Abstract
The skin flush response to niacin is abnormally blunted among a subset of patients with schizophrenia (SZ), preferentially associates with SZ compared to other mental illnesses, occurs frequently in nonpsychotic members of SZ-affected families, appears heritable, and shows evidence of genetic association. The niacin response abnormality (NRA) may prove to be a useful SZ endophenotype. Using a laser Doppler flowmeter, we undertook this study to estimate the prevalence of NRA in SZ (n = 70), bipolar disorder (BP, n = 59), and healthy control (HC, n = 87) groups, and to estimate its specificity for the illness. From the dose-response curves, we calculated the concentration of methylnicotinate required to elicit a half-maximal blood flow (MBF) response (EC50 value) and MBF value for each subject. The median log10EC50 of the SZ was above the third quartile of log10EC50 of either the HC or BP groups, whereas the MBF was significantly lower in the SZ than in the HC or BP groups. With a definition of NRA of having both EC50 above the ninetieth percentile of the control samples and MBF response below the sixtieth percentile for the control range, the NRA predicted SZ with 31% sensitivity and 97% specificity. Moreover, the NRA was not influenced by age, gender, race, and cigarette smoking. In summary, the NRA may define a SZ subtype with a clinically significant phospholipid signaling defect. Understanding its molecular origins may shed light on the pathophysiology of SZ and suggest new tools for its early diagnosis and treatment.
Key words: niacin-induced flush response, laser Doppler flowmeter, EC50, maximal blood flow, bipolar disorder, phospholipid-arachidonate-eicosanoid signaling
Introduction
Reduced sensitivity to the skin flush effect of niacin, a widely replicated finding in schizophrenia (SZ), is more prevalent among individuals with SZ and their family members than among other mentally ill comparison groups or healthy controls (HC).1–3
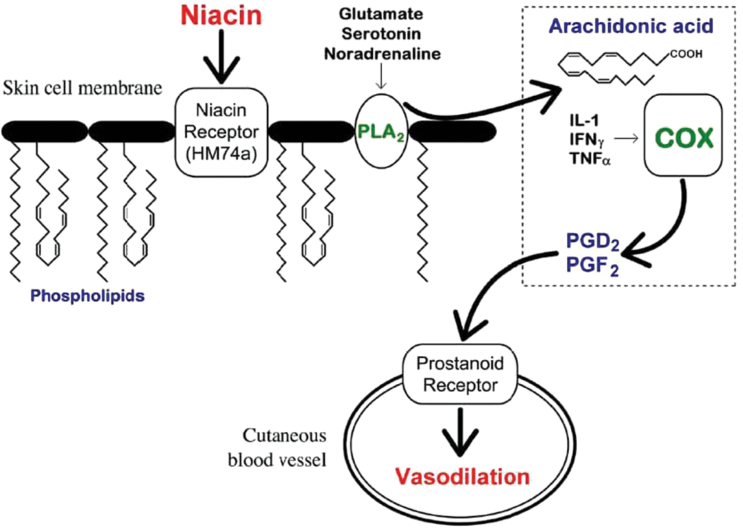
Although the etiology of SZ is unclear, the biochemical basis of niacin-evoked skin flushing is reasonably understood. As illustrated in figure 1, niacin interacts with a specific G-protein−coupled receptor, GPR109A (also known as HM74A),4,5 located on dermal macrophages and adipocytes6,7; its activation stimulates phospholipase A2 (PLA2)-mediated release of arachidonic acid (AA) from cell membranes.8 AA is then converted to the vasodilatory prostaglandins D2 (PGD2) and E2 (PGE2).9 The abnormal niacin response in SZ implies abnormal phospholipid-arachidonate-eicosanoid signaling pathways.10–12
Fig. 1.
The mechanism of niacin-induced skin flushing (adapted from Messamore et al 13). COX, cyclooxygenase; IL, interleukin; INF, interferon; PGD2, prostaglandin D2; PGF2, prostaglandin F2; PLA2, phospholipase A2; TNF, tumor necrosis factor.
Increased serum PLA2 activity is present in first-episode drug-naive patients with SZ.14 Elevated PLA2 activity has also been linked to SZ patients with the niacin response abnormality (NRA).15 Dose-response studies suggest that the abnormality entails diminished pharmacological sensitivity to niacin, and possibly also an attenuated ability to vasodilators in response to niacin.16
In all likelihood, the NRA identifies a physiologically distinct subgroup within the SZ syndrome. We here describe the use of quantitative laser Doppler flowmetry to ascertain the prevalence of this subtype, as well as its specificity for SZ compared to BP and HC reference groups.
Methods
Clinical Design
Patients with SZ and BP were recruited from outpatient treatment programs at the VA Pittsburgh Healthcare System (VAPHS). HC subjects were recruited through the local Pittsburgh community. Diagnoses were made according to the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (fourth ed., text rev.; DSM-IV-TR) criteria using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 4/2005 revision). A Global Assessment of Functioning (GAF) score was assigned to each enrolled subject following psychiatric assessment. The study was approved by the VA Pittsburgh Healthcare System Institutional Review Board.
Inclusion Criteria for SZ.
(a) SCID diagnosis of DSM-IV-TR SZ or schizoaffective disorder, (b) ages 18–65, and (c) duration of psychosis at least 5 years.
Inclusion Criteria for BP.
(a) SCID diagnosis of DSM-IV-TR Bipolar I Disorder but no known lifetime history of SZ or schizoaffective disorder, (b) ages 18–65 and (c) no known first-degree family history of SZ or schizoaffective disorder.
Inclusion Criteria for HC.
(a) No lifetime history of DSM-IV-TR Axis I disorder, (b) ages 18–65, and (c) no first-degree family history of psychotic disorder or mood disorder with psychotic features.
General Exclusion Criteria.
Regardless of subject type, those with the following characteristics were excluded: (a) DSM-IV-TR diagnosis of mental retardation or pervasive developmental disorder, (b) DSM-IV-TR Psychoactive Substance Dependence within the past 6 months or Substance Abuse within the past month, or, for SZ subjects, onset of the psychotic illness has a temporal relation to a substance use disorder, (c) history of significant head injury or, for SZ subjects, a temporal relation between illness onset and head injury, (d) significant history of, or current medical illness affecting the central nervous system, such as lupus or hypothyroidism, or hypertension or diabetes, (e) nonsteroidal or steroidal anti-inflammatory drugs (cyclooxygenase and PLA2 inhibitors, respectively) or niacin within the prior 10 days, (f) pregnancy, (g) significant neurologic disorder, and (h) lack of capacity to understand the study and to give written informed consent.
In order to increase the statistical power of sensitivity and specificity estimates, and to assess the extent to which this method can be replicated, we include in our analyses data obtained from an independently funded study conducted (by Dr Messamore) at the Portland VA Medical Center. Data from the Portland site were collected using identical inclusion/exclusion criteria for SZ and HC subjects and identical methods for eliciting and measuring the blood flow response to topical methylnicotinate (MN). The Portland data also include a group of subjects with mood disorders; members of this group had DSM-IV based diagnoses of BP or major depressive disorder (BP-MDD comparison group).
Quantification of Niacin Response
The cutaneous blood flow responses to graded topical doses of MN was measured according to Messamore et al.2 Cutaneous blood flow was determined by a laser Doppler flowmeter (PeriFlux System 5000, Perimed) equipped with an integrating flow probe that measures and averages blood flow from 7 spatially discrete tissue volumes. The dose-response data were analyzed by nonlinear curve fitting to calculate the EC50 value for MN-induced blood flow as well as the maximal blood flow (MBF) response to MN.
Statistical Analyses
Data Preparation and Assessment.
The data consisted of 8 blood flow response values, one for each MN concentration, per subject, for 70 SZ, 59 BP, and 87 HC subjects from Pittsburgh [and 90 SZ, 23 BP-MDD, and 93 HC subjects from the Portland site]. The distribution of the log10EC50 and MBF data was confirmed to be approximately normal by univariate histogram, quantile-quantile plots, a univariate correlation test of normality,17 bivariate scatterplots, and chi-square quantile-quantile plots and the Henze-Zirckler test 18 for the bivariate data.
Covariates.
Covariates considered were Smoke, Race, Gender, and Age. After the SZ group was divided into 2 subgroups based on their Log10EC50 and MBF measurements, the distribution of the covariate values between these 2 subgroups was examined. For categorical covariates (Smoke, Gender, Race), the null hypothesis of equal proportions of the covariate in each of the SZ subgroups was tested with Fisher Exact or chi-square tests, while the null hypothesis of equal Age distributions in the 2 SZ subgroups was tested with a Kolmogorov-Smirnov test.
Confirmation of Group Differences.
Though not our primary aim, we tested to confirm group differences in the (Log10EC50, MBF) vector by Hotelling T 2 test for each of 2 group pairs (HC vs SZ, BP vs SZ), with simultaneous Sidak CI.
Results
Normality
Quantile-quantile plots and the tests of univariate and multivariate normality confirmed that log10EC50 and MBF both were distributed with approximate univariate normality as well as bivariate normality for all subject groups, HC, SZ, and BP. MBF for the SZ group, whose correlation test of normality produced P = .08, was closest to rejecting normality due to more weight in each of the tails (SZ Box-Whisker plot in figure 2B). All of the outlying values plotted lie within the “outer fences” (3 × interquartile range beyond the first or third quartile). Also, the SZ group’s Henze-Zirkler test failed to reject bivariate normality (P = .22), and the chi-square Q-Q plot showed only one clear multivariate outlier, corresponding to the second highest value on the SZ group MBF boxplot.
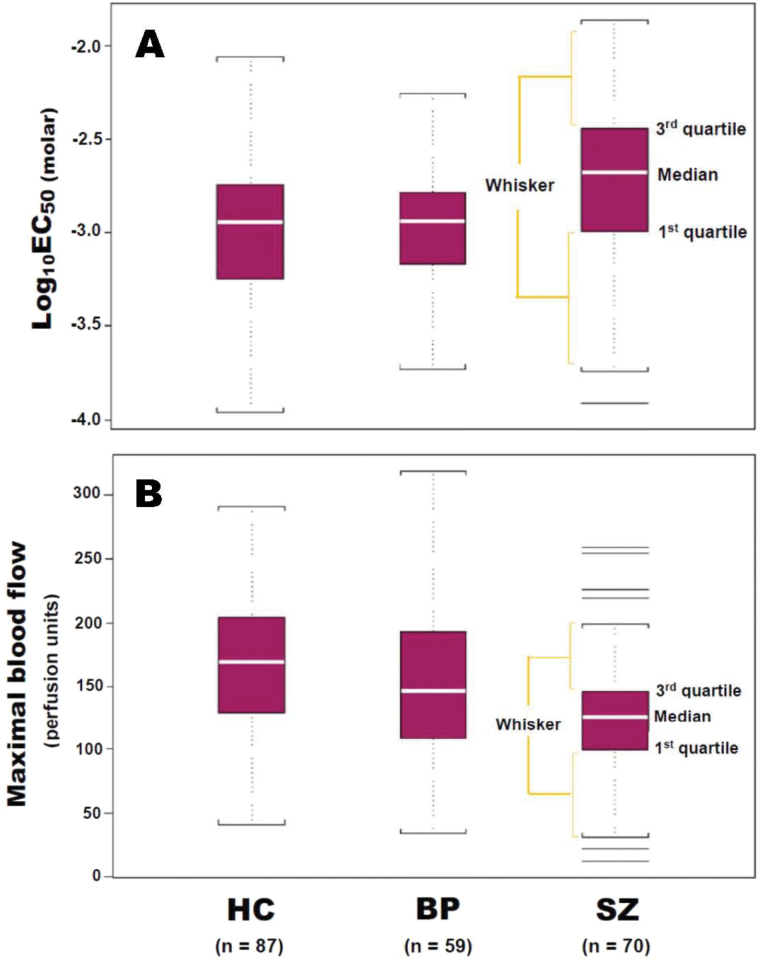
Fig. 2.
Box-Whisker plots of niacin-induced flushing response as expressed as Log10EC50 molar (A) and maximal blood flow (B) values in HC subjects, BP patients, and patients with chronicSZ. In SZ group (B), 4 lines above and 2 lines below the “outer fences” (3 × interquartile range beyond the first or third quartile) indicated outlying values, whose correlation test of normality produced P = .08, was closest to rejecting normality due to more weight in each of the tails. BP, bipolar disorder; HC, healthy controls; SZ, schizopherenia
Distributions
Descriptive statistics were computed for the HC group (log10EC50: mean = −2.97, SD = 0.38; MBF: mean = 167 perfusion units [PU], SD = 54), SZ group (log10EC50: mean = −2.74, SD = 0.44; MBF: mean = 125 PU, SD = 49), and BP group (log10EC50: mean = −2.97, SD = 0.33; MBF: mean = 152 PU, SD = 58).
Displays of log10EC50 distributions for the HC, BP, and SZ groups are shown in figure 2A. Approximately 75% of HC or BP subjects and 50% of SZ had log10EC50 values of 2.0mM (log10EC50 = −2.7) or less. Distributions of MBF in response to niacin-induced flushing among HC, BP, and SZ groups are shown in figure 2B. The third quartile of MBF in SZ group lies below the medians of MBF in both HC and BP groups.
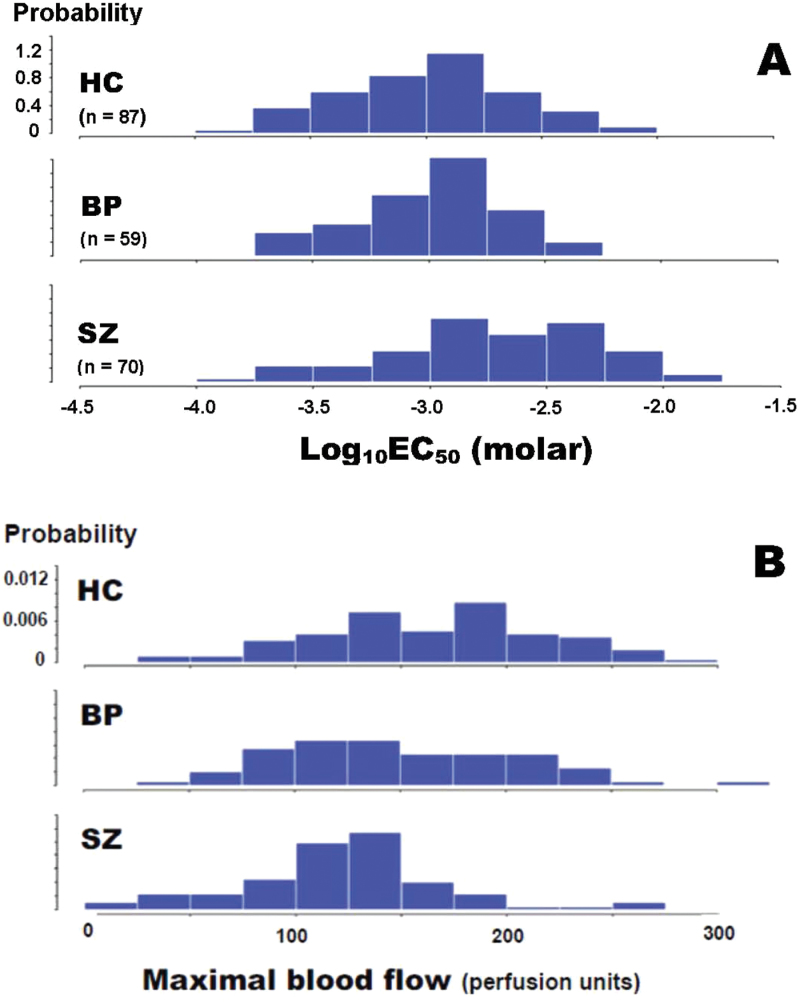
The histograms of niacin-induced flush response expressed as log10EC50 molar values among HC, BP, and SZ groups are shown in figure 3A. Note that all groups’ data are approximately normal with similar variances, but that the SZ group is shifted to the right by comparison with the HC and BP groups.
Fig. 3.
Histograms of niacin-induced flushing response as expressed as Log10EC50 molar (A) and maximal blood flow (B) values in HC subjects, BP patients, and patients with chronic SZ.
The histograms of MBF in response to niacin-induced flushing among HC, BP, and SZ groups are shown in figure 3B. Note that all groups’ data are approximately normal, but that the SZ group is shifted left by comparison with the HC and BP groups.
Abnormal Response by Log10EC50 and MBF
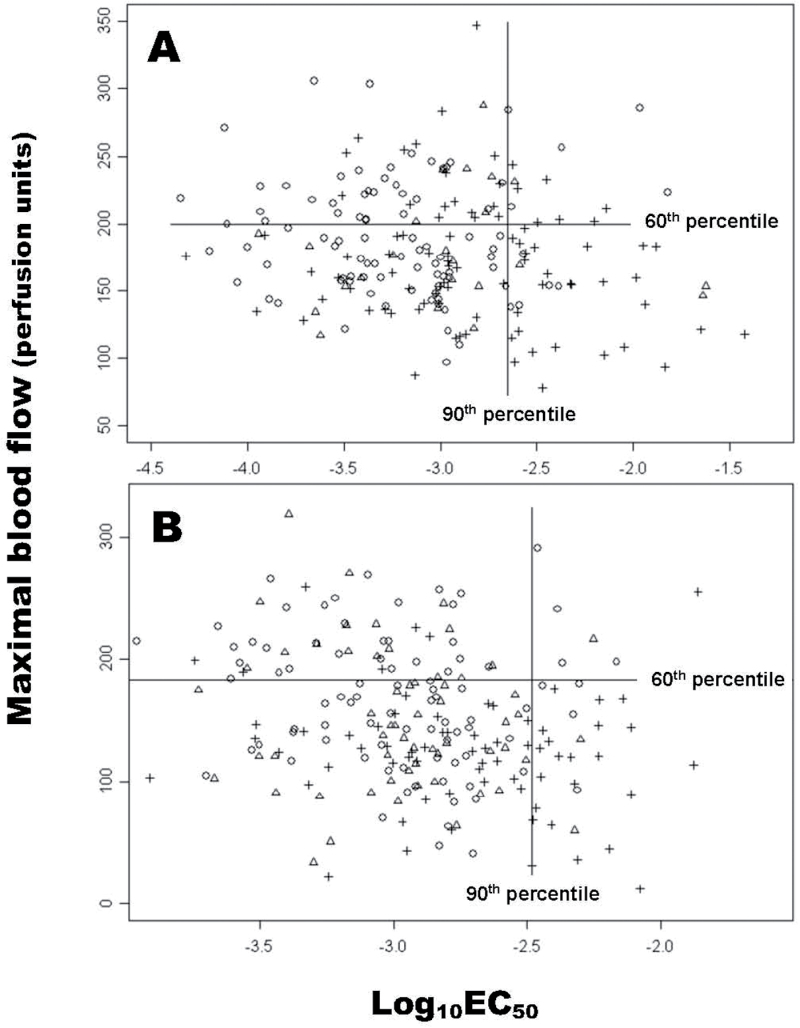
Based on the data shown in figures 2A and 3A, we define niacin subsensitivity as having a log10EC50 value equal or greater than ninetieth percentile of the HC group. We observed that the mean MBF of the SZ group (125 PU) was well below the mean of both HC (167 PU) and BP (152 PU) groups (figures 2B and 3B). To avoid false positive in HC and BP groups, we added having MBF below the 60th percentile of the HC group to define the abnormal niacin response.
Using the above criteria, a bivariate plot of log10EC50 and MBF from Pittsburgh data is shown in figure 4A. The vertical line represents the cutoff of ninetieth percentile of log10EC50 in HC group, whereas the horizontal line represents the sixtieth percentile of MBF in HC group. Thus, all the subjects in the lower right quadrant were defined as having an abnormal niacin response. The abnormal niacin response set contained 31.4% of the SZ patients and fewer than 5% of the HC (4.6%) and BP (3.39%) groups.
Fig. 4.
A bivariate plot of log10EC50 and maximal blood flow in healthy control (○) subjects, bipolar disorder (∆) patients, and patients with chronic schizophrenia (+) from Pittsburgh (A) and Portland (B) data, with a vertical line drawn at the 90 percentile of control log10EC50, and a horizontal line at the 60 percentile of control maximal blood flow.
A similar plot of log10EC50 and MBF from the Portland data is shown in figure 4B. A significantly higher rate of niacin subsensitivity is also demonstrated in SZ patients than in the HC or BP-MDD groups. Using the data obtained from both Pittsburgh and Portland sites, there is a general consensus that sensitivity of this physiologic marker is around 30%–35%, whereas the specificity exceeds 90% between SZ and HC groups, and between SZ and BP groups (table 1). A slightly lower than 90% specificity between the SZ and BP-MDD groups from the Portland site may be due to the smaller sample size (n = 23) or the heterogeneity of the mood disorder group.
Table 1.
Comparison of Sensitivity and Specificity of Blunted Niacin Response Between Schizophrenia and Control Groups
| Study Sites | SZ vs HC | SZ vs BP (or BP-MDD)a | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Pittsburgh | 0.31 | 0.95 | 0.31 | 0.97 |
| Portland | 0.32 | 0.95 | 0.32 | 0.87 |
Note: BP, clinically stable patients with bipolar disorders; HC, healthy controls; MDD, patients with major depressive disorder; SZ, clinically stable patients with chronic schizophrenia.
aAt Portland site, BP group also contained patients with MDD.
Covariate Effects
The covariates Smoke, Gender, Race, and Age had no impact on our effort to characterize the 2 SZ subgroups (SZnnr, SZ patients with normal niacin response; SZnra, SZ patients with NRA). None of the tests of the null hypothesis of equality of these distributions was rejected, under the liberal alpha (uncorrected) of .05.
In addition, all HC, BP, and SZnnr groups had imbalanced covariates (table 2), but with >90% of normal niacin sensitivity. The ratio of second-generation to first-generation antipsychotic treatment in SZ group was similar to those BP patients (table 2). Similarly, such high ratio of second-generation antipsychotic treatment was also present in both SZnnr and SZnra groups.
Table 2.
Covariate Subgroup Sizes
| Groups | N | Smoke (%) | Gender (%) | Race (%) | Age (y) | Antipsychotics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Male | Female | C | AA | Asian | 1st Quart. | Median | 3rd Quart. | First Gen | Second Gen | ||
| HC | 87 | 17 | 83 | 46 | 54 | 65 | 34 | 1 | 25 | 35 | 49 | — | — |
| BP | 59 | 59 | 41 | 53 | 47 | 50 | 49 | 1 | 41 | 46 | 52 | 5 | 95 |
| SZ | 70 | 70 | 30 | 71 | 29 | 39 | 60 | 1 | 43 | 50 | 56 | 12 | 89 |
| SZnnr | 46 | 63 | 37 | 83 | 17 | 44 | 54 | 2 | 40 | 48 | 55 | 11 | 91 |
| SZnra | 24 | 79 | 21 | 67 | 33 | 29 | 71 | 0 | 48 | 52 | 58 | 17 | 83 |
Note: AA, African American; BP, clinically stable patients with bipolar disorders; C, Caucasian; Gen, generation; HC, healthy controls; SZnnr, SZ with normal niacin response; SZnra, SZ with niacin response abnormality; SZ, clinically stable patients with chronic schizophrenia.
Correlations between Niacin Response and GAF Scores
To assess whether the abnormal niacin flush response is linked to neuropsychiatric manifestations of the illness, a GAF score was assigned to each of enrolled subjects. The values of GAF were not approximately normal within any group, with rejection of normality (P < .05) for all 3 groups’ correlation tests of normality. As expected, most severe was the crowding of HC values at the upper limit (P = .0008), while patient groups had smaller departures.
The correlations of GAF with Log10EC50 or MBF, within each group, were thus tested with Kendall’s tau. Alpha was set at .05/6 = .0083 for the 6 tests. The BP group had the only significant (positive) correlation between MBF and GAF (P = .0058), and it is notable that a wide range of GAF values were present in this group.
Group Comparisons
The Hotelling T 2 test of the (Log10EC50, MBF) vector found a significant difference for the HC vs SZ comparison (P < .0001). Sidak 97.5% CI for the mean HC-SZ difference of both Log10EC50 (−0.234) and MBF (42.3) were found to exclude 0. Similarly, the Hotelling T 2 test for the BP vs SZ comparison was significant (P = .0004), with Sidak 97.5% CI for the mean BP-SZ difference of Log10EC50 (−0.228) and of MBF (26.6) also both excluding 0. Thus, Log10EC50 was significantly higher, and MBF significantly lower, in the SZ than in either the HC or BP groups,
Discussion
SZ may represent a group of disorders with similar clinical picture but with distinct physiological abnormalities.19,20 These abnormalities include aberrant phospholipid signaling, for which niacin response may serve as a biomarker. Using a relatively large number of subjects and an objective measure of blood flow response, we found that both pharmacological sensitivity to niacin as well as its maximal efficacy can be reduced in SZ, and that 30% of SZ patients possess this abnormality. The abnormality appears highly specific for SZ. Even though patients with BP are at high risk of experiencing psychotic symptoms, the prevalence of NRA in our bipolar sample was identical to that in the HC group. Niacin response testing may prove useful in discerning BP from SZ, should our finding be replicated in further studies.
There was very good agreement between independently recruited patient groups, with respect to the prevalence of the abnormality in SZ and its specificity vs control and mentally ill comparison groups. Our estimates of the EC50 values for topical MN are also comparable to those reported by Ross et al,21 who also used laser Doppler flowmetry to measure the blood flow response to topical MN. They also found that the NRA had high specificity for SZ compared to HC and BP groups.21
The NRA in SZ does not appear to be an artifact of antipsychotic or other medications.22–24 There is no correlation between antipsychotic drug dose and niacin sensitivity in patients with SZ.16,25 Neither has a significant difference in niacin sensitivity been found between medicated vs unmedicated patients.26 Further, BP patients who take antipsychotic drug have a normal or even enhanced flush response.25 Taken together, our present data showing over 80% of SZ and BP patients treated with second-generation antipsychotic drugs (table 2) further support that niacin response is unlikely affected by the antipsychotic treatment. In addition, the niacin skin flush is not affected by local anesthetics27 or corticosteroid, anticholinergic, or antihistaminergic drugs.27,28
Prior studies have found that nicotine use has no effect on the niacin-induced flushing response.1,3,16,21,22,26 In our recent study,13 analysis of covariance also revealed no effect of smoking status on niacin efficacy. In the present study, all HC, BP, and SZnnr groups with imbalanced smoking status had normal niacin response (table 2), which suggests that smoking had no impact on our effort to characterize the 2 SZ subgroups. In addition, the niacin-induced flush response is not affected by coffee drinking23 or alcohol consumption.21,26
Early studies of the NRA suggested an association with more serious behavioral disturbances in SZ.29,30 Later, Smesny et al3 reported an association between impaired niacin response and higher measures of symptom severity among first-epidose SZ patients. Puri et al31 observed a strong association between the NRA and cerebral phospholipid metabolism among severely ill SZ patients. Messamore32 found that reduced niacin sensitivity in SZ is significantly correlated with reduced global functioning.
However, others have failed to demonstrate any significant correlations between niacin flush response and the SNAS, SAPS, BPRS, or similar psychiatric rating scales.15,33,34
In the present study, no significant correlations were found between niacin sensitivity and GAF scores in the SZ group. Our inability to replicate Messamore’s finding32 may be related to differences in the range of GAF scores encountered. Patients in our study were clinically stable outpatients with GAF scores above 40, whereas GAF scores in Messamore’s study had wider dispersion, with many scores below 40
In summary, our data are consistent with the view that the NRA is a physiological subtype that appears specific for SZ, when compared to the general population or to those with BP. We have also demonstrated that it is possible to obtain good concordance between different measurement sites using quantitative dose-response studies to identify this SZ subtype. Recent work suggests that the NRA may associate with genetic markers34,35 or other phospholipid-related abnormalities in SZ.31,36 Better understanding of this biomarker could eventually lead to the identification of a risk-conferring gene, or be used to predict preferential response to treatment, or could contribute to a rational deconstruction of the complex diagnosis of SZ into physiologically informed components.
Funding
This work was supported in part by Department of Veterans Affairs (Merit Reviews 1I01CX000110 and Senior Research Career Scientist Award toJ K Y ); VA VISN4 Mental Illness Research, Education and Clinical Center (MIRECC Director: D. Oslin; Associate Director: G. Haas); and the VA Pittsburgh Healthcare System.
Acknowledgments
The authors are grateful to Jesse Colon and Carol Korbanic for their technical assistance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Liu CM, Chang SS, Liao SC, et al. Absent response to niacin skin patch is specific to schizophrenia and independent of smoking. Psychiatry Res. 2007;152:181–187. [DOI] [PubMed] [Google Scholar]
- 2. Messamore E, Hoffman WF, Janowsky A. The niacin skin flush abnormality in schizophrenia: a quantitative dose-response study. Schizophr Res. 2003;62:251–258 [DOI] [PubMed] [Google Scholar]
- 3. Smesny S, Berger G, Rosburg T, et al. Potential use of the topical niacin skin test in early psychosis – a combined approach using optical reflection spectroscopy and a descriptive rating scale. J Psychiatr Res. 2003;37:237–247. [DOI] [PubMed] [Google Scholar]
- 4. Pike NB. Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J Clin Invest. 2005;115:3400–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zellner C, Pullinger CR, Aouizerat BE, et al. Variations in human HM74 (GPR109B) and HM74A (GPR109A) niacin receptors. Hum Mutat. 2005;25:18–21. [DOI] [PubMed] [Google Scholar]
- 6. Urade Y, Ujihara M, Horiguchi Y, et al. The major source of endogenous prostaglandin D2 production is likely antigen-presenting cells. Localization of glutathione-requiring prostaglandin D synthetase in histiocytes, dendritic, and Kupffer cells in various rat tissues. J Immunol. 1989;143:2982–2989. [PubMed] [Google Scholar]
- 7. Benyó Z, Gille A, Bennett CL, et al. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol. 2006;70:1844–1849. [DOI] [PubMed] [Google Scholar]
- 8. Tang Y, Zhou L, Gunnet JW, et al. Enhancement of arachidonic acid signaling pathway by nicotinic acid receptor HM74A. Biochem Biophys Res Commun. 2006;345:29–37 [DOI] [PubMed] [Google Scholar]
- 9. Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35. [DOI] [PubMed] [Google Scholar]
- 10. Morrow JD, Parsons WG, 3rd, Roberts LJ., 2nd Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 1989;38:263–274. [DOI] [PubMed] [Google Scholar]
- 11. Skosnik PD, Yao JK. From membrane phospholipid defects to altered neurotransmission: is arachidonic acid a nexus in the pathophysiology of schizophrenia? Prostaglandins Leukot Essent Fatty Acids. 2003;69:367–384. [DOI] [PubMed] [Google Scholar]
- 12. Maciejewski-Lenoir D, Richman JG, Hakak Y, et al. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol. 2006;126:2637–2646. [DOI] [PubMed] [Google Scholar]
- 13. Messamore E, Hoffman WF, Yao JK. Niacin sensitivity and the arachidonic acid pathway in schizophrenia. Schizophr Res. 2010;122:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smesny S, Kinder D, Willhardt I, et al. Increased calcium-independent phospholipase A2 activity in first but not in multiepisode chronic schizophrenia. Biol Psychiatry. 2005;57:399–405. [DOI] [PubMed] [Google Scholar]
- 15. Tavares H, Yacubian J, Talib LL, et al. Increased phospholipase A2 activity in schizophrenia with absent response to niacin. Schizophr Res. 2003;61:1–6. [DOI] [PubMed] [Google Scholar]
- 16. Messamore E. Relationship between the niacin skin flush response and essential fatty acids in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:413–419. [DOI] [PubMed] [Google Scholar]
- 17. Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 4th ed. Upper Saddle River, New Jersey: Prentice Hall; 1998:22–23. [Google Scholar]
- 18. Henze N, Zirckler B. A class of invariant consistent tests for multivariate normality. Commun Statist – Theory Meth. 1990;19:3595–3617. [Google Scholar]
- 19. Garver DL, Holcomb JA, Christensen JD. Heterogeneity of response to antipsychotics from multiple disorders in the schizophrenia spectrum. J Clin Psychiatry. 2000;61:964–72. [DOI] [PubMed] [Google Scholar]
- 20. Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. [DOI] [PubMed] [Google Scholar]
- 21. Ross BM, Hughes B, Turenne S, et al. Reduced vasodilatory response to methyl-nicotinate in schizophrenia as assessed by laser doppler flowmetry. Eur Neuropsychopharma. 2004;14:191–197. [DOI] [PubMed] [Google Scholar]
- 22. Chang SS, Liu CM, Lin SH, et al. Impaired flush response to niacin skin patch among schizophrenia patients and their nonpsychotic relatives: the effect of genetic loading. Schizophr Bull. 2009;35:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin SH, Liu CM, Chang SS, et al. Familial aggregation in skin flush response to niacin patch among schizophrenic patients and their nonpsychotic relatives. Schizophr Bull. 2007;33:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maclean R, Ward PE, Glen I, et al. On the relationship between methylnicotinate-induced skin flush and fatty acids levels in acute psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:927–933. [DOI] [PubMed] [Google Scholar]
- 25. Hudson CJ, Lin A, Cogan S, et al. The niacin challenge test: clinical manifestation of altered transmembrane signal transduction in schizophrenia. Biol Psychiatry. 1997;41:507–513. [DOI] [PubMed] [Google Scholar]
- 26. Shah SH, Vankar GK, Peet M, et al. Unmedicated schizophrenic patients have a reduced skin flush in response to topical niacin. Schizophr Res. 2000;43:163–164. [PubMed] [Google Scholar]
- 27. Winkelmann RK, Wilhelmj CM, Horner FA. Experimental studies on dermographism. Arch Dermatol. 1965;92:436–442. [PubMed] [Google Scholar]
- 28. Wilkin JK, Fortner G, Reinhardt LA, et al. Prostaglandins and nicotinate-provoked increase in cutaneous blood flow. Clin Pharmacol Ther. 1985;38:273–277. [DOI] [PubMed] [Google Scholar]
- 29. Glen AI, Cooper SJ, Rybakowski J, et al. Membrane fatty acids, niacin flushing and clinical parameters. Prostaglandins Leukot Essent Fatty Acids. 1996;55:9–15. [DOI] [PubMed] [Google Scholar]
- 30. Lin A, Hudson CJ. The niacin challenge test in schizophrenia: past, present and future. Prostaglandins Leukot Essent Fatty Acids. 1996;55:17–19. [DOI] [PubMed] [Google Scholar]
- 31. Puri BK, Richardson AJ, Counsell SJ, et al. Negative correlation between cerebral inorganic phosphate and the volumetric niacin response in male patients with schizophrenia who have seriously and dangerously violently offended: a (31)P magnetic resonance spectroscopy study. Prostaglandins Leukot Essent Fatty Acids. 2007;77:97–99. [DOI] [PubMed] [Google Scholar]
- 32. Messamore E. Niacin subsensitivity is associated with functional impairment in schizophrenia. Schizophr Res. 2012;137:180–184. [DOI] [PubMed] [Google Scholar]
- 33. Smesny S, Klemm S, Stockebrand M, et al. Endophenotype properties of niacin sensitivity as marker of impaired prostaglandin signalling in schizophrenia. Prostaglandins Leukot Essent Fatty Acids. 2007;77:79–85. [DOI] [PubMed] [Google Scholar]
- 34. Covault J, Pettinati H, Moak D, et al. Association of a long chain fatty acid-CoA ligase 4 gene polymorphismwith depression and with enhanced niacin-induced dermal erythema. Am. J. Med. Genet. Part B. 2004;127:42–47. [DOI] [PubMed] [Google Scholar]
- 35. Lien YJ, Huang SS, Liu CM, et al. A genome-wide quantitative linkage scan of niacin skin flush response in families with schizophrenia. Schizophr Bull. 2013;39:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hudson C, Gotowiec A, Seeman M, et al. Clinical subtyping reveals significant differences in calcium-dependent phospholipase A2 activity in schizophrenia. Biol Psychiatry. 1999;46:401–405. [DOI] [PubMed] [Google Scholar]