global Impression Scale. In: ECDEU Assessment Manual for Psychopharmacology-Revised. Rockville, MD: US Department of Health, Education and Welfare, ADAMHA, MIMH Psychopharmacology Research Branch; 1976:218–222. [Google Scholar]
- 35. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 2):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 36. Mao J, Mareci T, Scott K, Andrew E. Selective inversion radiofrequency pulses by optimal control. J Magn Reson. 1986;70:310–318. [Google Scholar]
- 37. Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage. 2004;21:1762–1771. [DOI] [PubMed] [Google Scholar]
- 38. Schubert F, Seifert F, Elster C, et al. Serial 1H-MRS in relapsing-remitting multiple sclerosis: effects of interferon-beta therapy on absolute metabolite concentrations. MAGMA. 2002;14:213–222. [DOI] [PubMed] [Google Scholar]
- 39. Elster C, Schubert F, Link A, Walzel M, Seifert F, Rinneberg H. Quantitative magnetic resonance spectroscopy: semi-parametric modeling and determination of uncertainties. Magn Reson Med. 2005;53:1288–1296. [DOI] [PubMed] [Google Scholar]
- 40. Elster C, Link A, Schubert F, Seifert F, Walzel M, Rinneberg H. Quantitative MRS: comparison of time domain and time domain frequency domain methods using a novel test procedure. Magn Reson Imaging. 2000;18:597–606. [DOI] [PubMed] [Google Scholar]
- 41. Mlynárik V, Gruber S, Moser E. Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14:325–331. [DOI] [PubMed] [Google Scholar]
- 42. Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. [DOI] [PubMed] [Google Scholar]
- 43. Ashburner J, Friston K. Multimodal image coregistration and partitioning–a unified framework. Neuroimage. 1997;6:209–217. [DOI] [PubMed] [Google Scholar]
- 44. Tayoshi S, Sumitani S, Taniguchi K, et al. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS). Schizophr Res. 2009;108:69–77. [DOI] [PubMed] [Google Scholar]
- 45. Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–67. [DOI] [PubMed] [Google Scholar]
- 46. Gallinat J, Kunz D, Senkowski D, et al. Hippocampal glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology (Berl). 2006;187:103–111. [DOI] [PubMed] [Google Scholar]
- 47. Hurd R, Sailasuta N, Srinivasan R, Vigneron DB, Pelletier D, Nelson SJ. Measurement of brain glutamate using TE-averaged PRESS at 3T. Magn Reson Med. 2004;51:435–440. [DOI] [PubMed] [Google Scholar]
- 48. Thompson RB, Allen PS. Sources of variability in the response of coupled spins to the PRESS sequence and their potential impact on metabolite quantification. Magn Reson Med. 1999;41:1162–1169. [DOI] [PubMed] [Google Scholar]
- 49. Choi C, Coupland NJ, Bhardwaj PP, Malykhin N, Gheorghiu D, Allen PS. Measurement of brain glutamate and glutamine by spectrally-selective refocusing at 3 Tesla. Magn Reson Med. 2006;55:997–1005. [DOI] [PubMed] [Google Scholar]
- 50. Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62:1396–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. da Silva Alves F, Boot E, Schmitz N, et al. Proton magnetic resonance spectroscopy in 22q11 deletion syndrome. PLoS ONE. 2011;6:e21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rüsch N, Tebartz van Elst L, Valerius G, et al. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophr Res. 2008;99:155–163. [DOI] [PubMed] [Google Scholar]
- 53. Hutcheson NL, Reid MA, White DM, et al. Multimodal analysis of the hippocampus in schizophrenia using proton magnetic resonance spectroscopy and functional magnetic resonance imaging. Schizophr Res. 2012;140:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Elst LT, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:724–730. [DOI] [PubMed] [Google Scholar]
- 55. Abbott C, Bustillo J. What have we learned from proton magnetic resonance spectroscopy about schizophrenia? A critical update. Curr Opin Psychiatry. 2006;19:135–139. [DOI] [PubMed] [Google Scholar]
- 56. Aoyama N, Théberge J, Drost DJ, et al. Grey matter and social functioning correlates of glutamatergic metabolite loss in schizophrenia. Br J Psychiatry. 2011;198:448–456. [DOI] [PubMed] [Google Scholar]
- 57. Ota M, Ishikawa M, Sato N, et al. Glutamatergic changes in the cerebral white matter associated with schizophrenic exacerbation. Acta Psychiatr Scand. 2012;126:72–78. [DOI] [PubMed] [Google Scholar]
- 58. Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158. [DOI] [PubMed] [Google Scholar]
- 59. Doherty JD, Simonovic M, So R, Meltzer HY. The effect of phencyclidine on dopamine synthesis and metabolic in rat striatum. Eur J Pharmacol. 1980;65:139–149. [DOI] [PubMed] [Google Scholar]
- 60. Hertel P, Mathé JM, Nomikos GG, Iurlo M, Mathé AA, Svensson TH. Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav Brain Res. 1995;72:103–114. [DOI] [PubMed] [Google Scholar]
- 61. Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162:394–396. [DOI] [PubMed] [Google Scholar]
- 63. Stone JM, Dietrich C, Edden R, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17:664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohrmann P, Siegmund A, Suslow T, et al. Evidence for glutamatergic neuronal dysfunction in the prefrontal cortex in chronic but not in first-episode patients with schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr Res. 2005;73:153–157. [DOI] [PubMed] [Google Scholar]
- 65. Bustillo JR, Chen H, Jones T, et al. Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry. 2014;71:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tunc-Skarka N, Weber-Fahr W, Hoerst M, Meyer-Lindenberg A, Zink M, Ende G. MR spectroscopic evaluation of N-acetylaspartate’s T2 relaxation time and concentration corroborates white matter abnormalities in schizophrenia. Neuroimage. 2009;48:525–531. [DOI] [PubMed] [Google Scholar]
- 67. Du F, Cooper A, Cohen BM, Renshaw PF, Öngür D. Water and metabolite transverse T2 relaxation time abnormalities in the white matter in schizophrenia. Schizophr Res. 2012;137:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ongür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. [DOI] [PubMed] [Google Scholar]
- 70. Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. [DOI] [PubMed] [Google Scholar]