Abstract
Increasing evidence suggests that olfactory dysfunction is an endophenotype of schizophrenia, and thus the olfactory system can be studied both in relation to this sensory dysfunction and also as a means of examining pathophysiologic mechanisms of schizophrenia. In this study, we examined human olfactory neuroepithelial (ON) biopsy tissues and their in vitro culture cells for ligand-induced guanine nucleotide-binding protein (G protein) activation and downstream signaling. We assessed the binding of a nonhydrolyzable GTP analogue [35S]GTPγS binding to specific G protein subtypes in response to odorants, dopamine, or serotonin in ON cell membranes from matched schizophrenia-control subjects. In response to odorant mixtures, we found decreased [35S]GTPγS binding to Gαs/olf in schizophrenia patients. These changes were not mediated by mRNA expression of key molecules of G protein coupling, including adenylate cyclase III (ACIII), protein kinase A (PKA), protein kinase Cγ (PKCγ), or Gαs or Gαolf in ON cells or ON biopsy tissues. In contrast, dopamine (DA)- and serotonin (5HT)-induced S35-GTPγS binding to Gαs/olf and Gαq/11 were significantly increased in schizophrenia cases, while these parameters were strikingly reduced by in vitro treatment with antipsychotics. Patients with schizophrenia exhibit increases in electrolfactogram (EOG) recordings, suggesting enhanced odorant-induced activation. Our results of decreased odorant-induced G protein activation may point further downstream for underlying mechanisms for increased EOG measures. Increased G protein activation in response to DA and 5HT may suggest increased postreceptor DA or 5HT signaling as an additional mechanism of dopaminergic or serotonergic dysregulation in schizophrenia.
Key words: schizophrenia, olfactory, G protein, dopamine, serotonin
Introduction
G protein–coupled receptors (GPCRs) include multiple neurotransmitter/neuropeptide systems implicated in the pathophysiology of schizophrenia. Dopamine receptor type 2 (D2R) is a molecular target for virtually all antipsychotics1 and recent neuroimaging evidence indeed demonstrates evidence for alterations in DA signaling.2 Serotonin receptor type 2 (5HT2R) also plays an important role in therapeutics of psychosis as well as in various physiological alterations associated with schizophrenia.3 In addition, schizophrenia patients exhibit many neurophysiological dysregulations, such as auditory, visual, and olfactory function, for which GPCRs may play an important role. Nevertheless, study of G protein signaling in neural cells of patients with schizophrenia has been limited.
The olfactory neuroepithelial (ON) biopsy approach offers a unique opportunity to investigate neural cells derived from patients without genetic reprogramming.4 When propagated in vitro, these cells express D2R and 5HT2 receptors as well as odorant receptors5 and permit us to investigate receptor-specific activation of G protein signaling in neural cells of patients. Moreover, ON cells are derived from the olfactory system, and thus molecular signaling in these cells can also be interpreted in the context of circuit function of the olfactory system. In this study, we examined GPCR signaling in response to odorants in the context of olfactory dysfunction observed in schizophrenia patients and in response to DA or 5HT.
Increasing evidence suggests that olfactory dysfunction is an endophenotype of schizophrenia6–12 and as such, biological underpinnings for olfactory dysfunction may offer clues to pathophysiologic mechanisms affecting other brain regions.4 Olfactory function reflects the activity of the circuitry connecting olfactory receptor neurons (ORNs), the olfactory bulb, and the primary olfactory cortex. Several research methodologies permit investigating each segment of the circuitry: ORNs are accessible via ON biopsy,5,13,14 and circuit activity can be measured via electrolfactogram (EOG)15 and olfactory evoked potentials (EP).16 Indeed, patients with schizophrenia have demonstrated alterations in EP and EOG recordings16,17 as well as in neuronal differentiation in the ON.18
Schizophrenia patients exhibit decreases in EP recordings, consistent with olfactory dysfunction as observed in psychophysical assessment.16 However, EOG recordings, which represent action potentials of ORNs in the ON, are increased in schizophrenia patients compared to healthy subjects,15 suggesting increases in odorant-induced activation of ORNs. Notably, individuals at high risk for schizophrenia also showed decreased amplitude of event-related potentials19 and similar changes in EOG recordings to patients with schizophrenia (data not shown), suggesting that this dysregulation is integral to olfactory dysfunction in schizophrenia.
Odorant signaling is initiated by binding of odorants to odorant receptors that activate Gαolf, which in turn triggers downstream signaling.20 Alterations in odorant signaling might, therefore, be determined at the level of odorant receptors and/or G protein coupling and its downstream signaling.
Dopamine and 5HT receptor signaling, whose functions are also mediated by G protein–coupled receptors (GPCRs), have been implicated in the pathophysiology of schizophrenia.21,22 In addition, metabotropic glutamatergic receptors, also signaled by G protein coupling, have been reported to be altered in postmortem studies of schizophrenia23 and have been investigated as a possible therapeutic target.23,24 More recently, molecular imaging studies with PET or SPECT examined 18F/11C-L-DOPA uptake and found significant increases in schizophrenia, suggesting presynaptic dysregulation of DA signaling in schizophrenia patients and individuals at risk for schizophrenia.25–27 At the postsynaptic receptor level, multiple groups have examined the expression levels of receptors and binding affinities for DA and 5HT and reported inconsistent changes in postmortem brains of schizophrenia patients.1,28 Beyond the receptor level, however, GPCR signaling has not been extensively studied.
The goal of this study was to examine G protein activation in ON cells as a mechanism for increased EOG measures, which may reflect activation of odorant-induced and G protein activation–mediated enhancement of intracellular calcium influx. We hypothesized that the observed alterations in odorant signaling are determined downstream to receptor activation, ie, G protein activation, activation of effectors, and further downstream. Indeed, recent behavioral threshold sensitivity data in schizophrenia patients is consistent with this hypothesis.29
ON cells, derived from olfactory biopsies from living subjects with schizophrenia, have previously been examined for alterations in cellular and molecular characteristics.14,30 In this study, we examined dysregulations of GPCR signaling in ON cells from patients with schizophrenia.
Methods
Human Olfactory Neuroepithelial Biopsies
Olfactory epithelial biopsies were obtained from 27 individuals with schizophrenia and 27 nonpsychiatric controls (see table 1 for subgroups of subjects included in each assay) who were recruited and assessed in the Schizophrenia Research Center at the University of Pennsylvania. Olfactory tissues were obtained in collaboration with the Department of Otorhinolaryngology at the University of Pennsylvania. Subjects were informed of the nature and potential risks of participation in the study and provided written informed consent for participation. Work with human tissue described in this manuscript was conducted in accordance with the Declaration of Helsinki according to protocols approved by the Institutional Review Board of the University of Pennsylvania (supplemental methods).
Table 1.
Demographic Distribution for Each Assay
| Mix A Odorant Stimulation Experiments | |||
|---|---|---|---|
| Schizophrenia (n = 17) | Control (n = 17) | ||
| Age | |||
| Mean | 36.3 | 34.9 | |
| SD | 9.6 | 9.8 | |
| Sex, n (%) | |||
| Males | 12 (70) | 12 (70) | |
| Females | 5 (30) | 5 (30) | |
| Race, n (%) | |||
| African American | 12 (70) | 9 (52) | |
| Caucasian | 3 (18) | 7 (41) | |
| Other | 1 (6) | 2 (12) | |
| Medication status, n (%) | |||
| Antipsychotics | 12 (70) | 0 (0) | |
| Protein and mRNA experiments | |||
| Schizophrenia (n = 17) | Control (n = 17) | ||
| Age | |||
| Mean | 43.1 | 41.4 | |
| SD | 9.2 | 10.5 | |
| Sex, n (%) | |||
| Males | 12 (70) | 12 (70) | |
| Females | 5 (30) | 5 (30) | |
| Race, n (%) | |||
| African American | 15 (88) | 14 (82) | |
| Caucasian | 2 (12) | 3 (18) | |
| Other | 0 (0) | 0 (0) | |
| Medication status, n (%) | |||
| Antipsychotics | 15 (88) | 0 (0) | |
| DA, 5HT, and Mix B odorant stimulation experiments | |||
| Schizophrenia (n = 10) | Control (n = 10) | ||
| Age | 32.3 | 32.9 | |
| Mean | 8.8 | 8.5 | |
| SD | |||
| Sex, n (%) | 8 (80) | 8 (80) | |
| Males | 2 (20) | 2 (20) | |
| Females | |||
| Race, n (%) | 5 (50) | 3 (30) | |
| African American | 3 (30) | 6 (60) | |
| Caucasian | 2 (20) | 1 (10) | |
| Other | |||
| Medication status, n (%) | Antipsychotics | 6 (60) | 0 (0) |
Olfactory biopsies were obtained as previously described.31 Briefly, the nasal cavity was anesthetized with pontocaine spray. After 15 minutes, two 1-mm3 biopsies were obtained with giraffe forceps, one from the high middle turbinate and the other from the opposed septum and transferred to culture media for transport to the laboratory.
Culture of Olfactory Neuroepithelial Cell Lines
ON cells were prepared as previously described.5 At confluence, cells were passaged and frozen in cell freezing medium (5% FBS in DMSO) and stored at −140°C in liquid nitrogen vapor phase freezer for subsequent studies (supplemental methods).
Odorant, Dopamine, and 5HT Receptor–Mediated G Protein Activation
As a measure of the functionality of various G protein–coupled receptors, we examined G protein activation in ON culture cells derived from olfactory biopsies of patients with schizophrenia and age- and sex-matched control using 2 odorant mixes: Mix A (citralva, hedione, geraniol, phenethylalcohol, citronella, eugenol, menthone) (17 schizophrenia-control pairs) and Mix B (lyral, lilial, triethylamine, ethyl vanillin, isovaleric acid, phenylethylamine) (10 schizophrenia-control pairs). Immunoprecipitation was performed as previously described.32 Cell membranes were incubated at 4°C with anti-Gαs/olf, -Gαi, -Gαo, or -Gαq/11 followed by 1 hour incubation with 25 µl protein A/G–conjugated agarose. The levels of immunoprecipitated [35S]GTPγS-bound Gα proteins were measured by scintillation spectrometry (supplemental methods).
Western Blotting
ON cell lines from 13 schizophrenia-control pairs were grown to 90% confluence, homogenates were extracted in RIPA buffer and processed through 2 freeze-thaw cycles. Twenty microgram of protein extracts were loaded onto 7.5% Tris-glycine gels (Biorad), run under denaturing conditions at 200V and transferred to PVDF membrane (Millipore). Membranes were blocked for 1 hour at RT in 5% milk/TBS with 0.05% Tween-20 (TBST) and probed overnight at 4°C with antibodies against Gαs/olf (1:100; sc-55545, Santa Cruz), ACIII (1:500; sc-588, Santa Cruz), PKCγ (1:500; sc-211, Santa Cruz), PKA (1:1000; C-α #4782, Cell Signaling), and β-actin (1:10000; A2228, Sigma Aldrich). Blots were washed 5×10 minutes in TBST, incubated in HRP-conjugated secondary antibody for 1 hour at RT, (1:5000 anti-rabbit or anti-mouse, Jackson Immunoresearch Laboratories), washed again 5×10 minutes in TBST and developed using ECL or ECL plus (GE Healthcare).
Quantitative Real Time PCR From ON Cells
Total RNA was extracted from the ON cells of 15 schizophrenia-control pairs using Trizol (Life Technologies) and RNeasy mini kit (Qiagen) following the manufacturer’s protocol. RNA was further purified by DNase I digestion (DNase I, 30U/100mg total RNA, Qiagen). Total RNAs were reverse transcribed using High Capacity cDNA Reverse Transcriptase kit (Life Technologies). Twenty-five nanograms of total RNA equivalent cDNA from each sample was used for quantitative real-time PCR (qPCR) in duplicate wells using 2× SYBR Green PCR master mix (Life Technologies) using gene specific primers for CNGA2, PRACA, PRKCG, GNAS, GNAL (supplementary methods) using ABI 7300 Sequence Detection System. Data from each qPCR run were analyzed using ABI Prism Software version 1.2.3.
Results
Odorant-Induced G protein Activation Is Decreased in Patients With Schizophrenia
ON culture cells express odorant receptors and these receptors can be activated by odorants in vitro.5 Binding of odorants to their receptors activates a subset of G proteins, which can then be monitored in ON and measured by the binding of a nonhydrolyzable GTP analogue, GTPγS into specific Gα proteins. To establish this experimental paradigm in human ON cells, we isolated membrane fractions from the ON cells of 3 healthy control subjects, which were then incubated with varying concentrations of Mix A (citralva, hedione, geraniol, phenethylalcohol, citronella, eugenol, menthone) and Mix B (lyral, lilial, triethylamine, ethyl vanillin, isovaleric acid, phenylethylamine) in the presence of [35S]GTPγS. Odorant-stimulated samples were then immunoprecipitated with antibodies for specific Gα classes, Gαs/Gαs/olf, Gαi, Gαo, Gαq/11, Gα12, and Gα13. Mix A increased [35S]GTPγS binding to Gα/olf, Gαi, Gαo, and Gα13 by 142%–192% (Gα/olf: P < .00001; Gαi: P < .00001; Gαo: P < .00001; Gαq/11: P = .077173; Gα12: P = .05903; Gα13: P < .00001), whereas Mix B increased [35S]GTPγS binding to Gα/olf, Gαo, and Gα13 by 75.1%–148.2% (Gα/olf: P < .00001; Gαi: P = .067564; Gαo: P < .00001; Gαq/11: P = .31904; Gα12: P = .10527; Gα13: P < .00142).
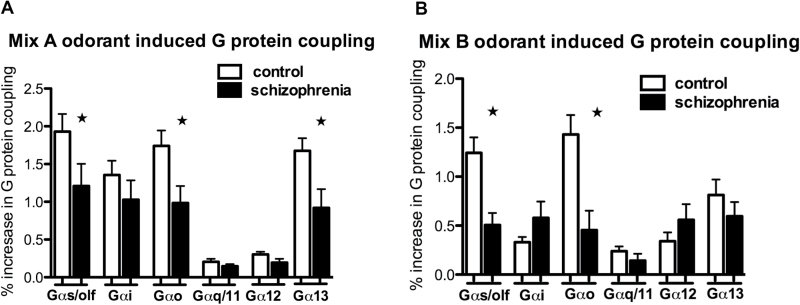
We then examined ON cells derived from a subset of patients with schizophrenia and their age- and sex-matched controls (table 2). Mix A-induced incorporation of [35S]GTPγS was decreased for G protein subunits, Gαs/olf (student’s t test; 2 tailed t(16) = 2.38, P = .03) and Gαo (t(16) = 2.56, P = .02) and Gα13 (t(16) = 2.34, P = .03) in the schizophrenia group (17 matched subject pairs) (Figure 1A). A subgroup of the same cohort (10 matched subject pairs) was also examined for G protein activation in response to odorant Mix B (figure 1B). Interestingly, we observed similar decreases in Mix B-induced [35S]GTPγS binding to Gαs/Gαolf (t(8) = 2.82, P = .02) as well as Gαo (t(8) = 2.69, P = .03).
Table 2.
Assay Subgroups
| Mix A Stimulation | Mix B Stimulation | Protein | mRNA | DA-Induced G Protein | 5HT-Induced G Protein | |
|---|---|---|---|---|---|---|
| Pair 1 | X | X | X | X | ||
| Pair 2 | X | X | X | X | ||
| Pair 3 | X | X | X | X | ||
| Pair 4 | X | X | X | X | ||
| Pair 5 | X | X | X | X | ||
| Pair 6 | X | X | X | X | ||
| Pair 7 | X | X | X | X | ||
| Pair 8 | X | X | X | X | ||
| Pair 9 | X | X | X | X | ||
| Pair 10 | X | X | X | X | ||
| Pair 11 | X | X | X | |||
| Pair 12 | X | X | X | |||
| Pair 13 | X | X | X | |||
| Pair 14 | X | X | X | |||
| Pair 15 | X | X | X | |||
| Pair 16 | X | X | X | |||
| Pair 17 | X | X | X | |||
| Pair 18 | X | |||||
| Pair 19 | X | |||||
| Pair 20 | X | X | ||||
| Pair 21 | X | X | ||||
| Pair 22 | X | |||||
| Pair 23 | X | |||||
| Pair 24 | X | X | ||||
| Pair 25 | X | X | ||||
| Pair 26 | X | |||||
| Pair 27 | X |
X denotes the use of the specific matched pair in the assay indicated in the column headings.
Fig. 1.
Odorant-induced G protein activation is decreased in patients with schizophrenia. Mix A odorant-induced receptor-G protein coupling was determined by [35S]GTPγS binding to selective Gα proteins in cell membranes obtained from olfactory neuroepithelial (ON) cells from 17 matched schizophrenia and age/sex-matched controls (A). Mix A-induced [35S]GTPγS incorporation into Gαs/olf, Gαo, and Gα13 was decreased in schizophrenia. Mix B odorant-induced coupling was examined in a subset of subjects from (A): 9 matched schizophrenia and age/sex-matched controls. Mix B-induced incorporation of [35S]GTPγS into Gαs/olf, Gαo and, to a lesser extent, Gα13 was decreased in schizophrenia (B). Data represented as means ± SEM (bars) of the percent increase in bound [35S]GTPγS elicited by odorant Mix A or Mix B. Statistical significance was assessed paired student’s t test, 2 tailed; *P < .05.
Expression Levels of Odorant Signaling Proteins Are Altered in ON Cells From Schizophrenia Patients
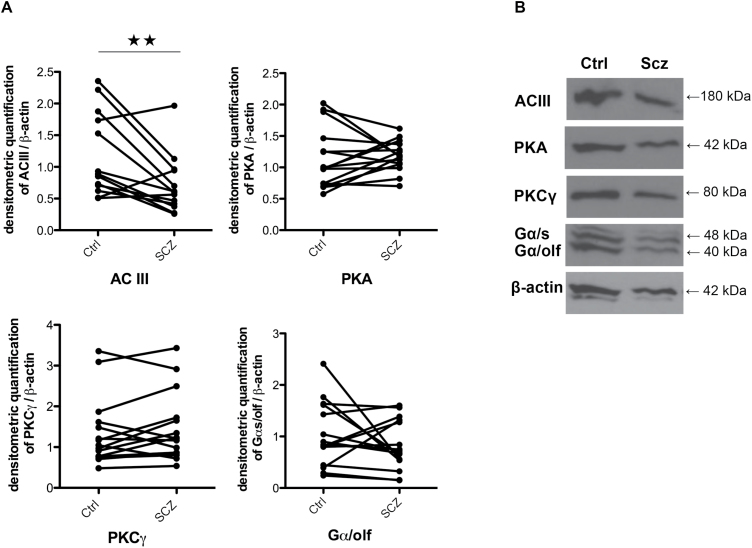
To assess whether the expression levels of signaling proteins in the odorant signaling cascade are altered in ON cells from schizophrenia patients, we examined expression of ACIII, PKA, PKCγ, and Gαs/olf, in ON cell lysates by western blot in a subgroup of 13 matched subject pairs (table 2). Gαs/olf is the main Gα subunit coupled with odorant receptors and Gαs/olf in turn activates ACIII, the effector of odorant signaling, producing cAMP (figure 2A). We found a significant decrease in ACIII (t(13) = 3.121, P = .009), which can lead to decreased cAMP formation (representative western blot figure 2B). No between-group differences were seen in PKA or PKCγ. We next examined mRNAs for key signaling molecules in ON cells.
Fig. 2.
ACIII protein level is decreased in patients with schizophrenia. ON cell lysates prepared from 15 age/sex-matched schizophrenia and control pairs were used to assess ACIII, PKA, PKCγ, and Gαs/olf protein levels by immunoblotting. Representative immunoblot (A) and summarized densitometric quantification of ACIII, PKA, PKCγ, and Gαs/olf protein band density normalized by β-actin quantity (B). Data represented as means ± SEM (bars) of the ratios. Statistical significance was assessed paired student’s t test, 2 tailed; **P < .01.
mRNAs of Odorant Signaling Molecules Are Unaltered in Ex Vivo ON Cells
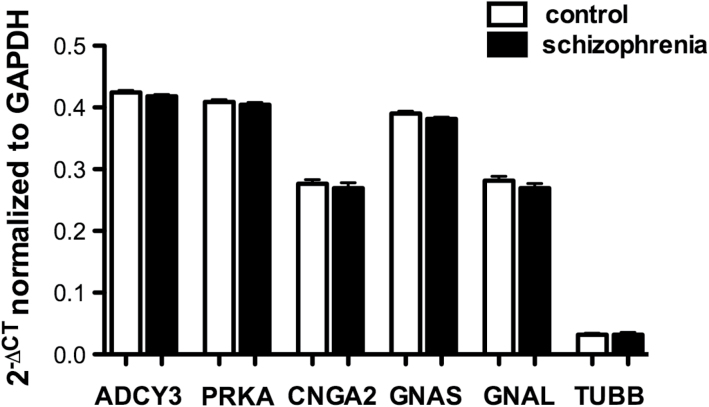
In vitro culture cells were derived from biopsy tissues from 10 schizophrenia patients and 10 age- and sex-matched control subjects. We assessed RNA expression of the transcripts for 5 genes which play critical roles in odorant signaling; cAMP-dependent protein kinase A (PRKACB), protein kinase Cγ (PRKCG) adenylate cyclase III (ADCY3), Guanine Nucleotide–Binding Protein (G protein), Alpha Activating Activity Polypeptide, Olfactory Type (GNAS) and Guanine Nucleotide–Binding Protein (G protein), Alpha Activating Activity Polypeptide and Olfactory Type (GNAL) or b-tubulin III (TUBB) in ON cells derived from each of the subjects. There were no between-group differences for PRKACB, ADCY3, GNAS, or GNAL transcripts between schizophrenia patients and their matched controls (figure 3).
Fig. 3.
mRNAs of odorant signaling molecules are unaltered in cultured ON cells. mRNA expression was examined in ON culture cells derived from olfactory biopsies from 10 age/sex-matched schizophrenia and control pairs. Amplified transcripts from OE cells were examined for ADCY3, PRKACB, GNAS, and GNAL. Data represented as means ± SEM (bars). Statistical significance was assessed paired student’s t test, 2 tailed, P < .05.
Increased DA- and 5HT-Induced G Protein Activation in ON of Schizophrenia Patients Is Attenuated by Antipsychotics
G protein signaling transduces various receptor systems, sharing some of molecular components in common. To test whether dysregulations in G protein signaling observed in response to odorants are shared by other receptor systems, we examined DA- and 5HT-mediated G protein activation, which are of particular interest in both the pathophysiology and treatment of schizophrenia.
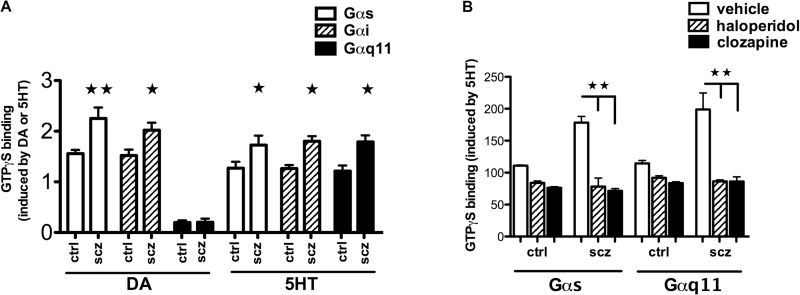
To that end, we incubated ON membranes derived from the same 10 matched control-schizophrenia pairs (table 2) with DA or 5HT for 5 minutes in the presence of [35S]GTPγS and assessed the level of [35S]GTPγS incorporation into Gα subunits as described previously.32 DA (1 µM) induces [35S]GTPγS binding to Gαs/olf, Gαi, and modestly to Gαq/11. DA-induced activation of both Gαs and Gαi was significantly increased in ON cells of schizophrenia patients. Similarly, 5HT increases [35S]GTPγS binding to Gαs, Gαi, and Gαq/11. 5HT-induced Gαs, Gαi, and Gαq/11 activation was heightened in ON cells of schizophrenia patients (figure 4A).
Fig. 4.
Increased DA- and 5HT-mediated G protein coupling in schizophrenia patients is normalized by chronic treatment with antipsychotics. ON cell membranes from 10 age/sex-matched schizophrenia and control pairs were stimulated with DA or 5HT in the presence of [35S]GTPγS. [35S]GTPγS-bound Gα proteins were immunoprecipitated with antibodies to various Gα proteins. Both DA- and 5HT-induced [35S]GTPγS incorporation were higher in ON cells from schizophrenia subjects (A). The effects of 5-day treatment with 100nM haloperidol, 2 µM clozapine, or vehicle on 5HT-induced G protein coupling were examined in 3 age/sex-matched schizophrenia and control pairs. 5HT-induced [35S]GTPγS binding to Gαs/olf and Gαq/11 was examined. Antipsychotic treatments significantly decreased [35S]GTPγS binding to Gαs and Gαq11 in ON cells from schizophrenia and control subjects. [35S]GTPγS binding was higher in schizophrenia patients without antipsychotic treatment (B). Data represented as means ± SEM (bars). Statistical significance was assessed paired student’s t test, 2 tailed. *P < .05, **P < .01.
Given that antipsychotics block these receptors, we tested the effects of antipsychotics on 5HT-induced G protein activation in ON culture cells. To assess the acute effects of antipsychotics, ON cells were incubated with 10 µM haloperidol or clozapine for 30 minutes and cell membranes were incubated with [35S]GTPγS and 5HT. As predicted, activation of Gαs and Gαq11 was strikingly suppressed by both antipsychotic agents. To evaluate the effects of chronic treatment, ON cells were treated with haloperidol or clozapine for 5 days and G protein activation was then measured by [35S]GTPγS incorporation induced by 1 µM 5HT. Similar to the acute effects of haloperidol and clozapine, chronic exposure to either antipsychotic decreased 5HT-induced Gαs/olf and Gαq11 activation but increased basal [35S]GTPγS binding to Gαs/olf and Gαq/11 (figure 4B).
Discussion
The goal of this study was to investigate G protein coupling as a locus of dysregulation in GPCR signaling in neural cells of patients with schizophrenia. Data presented here demonstrate ligand-specific dysregulations in G protein coupling in ON cells from schizophrenia patients: notably decreases in response to odorants but increases to DA and 5HT.
Increased EOG findings in schizophrenia patients predict greater neuronal excitation resulting from enhanced odorant signaling. Decreased G protein activation as we observed in schizophrenia patients, however, would reduce cAMP formation leading to decreased excitability of ORNs. In addition, we found a decrease in ACIII expression in patients, which can further lower cAMP formation. Our results together indicate that the activity of the proximal segment of the odorant signaling, ie, odorant receptors, G proteins, and ACIII, is lowered. Thus, the alterations in this segment may not be the primary cause for EOG abnormalities in schizophrenia.
It follows that increased EOG measures may result from enhanced activity of the segment of odorant signaling pathway that is downstream to G protein and ACIII. In this case, one explanation for decreased G protein coupling as observed in ON cells of patients could be that it is a compensatory mechanism. Downstream to the activation of Gαolf are activation of CNG channels and voltage-dependent [Ca++] and [Na+] channels, which regulate neuronal activity of ORNs. Given that voltage sensitive [Ca++] and [Na+] channels have been implicated for the pathophysiology of schizophrenia, it will also be important to examine these channels in ON cells and in postmortem brains of schizophrenia patients.
It would be interesting to examine the extent to which the G protein dysregulation observed in ON cells might be reflected in other sensory modalities in patients. If G protein dysregulation is inherent to the illness, it is possible that similar changes could be found in other sensory modalities, such as in gustatory function. If, on the other hand, it is a compensatory response to the changes in the further downstream segment of the pathway, it is less likely to be observed in other sensory modalities.
Our results do not elucidate the molecular underpinnings for decreased odorant-induced G protein coupling in schizophrenia. However, there are several possibilities to be considered. Reduced odorant-induced G protein activation may reflect changes in the quantity of odorant receptors, odorant binding affinity, G protein expression, and/or receptor-G protein coupling efficiency. While it is possible that our findings could be due to reduced expression of odorant receptors it is of note that, humans have more than 300 different odorant receptors, each of which responds to specific odorants or combinations of odorants.33,34 If increased EOG measures are caused by altered expression of odorant receptors, schizophrenia should be associated with altered expression of hundreds of odorant receptor genes, which is not a likely scenario.
Decreased G protein activity could be due to altered expression of key molecules in the G protein signaling pathway. Alternatively, the altered G protein activation may be a result of differential levels of heteromeric GPCR complexes that are known to exhibit different signaling properties35 although currently there is no concrete evidence to support the existence of heteromeric GPCR complexes in olfactory neuroepithelium. RNA quantification in ON cells, however, fails to show any dysregulation in PRKACB, PRKCG, ADCY3, GNAS, or GNAL, demonstrating that the observed dysregulation in G protein coupling is not caused by alterations at the mRNA level. Neither did we find discernible changes in Gα expression levels in ON cells derived from schizophrenia patients. Interestingly, decreased Gαs/olf was found in a cohort of individuals who are at clinically high risk for schizophrenia (Borgmann-Winter et al, under review). Thus, it will be important to further investigate Gαs/olf protein expression levels in a larger sample size and to delineate progressive changes in G protein expression in the clinical risk period for onset of schizophrenia. DA and 5HT receptors are of particular interest as they are critical for the pathophysiology and therapeutics of schizophrenia. In contrast to odorant-induced signaling, DA- and 5HT-induced G protein activation were increased, indicating that G protein signaling dysregulation in schizophrenia is receptor specific.
Schizophrenia patients exhibit increased DA-induced Gαs/olf and Gαi but not Gαq/11 activation suggesting enhancement of DR1-, D2R-, and D1R-like signaling. The dopamine hypothesis of schizophrenia has been extensively investigated by molecular brain imaging of living subjects.2 Meta-analyses have shown that DOPA uptake was overall increased, indicating a presynaptic dysregulation in the DA system.36–38 At the postsynaptic level, however, evidence has been inconsistent for the levels of D2R and D3R in postmortem or molecular imaging studies.38 Interestingly, postmortem studies of patients with schizophrenia have demonstrated a number of alterations in expression levels of dopamine pathway-mediated proteins and mRNA, such as G protein–coupled receptor kinase GRK,39 and the transcript for calcyon which mediates DA and Gq11 crosstalk in the cortex and thalamic nuclei of patients with schizophrenia.40,41 Our findings indicating heightened G protein coupling in ON cells suggest postsynaptic DA receptor function as a potential mechanism via which DA function is increased. It will be important to further examine such changes in postsynaptic DA function in postmortem brains of schizophrenia patients.
We considered whether altered G protein signaling could be mediated by antipsychotics. This scenario seems unlikely since in vitro antipsychotic treatment (acute or chronic) produced opposite effects on G protein coupling to those observed in patients. Furthermore, no significant correlations between antipsychotic use at the time of biopsy and the alterations in odorant-stimulated G protein coupling observed in patients were found (supplemental data). Smoking is another factor that could affect G protein coupling. Interestingly, subjects with schizophrenia who are smokers have increased Mix A-induced Gαo coupling compared to nonsmokers (supplemental data). No differences are seen between smokers and nonsmokers in control subjects. Interestingly, the increased Mix A-induced Gαo coupling associated with smoking in patients with schizophrenia is in the opposite direction of the changes we saw in G protein coupling in subjects with schizophrenia in which we observed decreased Mix A-induced Gαo coupling. A paradoxical effect of smoking specifically in patients with schizophrenia has been observed in psychophysical measures of olfactory function such as olfactory identification.42,43 While subjects were matched for age and sex, our sample sizes precluded an assessment of race as a possible confound, although to our knowledge no olfactory studies to date have identified race as a mediator in olfactory assessments.
The pervasive nature of olfactory dysfunction in schizophrenia indicates that there are fundamental neurobiological alterations in the olfactory circuitry of schizophrenia patients. In addition, endophenotypic manifestation of these neurobiological alterations suggests that they may be integral to the overall disease pathology affecting multiple brain regions. Our findings in ON cells are consistent with the notion that altered G protein coupling may potentially be a compensatory mechanism for increased ORN activity noted in EOG study of schizophrenia patients. Further, they point to G protein coupling as a locus of dysregulation in schizophrenia, which may affect various intracellular signaling pathways in a receptor-specific manner.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the National Institutes of Mental Health (R01MH059852 to B.I.T. and C.-G.H and K23MH079498 to K.E.B.-W).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Seeman P. Schizophrenia as a brain disease. The dopamine receptor story. Arch Neurol. 1993;50:1093–1095. [DOI] [PubMed] [Google Scholar]
- 2. Bonoldi I, Howes OD. The enduring centrality of dopamine in the pathophysiology of schizophrenia: in vivo evidence from the prodrome to the first psychotic episode. Adv Pharmacol. 2013;68:199–220. [DOI] [PubMed] [Google Scholar]
- 3. Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. [DOI] [PubMed] [Google Scholar]
- 4. Borgmann-Winter K, Willard SL, Sinclair D, et al. Translational potential of olfactory mucosa for the study of neuropsychiatric illness. Transl Psychiatry. 2015;5:e527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borgmann-Winter KE, Rawson NE, Wang HY, et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience. 2009;158:642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamath V, Turetsky BI, Calkins ME, et al. Olfactory processing in schizophrenia, non-ill first-degree family members, and young people at-risk for psychosis. World J Biol Psychiatry. 2014;15:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moberg PJ, Kamath V, Marchetto DM, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2014;40:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18:399–415. [DOI] [PubMed] [Google Scholar]
- 9. Kamath V, Turetsky BI, Calkins ME, et al. The effect of odor valence on olfactory performance in schizophrenia patients, unaffected relatives and at-risk youth. J Psychiatr Res. 2013;47:1636–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gill KE, Evans E, Kayser J, et al. Smell identification in individuals at clinical high risk for schizophrenia. Psychiatry Res. 2014;220:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turetsky BI, Kohler CG, Gur RE, Moberg PJ. Olfactory physiological impairment in first-degree relatives of schizophrenia patients. Schizophr Res. 2008;102:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 13. Hahn CG, Han LY, Rawson NE, et al. In vivo and in vitro neurogenesis in human olfactory epithelium. J Comp Neurol. 2005;483:154–163. [DOI] [PubMed] [Google Scholar]
- 14. Brown AS, Borgmann-Winter K, Hahn CG, et al. Increased stability of microtubules in cultured olfactory neuroepithelial cells from individuals with schizophrenia [published online ahead of print October 24, 2013]. Prog Neuropsychopharmacol Biol Psychiatry. doi: 10.1016/j.pnpbp.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turetsky BI, Hahn CG, Arnold SE, Moberg PJ. Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology. 2009;34:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003;53:403–411. [DOI] [PubMed] [Google Scholar]
- 17. Turetsky B. Olfactory Probes of Dysregulated cAMP Signaling in Schizophrenia. Paper presented at: 68th Society of Biological Psychiatry Annual Meeting2013; San Francisco, CA. [Google Scholar]
- 18. Arnold SE, Han LY, Moberg PJ, et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. [DOI] [PubMed] [Google Scholar]
- 19. Kayser J, Tenke CE, Kroppmann CJ, et al. Olfaction in the psychosis prodrome: electrophysiological and behavioral measures of odor detection. Int J Psychophysiol. 2013;90:190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ronnett GV, Moon C. G proteins and olfactory signal transduction. Annu Rev Physiol. 2002;64:189–222. [DOI] [PubMed] [Google Scholar]
- 21. Gray L, Scarr E, Dean B. Serotonin 1a receptor and associated G-protein activation in schizophrenia and bipolar disorder. Psychiatry Res. 2006;143:111–120. [DOI] [PubMed] [Google Scholar]
- 22. Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neurosci Biobehav Rev. 2014;45:233–245. [DOI] [PubMed] [Google Scholar]
- 23. Gupta DS, McCullumsmith RE, Beneyto M, Haroutunian V, Davis KL, Meador-Woodruff JH. Metabotropic glutamate receptor protein expression in the prefrontal cortex and striatum in schizophrenia. Synapse. 2005;57:123–131. [DOI] [PubMed] [Google Scholar]
- 24. Walker AG, Conn PJ. Group I and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics. Curr Opin Pharmacol. 2015;20:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 26. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. [DOI] [PubMed] [Google Scholar]
- 27. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mackay AV, Iversen LL, Rossor M, et al. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry. 1982;39:991–997. [DOI] [PubMed] [Google Scholar]
- 29. Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCurdy RD, Féron F, Perry C, et al. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82:163–173. [DOI] [PubMed] [Google Scholar]
- 31. Lowry LD, Pribitkin E. Collection of human olfactory tissue. In: Spielman AI, Brand JG, eds. Experimental Cell Biology of Taste and Olfaction. Boca Raton, FL: CRC Press; 1995:47–48. [Google Scholar]
- 32. Hahn CG, Umapathy , Wang HY, Koneru R, Levinson DF, Friedman E. Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39:355–363. [DOI] [PubMed] [Google Scholar]
- 33. Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. [DOI] [PubMed] [Google Scholar]
- 34. Malnic B, Godfrey PA, Buck LB. The human olfactory receptor gene family. Proc Natl Acad Sci USA. 2004;101:2584–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanders RD, Brian D, Maze M. G-protein-coupled receptors. Handb Exp Pharmacol. 2008:93–117. [DOI] [PubMed] [Google Scholar]
- 36. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, Part I: meta-analysis of dopamine active transporter (DAT) density. Schizophr Bull. 2013;39:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fusar-Poli P, Meyer-Lindenberg A. Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 2013;39: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Funk AJ, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Increased G protein-coupled receptor kinase (GRK) expression in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2014;159:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baracskay KL, Haroutunian V, Meador-Woodruff JH. Dopamine receptor signaling molecules are altered in elderly schizophrenic cortex. Synapse. 2006;60:271–279. [DOI] [PubMed] [Google Scholar]
- 41. Clinton SM, Ibrahim HM, Frey KA, Davis KL, Haroutunian V, Meador-Woodruff JH. Dopaminergic abnormalities in select thalamic nuclei in schizophrenia: involvement of the intracellular signal integrating proteins calcyon and spinophilin. Am J Psychiatry. 2005;162:1859–1871. [DOI] [PubMed] [Google Scholar]
- 42. McLean D, Féron F, Mackay-Sim A, et al. Paradoxical association between smoking and olfactory identification in psychosis versus controls. Aust N Z J Psychiatry. 2004;38:81–83. [DOI] [PubMed] [Google Scholar]
- 43. Moberg PJ, Kamath V, Marchetto DM, et al. Meta-analysis of olfactory function in schizophrenia, first-degree family members, and youths at-risk for psychosis. Schizophr Bull. 2014;40:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.