Abstract
Aims: The immunomodulatory and anti-inflammatory properties of mesenchymal stem cells (MSCs) have been proposed in several autoimmune diseases and successfully tested in animal models, but their contribution to psoriasis and underlying pathways remains elusive. Likewise, an increased or prolonged presence of reactive oxygen species and aberrant antioxidant systems in skin are known to contribute to the development of psoriasis and therefore effective antioxidant therapy is highly required. We explored the feasibility of using extracellular superoxide dismutase (SOD3)-transduced allogeneic MSCs as a novel therapeutic approach in a mouse model of imiquimod (IMQ)-induced psoriasis-like inflammation and investigated the poorly understood underlying mechanism. In addition, the chronicity and late-phase response of inflammation were evaluated during continued activation of antigen receptors by applying a booster dose of IMQ. Results: Subcutaneous injection of allogeneic SOD3-transduced MSCs significantly prevented psoriasis development in our IMQ-induced mouse model, likely through a suppression of proliferation and infiltration of various effector cells into skin with a concomitant modulated cytokine and chemokine expression and inhibition of signaling pathways such as toll-like receptor-7, nuclear factor-kappa B, p38 mitogen-activated kinase, and Janus kinase–signal transducer and activator of transcription, as well as adenosine receptor activation. Innovation and Conclusion: Our data offer a novel therapeutic approach to chronic inflammatory skin diseases such as psoriasis by leveraging immunomodulatory effects of MSCs as well as SOD3 expression. Antioxid. Redox Signal. 24, 233–248.
Introduction
Psoriasis is an immune-mediated chronic inflammatory disease involving skin and joints, or both, and hallmarks are abnormal epidermal differentiation, hyperproliferation, angiogenesis, and increased T-cell infiltrates. Psoriasis is associated with a proliferation of activated T helper cells 1 (Th1), Th17, and Th22 T cells with increased production of cytokines such as interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-17, and IL-22 in skin (35). Psoriatic cutaneous lesions are promoted and sustained by T-cell and neutrophil infiltration into both dermis and epidermis, which leads to inflammation, causing a reduction of global antioxidant capacity of the skin. It has been suggested that increased reactive oxygen species (ROS) production and an aberrant antioxidant system may be the main factors in pathogenesis of psoriasis (6, 17, 38, 57). ROS actively promote the secretion of inflammatory Th1 cytokines and infiltration and proliferation of neutrophils, which are directly involved in pathogenesis. Similarly, the cellular signaling pathways such as mitogen-activated protein (MAP) kinase/activator protein-1, nuclear factor-kappa B (NF-κB), and Janus kinase–signal transducer and activator of transcription (JAK-STAT) are also known to be redox sensitive (9) and have been proven to be involved in the progression of psoriasis (46, 55, 57). It is also known that JAK-STAT signaling pathways play a pivotal role in immune regulation as well as in inflammatory responses. Several cytokines and growth factors upregulated in psoriasis, such as IL-6, the IL-20 family of cytokines (IL-19, IL-20, IL-22, IL-24), and IL-23 are able to induce STAT activation (16, 44, 55). Therefore, the STAT pathway is one of the major targets for the treatment of Th17-derived diseases such as psoriasis.
Innovation.
The present study provides the first piece of evidence for application of extracellular superoxide dismutase (SOD3) in mesenchymal stem cells (MSCs) to treat psoriasis and reveals basic pathways of immune regulation. The use of SOD3-transduced MSCs is more effective than MSCs alone. Enhancing the immunomodulatory and antioxidant activities of MSCs such as transduction of SOD3 may be a powerful approach for treatment of psoriasis.
Mesenchymal stem cells (MSCs), which are multipotent progenitor cells, have potent immunosuppressive and anti-inflammatory effects through either cell–cell contacts or by secreting soluble factors (50) and thus can have clinical applications (11, 28). The immunomodulatory effect of MSCs has been exploited to treat diseases such as acute graft-versus-host disease, experimental encephalomyelitis, and diabetes (45). However, very little is known about the role of MSCs in chronic inflammatory skin diseases such as psoriasis. MSCs isolated from psoriatic patients have shown that the defective resident MSCs produced angiogenic and proinflammatory mediators, which led to reduction in antioxidant capacity of the cells, contributing to the development of skin lesions in psoriasis (8). More interestingly, skin obtained from the psoriatic lesion has shown to express reduced levels of extracellular superoxide dismutase (SOD3) (21).
Recently, our groups have shown that SOD3 can inhibit Ultraviolet B-induced skin inflammation, hyaluronic acid fragment-mediated skin inflammation, contact hypersensitivity, and allergic airway inflammation in mice models (21–23, 33). Ross et al. also demonstrated that SOD3 plays a key role in regulating inflammation in collagen-induced arthritis (43). Similarly, previous studies have proven that inhibition of superoxide ions by the NADPH oxidase inhibitor, gentian violet, could be beneficial in treatment of chronic skin diseases (3, 29). Therefore, these observations led us to investigate the role of SOD3, a powerful antioxidant enzyme, in MSC-mediated immune modulation of psoriasis, which shows similar pathogenesis to that of other autoimmune diseases. The basic pathway of immune regulation by MSCs in psoriasis is not known and the therapeutic implications of SOD3 in psoriasis are poorly understood.
In this study, we aimed to investigate the efficacy and pathways of immunomodulation of SOD3 overexpressed in MSCs using the mouse model of imiquimod (IMQ)-induced psoriasis-like inflammation. Generally, the IMQ-induced skin inflammation model is based on daily application of IMQ for 6 days (52). However, prolonged application of IMQ and its effects on immune homeostasis are not reported. We believe that prolonged IMQ application on skin may elicit late-phase inflammatory cascades and thus helps to understand the chronicity of disease during continued activation of antigen receptors. Therefore, mice also received daily IMQ application during the entire 12-day experimental period.
We used adenovirus-mediated SOD3 gene transfer to human cord blood-derived MSCs to achieve more potent therapeutic efficacy to treat psoriasis. Subcutaneous administration of SOD3-transduced MSCs reduced the psoriatic symptoms more potently than MSCs alone via exerting stronger immunomodulatory activity in both early and chronic late phases. We suggest that SOD3-transduced MSCs might serve as a novel therapeutic in the fight against psoriasis and other autoimmune diseases.
Results
SOD3-transduced MSCs significantly prevent the development and severity of psoriasis induced by IMQ in mice
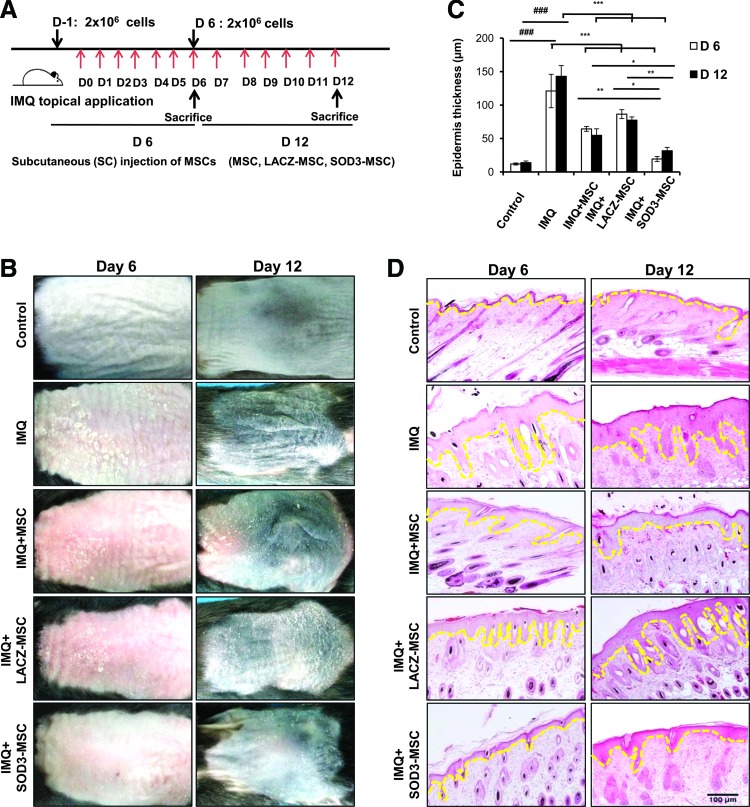
To investigate the role of MSCs and MSCs overexpressing SOD3, in psoriasis pathogenesis, mice were subcutaneously injected with MSCs or SOD3-transduced MSCs before 24 h and on the 6th day of IMQ application. IMQ was applied daily for 6 or 12 consecutive days. Mice were sacrificed on days 6 and 12 (Fig. 1A). Phenotypically, IMQ application on the back skin of the mice started to display signs of erythema, scaling, and thickening, followed by inflammation, which continuously increased in severity up to the end of the experiment. In both SOD3-transduced MSCs and MSC-treated groups, the psoriatic erythema, scaling, and thickening were highly reduced compared with the IMQ group. SOD3-transduced MSC-treated mice showed stronger inhibition of psoriasis phenotype compared with MSC-treated mice on both 6- and 12-day models (Fig. 1B). Hematoxylin and eosin (H&E) staining showed increased epidermal thickness and infiltration of mononuclear cells into the dermis with IMQ application. However, MSCs alone and SOD3-transduced MSCs showed decreases in epidermal thickness and mononuclear cell infiltration. The epidermal thickness was significantly reduced in the SOD3-transduced MSC group compared with the other groups (Fig. 1C, D).
FIG. 1.
Treatment with SOD3-transduced MSCs inhibits epidermal hyperproliferation, decreases acanthosis, and reduces the disease severity in psoriasis mouse model. (A) An experimental setup and (B) phenotypical presentation of mouse back skin after 6- and 12-day regimens are shown. Measurement of epidermal thickness (C) and hematoxylin and eosin staining of the back skin of mice (D). Data are represented as mean ± SD. ###p < 0.001 (control group vs. IMQ-treated group in all cases); *p < 0.05, **p < 0.01, ***p < 0.001 (IMQ-treated group vs. MSC-treated group in all cases). Scale bar, 100 μm. IMQ, imiquimod; MSCs, mesenchymal stem cells; SD, standard deviation; SOD3, extracellular superoxide dismutase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
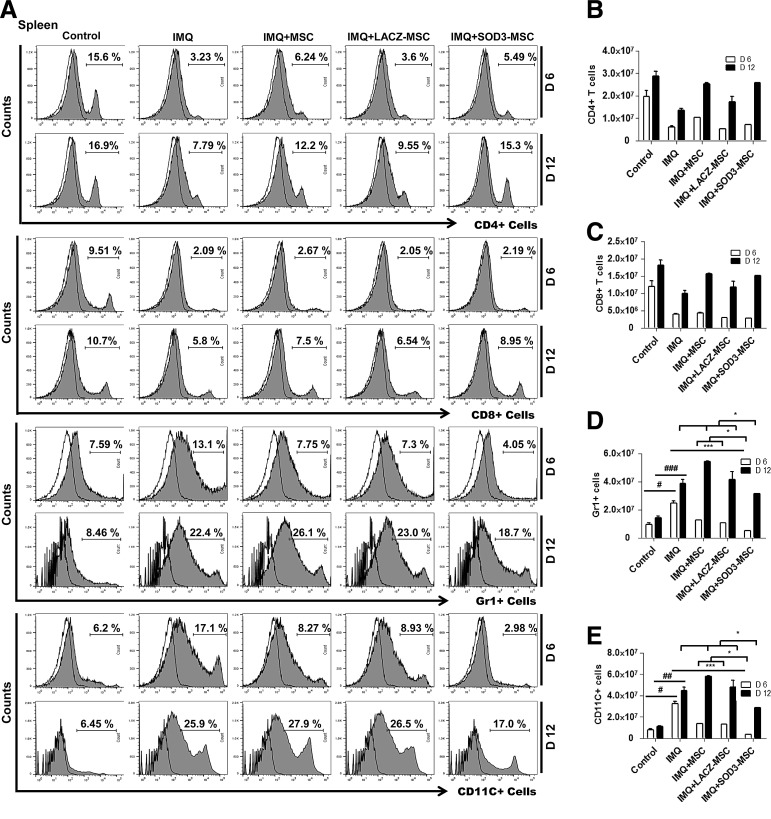
SOD3-transduced MSCs reduce the ROS level in skin and inhibit the infiltration of T cells, neutrophils, and dendritic cells more potently than MSCs alone in skin, spleen, and lymph nodes
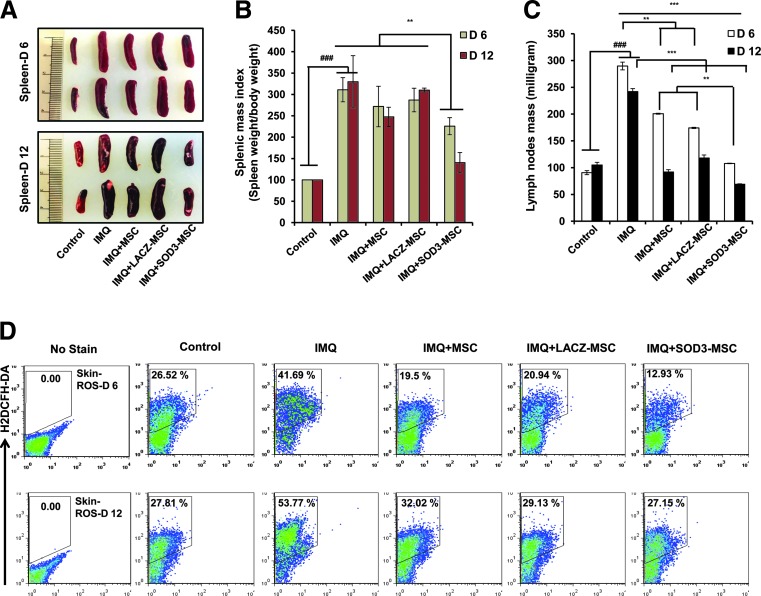
At the end of experiments, after 6 or 12 days of IMQ treatment, significantly enlarged spleens were observed in the mice. The splenic and lymph nodes (axillary, lateral axillary, and inguinal) masses were decreased in the SOD3-transduced MSCs compared with MSCs or IMQ alone-treated groups (Fig. 2A–C). To further investigate the effect of SOD3-transduced MSCs in IMQ-induced skin inflammation, we first evaluated the total ROS levels in single-cell suspension of skin by flow cytometry. As shown in Figure 2D, the levels of ROS were highly increased in the skin of IMQ-treated mice compared with control. Administration of SOD3-MSCs or MSCs markedly reduced the ROS level in skin. The SOD3-transduced MSC group had significantly less ROS compared with IMQ or MSC-treated groups.
FIG. 2.
SOD3-transduced MSCs effectively decrease spleen and lymph node masses and reduce the ROS level in skin. (A) Photomicrograph of spleens of control, IMQ alone, MSCs, and SOD3-transduced MSC-treated mice. (B, C) Spleen and lymph node masses were determined. (D) ROS production was measured in single-cell suspensions by staining with H2DCFH-DA (5 μM). Data are represented as mean ± SD. ###p < 0.001 (control group vs. IMQ-treated group for all cases); **p < 0.01, ***p < 0.001 (IMQ-treated group vs. MSC-treated group for all cases). ROS, reactive oxygen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
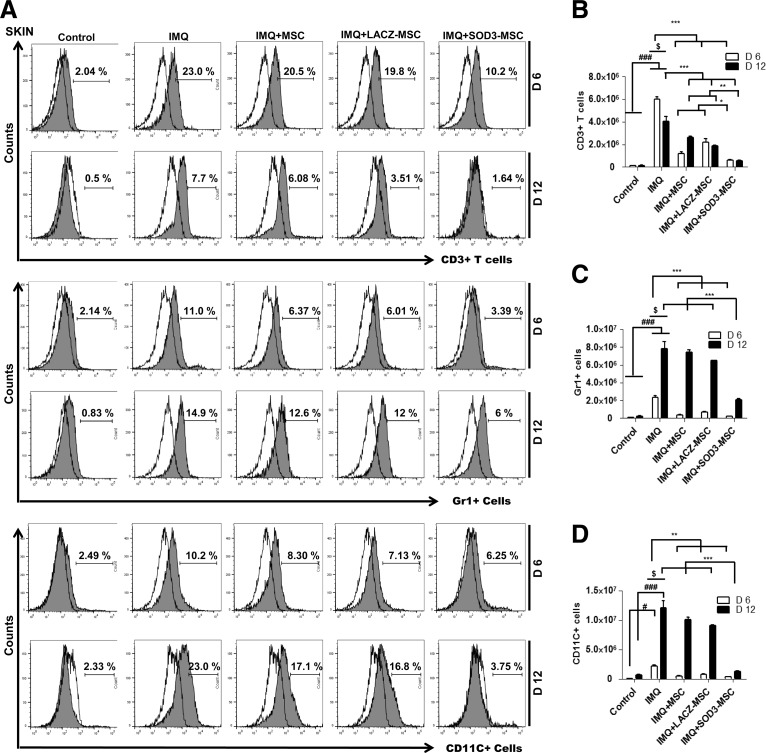
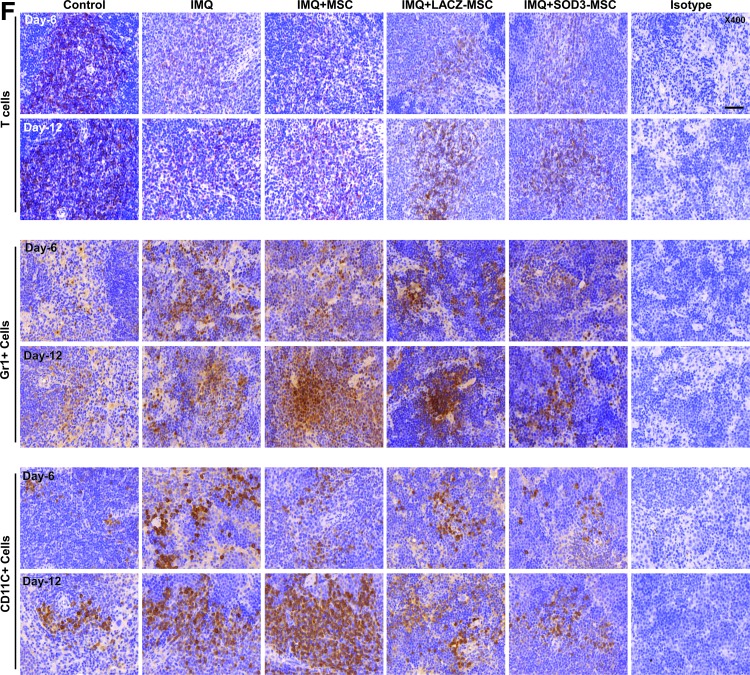
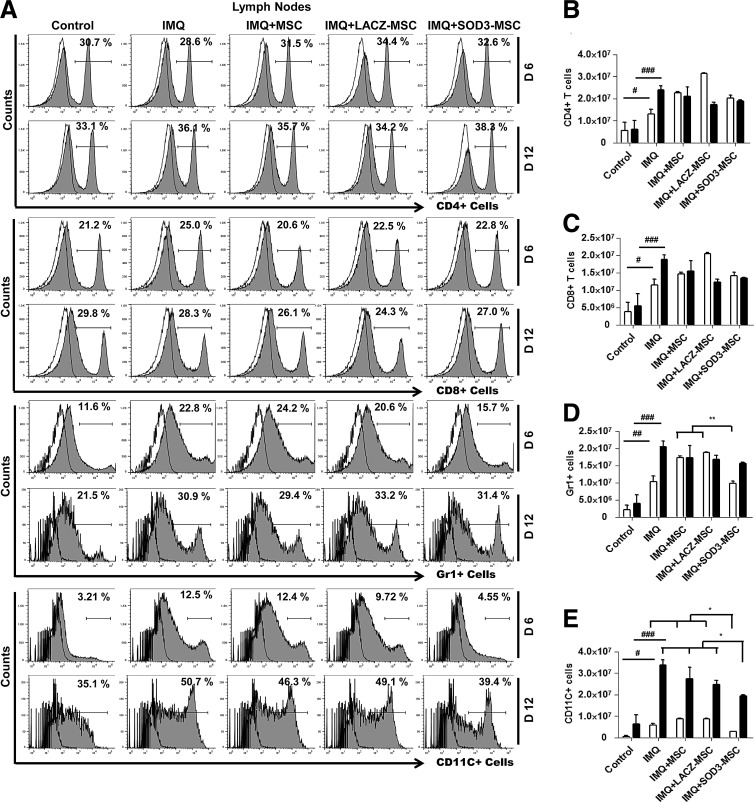
We next evaluated the cellular composition of the skin, spleen, and lymph nodes (T cells, neutrophils, and dendritic cells) by flow cytometry and immunohistochemistry (IHC) staining, and also determined the absolute number of infiltrating immune cells. The infiltration of T cells was higher in the back skin of IMQ-treated mice and highly reduced in MSCs or SOD3-transduced MSC-treated mice. The infiltration of T cells in skin of the 6-day model was higher than the 12-day model, which was significantly decreased in the SOD3-transduced MSC group (Fig. 3A upper two panels, B; Fig. 4A, D left 1st–2nd panels; the higher magnification of effector cells in skin with isotype controls is illustrated in Supplementary Fig S6; Supplementary Data are available online at www.liebertpub.com/ars). In spleen, the recruitment of T cells (CD4+ and CD8+ T cells) was not significantly different between MSC-treated, SOD3-transduced MSCs, or IMQ alone-treated mice. The infiltration of these cells was lower compared with control mice (Fig. 5A 1st–4th panels, B, C, and F upper two panels). However, the total T cells remained increased in lymph nodes of IMQ, MSCs, or SOD3-transduced MSC-treated mice in both 6- and 12-day models (Fig. 6A upper 1st–4th panels, B, and C).
FIG. 3.
Accumulation of T cells, neutrophils, and dendritic cells in the back skin is potentially reduced in the SOD3-transduced MSC group. (A) Representative histogram of expression of T cells, neutrophils, and dendritic cells. Open and filled histogram represents isotype controls and cells stained with specific indicated antibodies, respectively. Total cell counts and absolute number of T cells (B), neutrophils (Gr1+ cells) (C), and dendritic cells (CD11C+ cells) (D) in the skin. Data are represented as mean ± SD. #p < 0.05, ###p < 0.001 (control group vs. IMQ-treated group for all cases); *p < 0.05, **p < 0.01, ***p < 0.001 (IMQ-treated group vs. MSC-treated group for all cases); $p < 0.05 (day 6 vs. day 12).
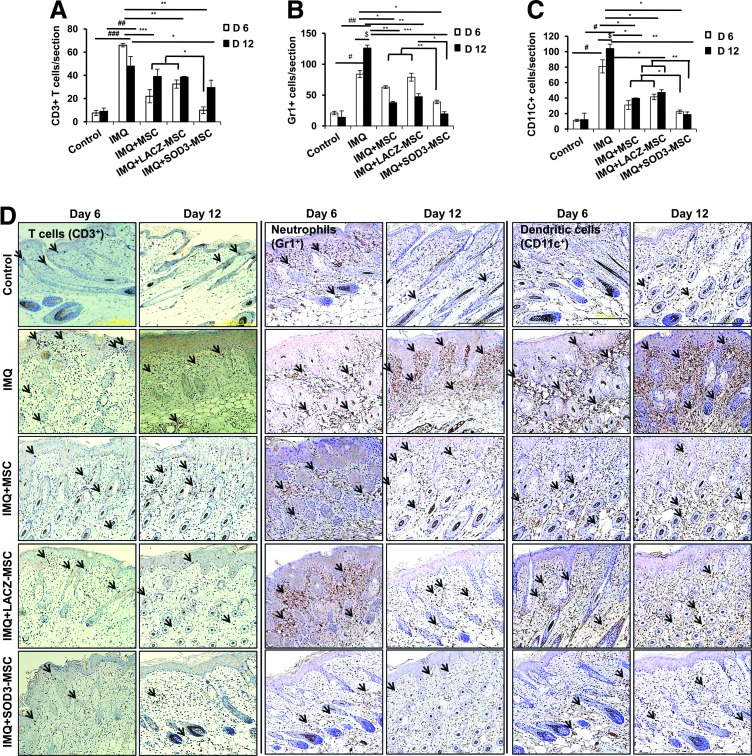
FIG. 4.
The effector cells that mainly infiltrate the dermis region of the skin are decreased in the SOD3-transduced MSC group. (A–D) IHC analysis of infiltrating immune cells in skin. (A) T-cell count, (B) Gr1+ cell count, and (C) CD11C+ cell count. (D) Representative images of the skin. The arrows indicate the infiltration of effector cells. Quantification of staining was performed by two researchers for two sections of two mice in each group. Data are represented as mean ± SD. #p < 0.05, ##p < 0.01, ###p < 0.001 (control group vs. IMQ-treated group for all cases); *p < 0.05, **p < 0.01, ***p < 0.001 (IMQ-treated group vs. MSC-treated group for all cases); $p < 0.05 (day 6 vs. day 12). Scale bar, 200 μm. IHC, immunohistochemistry. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 5.
SOD3-transduced MSCs effectively reduce neutrophil and dendritic cell recruitment in spleen compared with MSCs. (A) Representative histograms showing infiltrate populations in spleens. (B–E) Absolute cell numbers and (F) IHC staining for T cells, neutrophils (Gr1+), and dendritic cells (CD11C+) in spleen. Data are represented as mean ± SD. #p < 0.05, ##p < 0.01, ###p < 0.001 (control group vs. IMQ-treated group for all cases); *p < 0.05, ***p < 0.001 (IMQ-treated group vs. MSC-treated group for all cases); scale bar, 50 μm. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 6.
Neutrophils and dendritic cells infiltrating the lymph nodes after IMQ application are affected by MSCs that overexpress SOD3. (A) Representative histograms for CD4+ and CD8+ T cells, neutrophils, and dendritic cells in lymph nodes as evaluated by flow cytometry. (B–E) Absolute cell numbers of indicated infiltrating cells. Data are represented as mean ± SD. #p < 0.05, ##p < 0.01, ###p < 0.001 (control group vs. IMQ-treated group for all cases); *p < 0.05, **p < 0.01 (IMQ-treated group vs. MSC-treated group for all cases).
In contrast to T cells, infiltration of neutrophils (Gr1+ cells) and dendritic cells (CD11C+ cells) was significantly higher in skin, spleen, and lymph nodes of IMQ alone and MSC-treated mice compared with SOD3-transduced MSC-treated mice. In skin, the total number of neutrophils and dendritic cells was significantly more in 12 days of IMQ treatment. The number of these effector cells was potentially reduced both in SOD3-transduced MSCs and MSC-treated mice following a 6-day regimen. The SOD3-transduced MSC group showed relatively lower neutrophil infiltration compared with the MSC-treated group. At the 12-day regimen, the SOD3-transdued MSC group showed significant decrease in neutrophils and dendritic cell population compared with IMQ alone or MSC-treated groups (Fig. 3A 3rd–6th panels, C, D; Fig. 4B, C, and D 3rd–6th panels). Similarly, the SOD3-transduced MSC-treated group showed effective decrease in dendritic cell infiltration in the spleen of 6- and 12-day models. The number of neutrophils was potentially lower in SOD3-transduced MSC-treated mice compared with IMQ alone or MSC-treated mice following the 6- and 12-day regimens. Numbers of infiltrating neutrophils and dendritic cells remained unchanged and increased in the 12-day regimen between IMQ and MSC-treated groups (Fig. 5A 5th–8th panels, D, E, and F 3rd–6th panels). Interestingly, in lymph nodes, the total number of neutrophils and dendritic cells was significantly reduced only in the SOD3-transduced MSC group following the 6-day regimen, whereas the 12-day model had a relatively lower population of neutrophils and markedly reduced infiltrating dendritic cells in the SOD3-transduced MSC group compared with IMQ or MSC-treated groups (Fig. 6A 5th–8th panels, D, and E). These data show that SOD3-transduced MSCs have strong immunoregulatory functions compared with MSC-treated mice.
SOD3-transduced MSCs suppress the proliferation and differentiation of CD4+ T cells
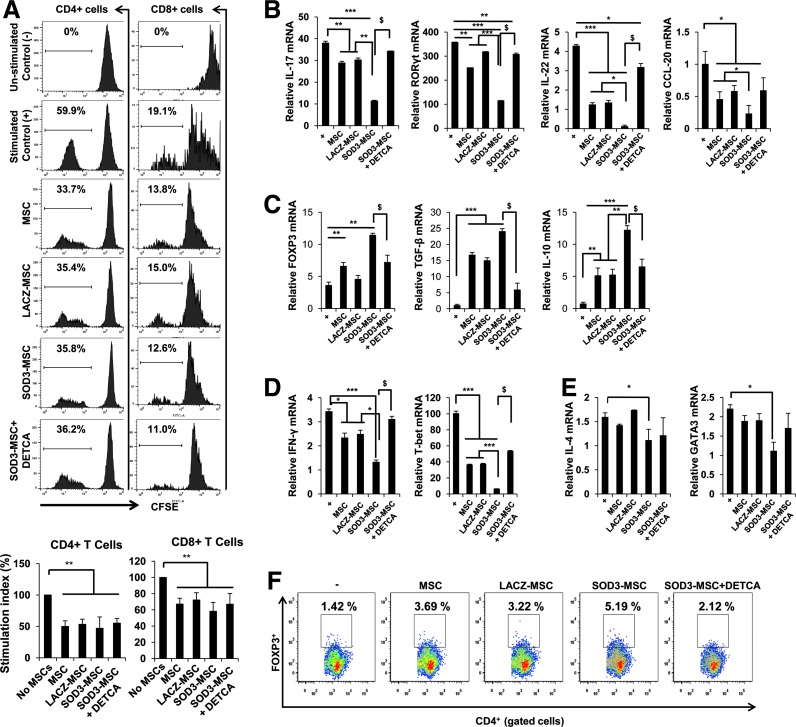
To determine whether SOD3-transduced MSCs can also suppress T-cell proliferative responses in vitro, we performed carboxyfluorescein diacetate succinimidyl ester–mixed lymphocyte reaction (CFSE-MLR) assay to examine CD4+ and CD8+ T-cell proliferation. The proliferation was sharply inhibited by both SOD3-transduced MSCs and MSCs, although SOD3-transduced MSCs did not show significant differences from the other MSC groups. The suppression of CD4+ and CD8+ T-cell proliferation by SOD3-transduced MSCs were not affected when treated with Cu/Zn SOD inhibitor, sodium diethyldithiocarbamate trihydrate (DETCA) (Fig. 7A).
FIG. 7.
SOD3-transduced MSCs affect the proliferation and differentiation of T cells. (A) Proliferation of CD4+ T cells and CD8+ T cells was measured by the CFSE-based MLR assay in spleens and lymph nodes isolated from wild-type C57BL/6 mice and analyzed by flow cytometry. The Cu/Zn SOD inhibitor, DETCA, was used at 10 μM. Data shown are representative of three independent experiments. For differentiation study, naïve CD4+ T cells isolated from mice were cocultured with MSCs or SOD3-transduced MSCs under the appropriate polarizing conditions for 4 days. Total RNA was isolated from the cells and quantitative real-time PCR was performed to determine the mRNA levels of T-cell lineage-specific master transcription factors and cytokines. (B) Th17 lineage markers; (C) Treg lineage markers; (D) Th1 lineage markers; (E) Th2 cell lineage markers; (F) Evaluation of CD4+Foxp3+ population in splenocytes when cocultured with MSCs, SOD3-transduced MSCs, and SOD3-transduced MSCs in the presence of Cu/Zn SOD inhibitor, DETCA (10 μM). Data are represented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, $p < 0.05. CFSE-MLR, carboxyfluorescein diacetate succinimidyl ester–mixed lymphocyte reaction; DETCA, sodium diethyldithiocarbamate trihydrate; PCR, polymerase chain reaction; Th, T helper. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Next, to evaluate whether SOD3-transduced MSCs could affect CD4+ T-cell differentiation, we examined the expression of key transcription factors in cultured mouse CD4+ T cells under the appropriate polarizing conditions. The mRNA levels of IL-17, IL-22, chemokine ligand (CCL)-20, and retinoic acid-related orphan receptor gamma (RORγt), which are signature markers for Th17 cells, were markedly suppressed by SOD3-transduced MSCs compared with MSCs alone. However, SOD3-transduced MSCs increased the mRNA levels of Foxp3, IL-10, and transforming growth factor-beta (TGF-β), which are signature markers for Treg-positive cells (Fig. 7B, C). Similarly, IFN-γ, T-bet, IL-4, and GATA3, which are signature molecules for Th1 and Th2 cell lineages, were also affected by overexpression of SOD3 in MSCs (Fig. 7D, E). SOD3-transduced MSCs also showed increased Treg cell population compared with MSC groups when cocultured with activated splenocytes for 3 days (Fig. 7F). SOD3-transduced MSC-mediated stronger inhibition of T-cell differentiation and increased Treg population were reversed when treated with the Cu/Zn SOD inhibitor, DETCA (10 μM), indicating that SOD3-transduced MSCs can greatly affect differentiation of CD4+ T cells.
SOD3-transduced MSCs strongly affect the expression of inflammatory mediators that play pivotal roles in psoriasis
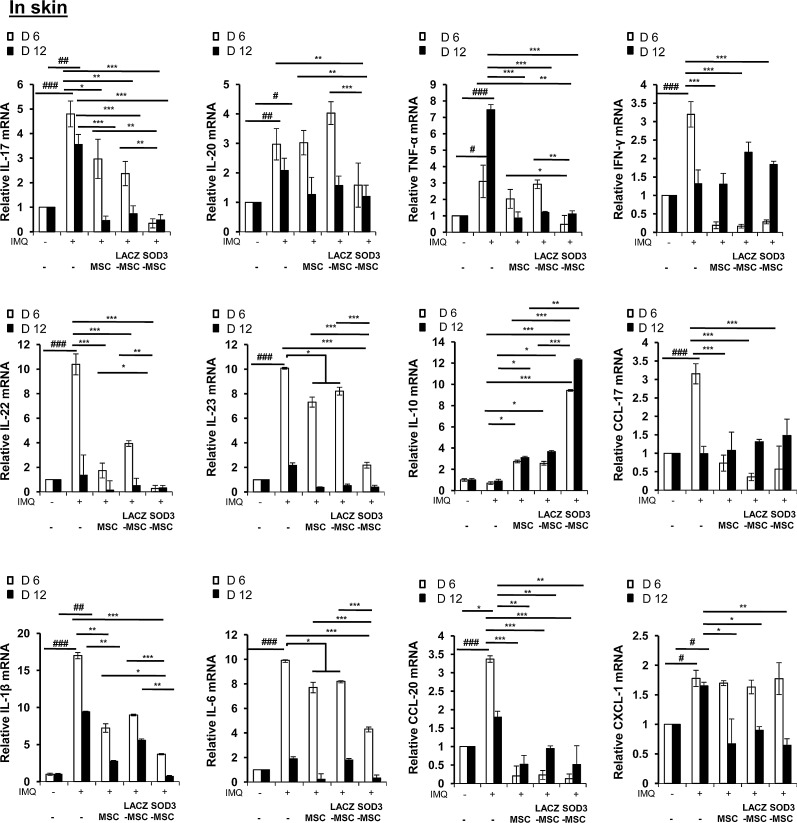
Next, we studied the effect of SOD3-transduced MSCs or MSCs on expression of proinflammatory mediators in the skin of a psoriatic mouse. Similar to the histological changes, quantitative real-time polymerase chain reaction (PCR) analysis also showed that the mRNA levels of T-cell-specific cytokines were increased in IMQ-treated mouse skin, whereas MSCs effectively decreased these mRNA levels. Consistent with the results of CD4+ T-cell differentiation, MSCs or SOD3-transduced MSCs inhibited the mRNA levels of IL-17A and IL-22, which are Th17 cell-specific cytokines. The suppression by SOD3-transduced MSCs was at a greater degree than by MSCs alone. Similarly, increased mRNA level of IL-20 was only inhibited by SOD3-transduced MSCs in the 6-day group, whereas marginal decrease in expression was observed in the 12-day group with no significant differences between the MSCs and SOD3-transduced MSC-treated mice.
Interestingly, upregulated mRNA levels of IL-6 and IL-23 after IMQ treatment were effectively reduced in the SOD3-transduced MSC group compared with the MSC group. In contrast, the anti-inflammatory cytokine, IL-10, was increased in the MSC-treated group with significantly higher expression in the SOD3-transduced MSC group in both 6-day and 12-day regimens. The mRNA level of chemokine, CXCL-1, remained elevated in all groups compared with control at 6-day regimen, but reduced in both MSCs and SOD3-transduced MSC groups following 12-day regimen. In both 6- and 12-day groups, MSCs and SOD3-transduced MSCs effectively and differentially inhibited the mRNA levels of other major inflammatory mediators in psoriasis, such as IL-1β, TNF-α, IFN-γ, CCL-17, and CCL-20 (Fig. 8). The induction of inflammatory mediators following 6- and 12-day regimens was differential and transient, despite continuous IMQ treatment.
FIG. 8.
Regulation of the expression of signature inflammatory mediators by SOD3-transduced MSCs. Total RNA was isolated from the mouse back skin from each group and quantitative real-time PCR was performed to evaluate proinflammatory mediators. Statistical significance is displayed for all the samples; error bar indicates SD; #p < 0.05, ##p < 0.01, ###p < 0.001 (control group vs. IMQ-treated group for all cases); *p < 0.05, **p < 0.01, ***p < 0.001 (IMQ-treated group vs. SOD3-transduced MSCs or MSC-treated group).
Toll-like receptor-7 activation, a downstream target NF-κB, MAP kinase signaling, and JAK-STAT signaling are affected by SOD3-transduced MSCs
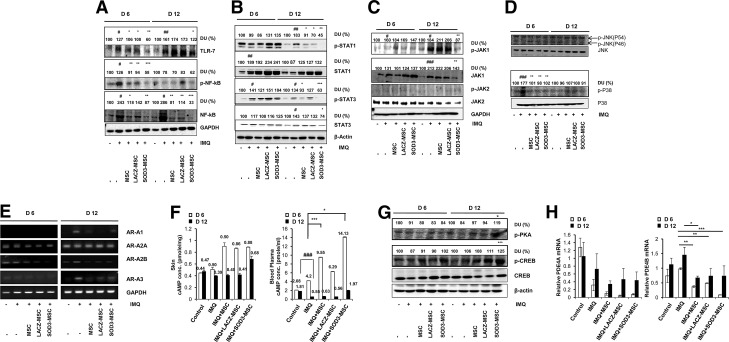
IMQ has been reported to exert its biological activity through toll-like receptor-7 (TLR-7) and/or TLR-8 activation, thereby activating NF-κB signaling (14, 26, 47). Therefore, we investigated the effects of MSCs and SOD3-transduced MSCs on activation of TLR-7 and its downstream NF-κB signaling at 6 and 12 days of treatments. As shown in Figure 9A, both MSCs and SOD3-transduced MSCs effectively inhibited TLR-7 activation and NF-κB expression at the protein level. SOD3-transduced MSCs strongly inhibited TLR-7 activation and NF-κB expression in the 6- and 12-day groups. In contrast, the MSC-treated groups did not show inhibition of the TLR-7 activation, but did show suppressed expression of total NF-κB in the 12-day group. However, the development of psoriasis was also diminished in MSC groups following 12 days of treatment, suggesting the effects of MSCs on multiple pathways of inhibition of psoriasis at different levels.
FIG. 9.
SOD3-transduced MSCs inhibit TLR-7 activation, NF-κB expression, MAP kinase, JAK-STAT signaling, and late-phase responses independent of TLR-7 activation. (A) Tissue extracts from mouse back skins were prepared and blotted to evaluate TLR-7 and NF-κB expression. (B–D) Phosphorylated STAT1, STAT3, JAK1, JAK2, JNK, and p38 levels were determined from mouse back skins. Western blot analysis was performed with antibodies specific for the molecules indicated. (E) The expression of adenosine receptors AR-A1, AR-A2A, AR-A2B, and AR-A3 mRNAs at days 6 and 12 in normal and IMQ-induced psoriatic mice skins was assessed by RT-PCR. GAPDH was used as a control. (F) The cAMP concentrations in tissue and blood plasma were measured by a cAMP ELISA kit (Enzo Life Sciences). (G) Phosphorylation of PKA substrate and CREB in control versus IMQ-induced psoriatic mice either treated with IMQ alone or SOD3-transduced MSCs. The numerical values on the blot represent % relative DU, as measured by ImageJ software (National Institutes of Health). Control group was set as 100% DU in each regimen. (H) Regulation of PDE4A and PDE4B mRNA expression by MSCs or SOD3-transduced MSCs in IMQ-induced psoriatic mouse models. Error bar indicates SD; #p < 0.05, ##p < 0.01, ###p < 0.001 (control group vs. IMQ-treated group for all cases); *p < 0.05, **p < 0.01, ***p < 0.001 (IMQ-treated group vs. SOD3-transduced MSCs or MSC-treated group). cAMP, cyclic adenosine monophosphate; CREB, cAMP response element-binding protein; DU, densitometric unit; JAK-STAT, Janus kinase-signal transducer and activator of transcription; MAP, mitogen-activated protein; NF-κB, nuclear factor-kappa B; PDE4, phosphodiesterase 4; RT, reverse transcriptase; TLR-7, toll-like receptor-7.
The cytokine-mediated JAK-STAT signaling pathway also regulates the immune response (2). Thus, we examined whether MSCs or SOD3-transduced MSCs could also regulate JAK-STAT signaling. Both MSCs and SOD3-transduced MSCs inhibited phosphorylation of STAT1 and STAT3 proteins only in the 12-day-treated group, whereas the SOD3-transduced MSCs showed a significant effect compared with MSCs alone. STAT1 and STAT3 phosphorylation was not inhibited in the 6-day-treated group, and total STAT1 expression remained elevated in the IMQ, MSCs, or SOD3-transduced MSC groups at both 6 and 12 days. However, elevated total STAT3 levels were effectively reduced only in the SOD3-transduced MSC group at 12 days of IMQ treatment (Fig. 9B). Moreover, it was recently reported that inhibition of JAK1 activation could be a more effective approach to reduce psoriasis pathogenesis (56). Our data showed that only SOD3-transduced MSCs inhibited the activation of JAK1 and total JAK1 protein levels, whereas we could not observe the phosphorylation of JAK2, and the level of total JAK2 protein remained unchanged in all groups. These data indicate that administration of SOD3-transduced MSCs may be more effective than MSCs alone in reducing psoriasis pathogenesis by targeting JAK1 activation (Fig. 9C).
MAP kinases play key roles in inflammatory signaling in mammalian cells and were shown to be responsible for epidermal hyperproliferation in psoriasis (18, 30, 54). In this study, IMQ induced phosphorylation of p38 in the 6-day groups and declined thereafter. The phosphorylation of p38 by IMQ treatment was inhibited both in the SOD3-transduced MSCs and MSCs alone groups. In contrast, we could not observe the phosphorylation of JNK, suggesting the involvement of p38 in psoriasis pathogenesis (Fig. 9D).
MSCs and SOD3-transduced MSCs also inhibit IMQ-induced inflammation independent of TLR-7 signaling through regulation of the late-phase inflammatory responses
IMQ is also shown to induce the activation of transcription factor NF-κB and the downstream production of proinflammatory cytokines, in the absence of TLR-7, through the adenosine receptor-dependent mechanism (48, 49, 53). To further investigate the cause and therapeutic effect of SOD3-transduced MSCs or MSCs in the IMQ-induced psoriasis mouse model, independent of TLR-7 signaling, we explored adenosine receptor activation and dependent mechanism. Our results showed that all known subtypes of adenosine receptors are expressed in the skin. We did not observe the significant expression of A1 and A3 in all groups, but expression levels of A2A and A2B were reduced in the MSC-administered groups compared with IMQ-treated groups at 6-day regimen. Interestingly, in the 12-day group, the increased expression of A1, A2B, and A3 by IMQ treatment was significantly reduced in the MSCs and SOD3-transduced MSC groups, although there were only slight differences in the MSCs and SOD3-transduced MSC groups, suggesting a role of MSCs in adenosine receptor expression balance and hence the inhibition of late-phase inflammation (Fig. 9E).
We also evaluated the downstream effects of adenosine receptor activation or inhibition. Our data showed that the levels of cyclic adenosine monophosphate (cAMP) were higher in tissue and blood plasma in the MSCs and SOD3-transduced MSC-treated groups at the 6-day regimen. SOD3-transduced MSCs showed a higher level of cAMP in blood plasma at the 6-day regimen and in both tissue and blood plasma samples at the 12-day regimen compared with the MSC groups (Fig. 9F).
Downstream of cAMP, the phosphorylation of cAMP response element-binding protein (CREB) was increased in MSCs and SOD3-transduced MSC-treated groups at 6 days and more significantly at 12 days of IMQ treatment. The SOD3-transduced MSC-treated group showed higher levels of phosphorylation of CREB compared with MSCs alone-treated group at both 6 and 12 days. Similarly, protein kinase A (PKA) phosphorylation was elevated in the MSC group and more significantly elevated in the SOD3-transduced MSC group at 12 days of treatment (Fig. 9G). Moreover, the endogenous inhibitors of cAMP, the phosphodiesterases (PDEs), specifically PDE4B mRNA expression, reduced in MSCs or SOD3-transduced MSC groups compared with the IMQ-treated group, whereas the mRNA levels of isoform PDE4A were also found to be downregulated along with the IMQ alone-treated group (Fig. 9H). Therefore, these results suggest the involvement of pathways other than TLR-7 and their regulation by SOD3-transduced MSCs or MSCs during the chronic phase of inflammation.
Discussion
In this study, we demonstrated the novel use of MSCs that overexpress SOD3, a powerful antioxidant enzyme, to prevent the severity and progression of psoriasis through the regulation of immune cell infiltration and functions, specifically dendritic cells, neutrophils, and Th17 cells, and by regulating epidermal functions, TLR-7-dependent and independent pathways, MAP kinases, and JAK-STAT pathways, which augment the inflammatory actions. In this study, we used allogeneic MSCs derived from human cord blood as several studies showed that immunosuppressant functions of MSCs are not major histocompatibility complex restricted and therefore could be more functional in clinical settings (1, 5, 12, 25, 42). Most studies showed that MSCs exert immunosuppressive effects and interfere with effector cell function through various molecules such as TGF-β, indoleamine-pyrrole 2,3-dioxygenase (IDO-1), heme oxygenase-1 (HO-1), prostaglandin E2 (PGE2), galectin-1, IL-1 receptor antagonist (IL-1Ra) (15, 31, 39), and many others, which may have not been entirely identified. The contribution of individual suppressive molecule is dependent on the experimental setting and on the species studied.
Overexpression of SOD3 in MSCs resulted in increased secretion of SOD3 with higher activity without affecting the proliferation ability and positive surface marker expression such as CD73, CD90, and CD105. ROS levels did not change much in SOD3-transduced MSCs in normal condition, whereas increased ROS levels in MSCs upon exposure to FAS ligand were significantly reduced in SOD3-transduced MSCs (Supplementary Figs. S1A–D and S2A, B). Interestingly, overexpression of SOD3 in MSCs affects the differentiation of MSCs into adipogenic and osteogenic lineages by promoting and weakly reducing the differentiation potential, respectively, with no effect on differentiation into chondrogenic lineage (Supplementary Fig. S3A–C). However, SOD3-MSCs showed enhanced immunomodulatory property compared with MSCs alone through increased expression of various immunosuppressive factors such as intracellular IL-1Ra, TGF-β, IL-10, HO-1, and IDO-1. Interestingly, priming MSCs with TNF-α and IFN-γ induced IDO-1 and IL-10 expression, which was further increased in SOD3-transduced MSCs. We did not observe the SOD3 effect on PGE2 secretion and galectin-1 expression in MSCs (Supplementary Fig. S4A–D). These results suggest the role of SOD3 in overall immunomodulation by MSCs.
We showed that SOD3-transduced MSCs or MSCs, when given subcutaneously before and after the application of IMQ following the experimental regimens, reduced inflammatory and activated T cells, neutrophils, and dendritic cell responses. Interestingly, we observed that SOD3-transduced MSCs inhibited the severity and development of the disease to a greater extent compared with MSCs alone. We believe that these results could help to shape future clinical strategies for the treatment of psoriasis or other inflammatory disorders such as atopic dermatitis and rheumatoid arthritis.
IMQ-induced skin inflammation involves the infiltration of mononuclear cells into the spleen, lymph nodes, dermis, and epidermis, resulting in thickened epidermis and dermis. The recruitment of such immune cells is crucial for psoriasis pathogenesis, which is mediated through expression of various proinflammatory cytokines and chemokines in the lesions (52). Our results showed that the expression levels of such inflammatory mediators were elevated in IMQ-induced mouse skin. However, in the SOD3-transduced MSC group, the expression of inflammatory mediators was effectively lowered when compared with other MSC groups in both experimental regimens. The immunomodulatory and reparative anti-inflammatory properties of MSCs have been evaluated in various autoimmune inflammatory models and have various conflicting results (10, 40). Therefore, in an attempt to augment the therapeutic efficacy of MSCs to prevent or treat psoriasis, we developed genetically modified MSCs, using adenoviral vector encoding SOD3. SOD3-transduced MSCs exerted more potent effect on epidermis and dermis homeostasis management by reducing the thicknesses of the epidermis and by inhibiting the infiltrations of various immune cells into the skin, spleen, and lymph nodes. These results suggest the enhanced immunomodulatory activity in MSCs through overexpression of SOD3.
In this study, we demonstrated that SOD3-transduced MSCs regulated CD4+ T-cell differentiation and immune responses, thereby reducing IMQ-induced skin inflammation. Our study showed that SOD3-transduced MSCs effectively inhibited the expression of Th cell lineage-specific transcription factors and cytokines. Among them, SOD3-transduced MSCs significantly affected IL-17, CCL-20, and RORγt (Th17 cell regulators) when compared with MSCs alone. In addition, SOD3-transduced MSCs increased the mRNA levels of Foxp3, cytokines IL-10, and TGF-β specific for Treg cells. The stronger immunomodulation by SOD3-transduced MSCs is partly due to the increased expression of HO-1 and TGF-β, which inhibits proinflammatory cytokines, while promoting IL-10 production and generation of immunosuppressive Treg subsets both in vitro and in vivo (Fig. 7F and Supplementary Figs. S4 and S5). The enhanced inhibitory effects of SOD3-transduced MSCs on CD4+ T-cell differentiation suggest the role of SOD3 and its beneficial effects when overexpressed in MSCs to prevent inflammatory disorders that are caused by aberrant immune responses, particularly the pathology mediated by Th17 subsets such as during psoriasis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, graft-versus-host disease, experimental encephalomyelitis, and atherosclerosis and the pathology mediated by Th2 subsets such as atopic dermatitis, allergy, and asthma.
In case of proliferative effects of MSCs and SOD3-transduced MSCs on T cells, we did not observe any significant differences, although they effectively suppressed the proliferation. In this study, overexpression of SOD3 in MSCs did not correspond to suppressive potency of MSCs. One of the potential explanations may be that the suppressive or immunomodulatory property of SOD3 is overpowered by the dominant presence of other immunosuppressive factors in MLR milieu. IMQ has been reported to exert its biological activity through TLR-7 and/or TLR-8 activation by promoting NF-κB signaling (14, 47). SOD3-transduced MSCs strongly inhibited TLR-7 activation and NF-κB expression at the protein level in both the 6- and 12-day groups. MSCs alone were found to be ineffective in the 12-day-treated groups compared with SOD3-MSC-administered mice. The severity of psoriasis was also less in MSC-administered mice, suggesting multiple pathways of inhibition and the involvement of overall immune responses.
In addition, our data also showed the involvement of MAP kinase pathways in IMQ-mediated inflammation, which were effectively inhibited in both the MSCs and SOD3-transduced MSC group, but only in the 6-day-treated group, suggesting the role of MAP kinases in initiation of early inflammatory processes. SOD3-transduced MSCs also effectively inhibited the activation of STAT proteins, specifically STAT1 and STAT3, and activation of JAK1. The JAK-STAT signaling pathways transduce cytokine-mediated signals and activate JAK-STAT proteins to regulate CD4+ T-cell differentiation and are associated with a wide range of inflammatory diseases. Therefore, regulation of aberrantly activated STAT signaling is important for the treatment of autoimmune disorders (4, 20). The strong inhibitory activity of SOD3-transduced MSCs on CD4+ T-cell differentiation was due to the combined effect of its regulation of multiple pathways at various levels, such as the expression of key transcription factors, expression of various proinflammatory cytokines, and activated STAT proteins. The development of psoriasis is known to be associated with STAT3, which is also necessary for Th17 cell development and activated by IL-17, IL-22, and IL-23 (34, 51). Our results showed that both STAT1 and STAT3 were activated with increased total STAT1 and STAT3 at both 6 and 12 days. Interestingly, the phosphorylations of STAT1 and STAT3 and total STAT3 protein expression levels were remarkably reduced in SOD3-transduced MSC-treated mice, suggesting an enhanced therapeutic efficacy of SOD3-transduced MSCs compared with MSCs alone.
In general, inhibiting JAK signaling has been shown to be effective in reducing disease symptoms, demonstrating therapeutic potential in treatment of inflammatory diseases. It has been shown that inhibition of JAK1 signaling blocks the activity of cytokines, such as IL-6, IL-23, and IL-22, and thus predicted to cause significant amelioration of psoriasis pathology (56). In our experimental model, SOD3-transduced MSC-treated mice showed strong suppression of these inflammatory mediators compared with IMQ or MSCs alone-treated mice. Therefore, we investigated other aspects of psoriasis after treatments with MSCs or SOD3-transduced MSCs. Both MSCs and SOD3-transduced MSC-treated mice showed significant reduction of IL-17 and IL-22 in the skin. Only SOD3-transduced MSCs showed more significant reduction of IL-6 and IL-23 compared with MSCs or IMQ alone-treated groups in the 6-day regimen. This may be due to stronger inhibition of redox-sensitive pathways, such as activator protein-1, NF-κB, and JAK-STAT, and the overall reduced inflammatory environment by SOD3-transduced MSCs. However, at this present situation, the precise mechanism is unknown and thus warrants further study to address this notion. Accordingly, SOD3-transduced MSCs showed strong inhibition of JAK1 phosphorylation and total JAK1 expression at protein levels. Inhibition of JAK1 and JAK1-mediated cytokine activities, specifically IL-6, IL-23, and IL-22, and significantly impairing cytokine-dependent STAT1 and STAT3 phosphorylation by SOD3-transduced MSCs further illustrated the strong inhibition of psoriasis progression by SOD3-transduced MSCs.
IMQ is also reported to regulate adenosine receptors and inhibit adenylyl cyclase activity independent of TLR activation, thereby promoting the inflammatory actions (53). The inflammatory environment has a strong impact on the effect of activation of adenosine receptors in regulating immune cells. Previously reported data have shown that late activation of adenosine receptors contributes to increased Th17 responses in an experimental autoimmune uveitis model (27). In agreement with these findings, our results also showed activation of adenosine receptors in the IMQ-induced psoriasis model following the 12-day regimen, where the initiation of inflammation had already been established. The effect of adenosine receptor activation on chronic inflammatory responses was possibly related to the combined effects of T-cell activation, proinflammatory environment, such as cytokines, and TLR activation by ligands. Our data provide the evidence that MSCs and SOD3-transduced MSCs can regulate the expression of all four adenosine receptor subtypes. The results showed elevated levels of adenosine receptor subtypes in the 12-day-treated groups, which were inhibited to a similar extent by administration of MSCs and SOD3-transduced MSCs. However, the downstream of receptors, the cAMP gradient, and the phosphorylation of PKA and CREB were higher in SOD3-transduced MSC-administered mice compared with MSCs alone. The cAMPs have been reported to regulate T-cell functions and maintain immune homeostasis by suppressing the release of proinflammatory mediators such as TNF-α, IL-17, and IFN-γ and by promoting the release of IL-10 (7, 32, 36). The precise mechanism of activation of all adenosine receptor subtypes by IMQ, augmented inflammatory responses in psoriasis and their regulation by MSCs require further investigation. Furthermore, the endogenous cAMP-degrading enzyme, PDE4, has been associated with proinflammatory responses via decreasing the cAMP concentration (7). Our data showed a reduced level of PDE4 in both MSC and SOD3-MSC-treated mice compared with IMQ-treated mice. One mechanism of inhibition of the inflammatory response in psoriasis, by MSCs or SOD3-transduced MSCs, may be related to inhibition of PDE4.
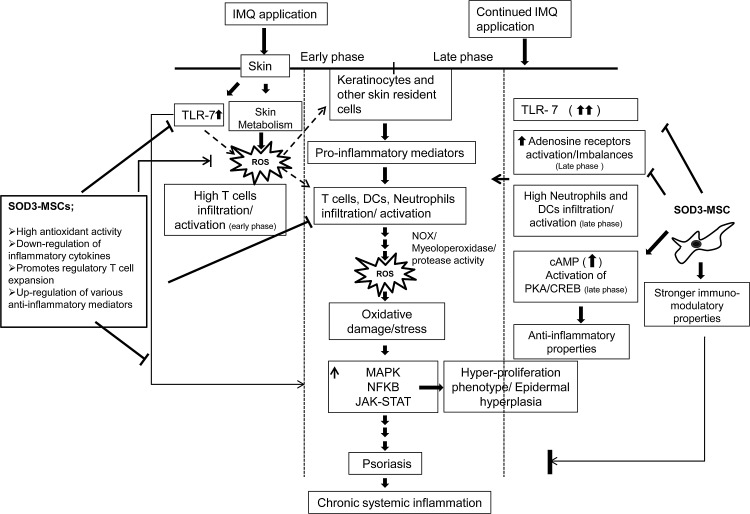
In summary, we demonstrated that administration of genetically modified SOD3-MSCs effectively prevented the development of psoriasis through regulation of multiple pathways. Early cellular interaction between epidermal cells and infiltrated T cells in skin and higher expression of inflammatory mediators by IMQ treatment in the 6-day regimen, which declined afterward, suggest the initiation of psoriasis, which was effectively inhibited by SOD3-transduced MSCs via negatively modulating activation of TLR-7, MAP kinase, and NF-κB pathways. In the 12-day regimen, where the inflammation had already been established, mainly neutrophils and dendritic cell infiltration and activation of adenosine receptor pathways were involved in disease maintenance, despite the activation of TLR-7. SOD3-transduced MSCs strongly inhibited the disease progression by negatively modulating the JAK-STAT pathway, suppressing the recruitment of neutrophils and dendritic cells in skin, spleen, and lymph nodes, upregulating cAMP gradient, and inhibiting the pathogenic adenosine receptor activation (Fig. 10). Our data strongly suggest that overexpression of SOD3 in MSCs provides a new enhanced therapeutic application for the treatment of chronic inflammatory disorders. However, further experiments are needed to understand the detailed mechanism of regulation in physiopathology and treatment based on antioxidant strategies.
FIG. 10.
A scheme for SOD3-transduced MSC therapy in IMQ-induced psoriasis-like skin inflammation in mice. IMQ induced skin inflammation in mice by TLR-7 and pathogenic adenosine receptor activation and alters the skin metabolism, thereby activating skin resident cells, which leads to recruitment and infiltration of immune cells into the skin, producing proinflammatory molecules and ROS. Subsequently, these molecules contribute to psoriasis, followed by chronic systemic inflammation. SOD3-transduced MSC therapy counteracts the IMQ-induced inflammatory network by both stronger antioxidant and immunomodulatory activities.
Materials and Methods
Mice and IMQ treatment
C57BL/6 mice were purchased from Central Laboratory Animal, Inc., and kept under specific pathogen-free conditions. Mice were provided with standard laboratory mouse chow and water. The mice used for the study were 8 weeks of age. The mice were treated according to the regulations of the Catholic Ethics Committee of the Catholic University of Korea, which conform to the National Institutes of Health guidelines.
The mice received a daily topical dose of 62.5 mg of commercially available IMQ cream (5%) (Aldara; 3M Pharmaceuticals) on the shaved back skins for 6 days and received a booster dose for 12 consecutive days to achieve the optimal chronic and aggressive inflammation.
Isolation, culture, and characterization of MSCs and construction of recombinant adenoviral vectors
Human umbilical cord blood-derived MSCs were generated according to previously described methods (13, 41). Details can be found in the Supplementary Materials and Methods section.
Subcutaneous injection of MSCs
The regimens of immunosuppressive therapy with MSCs were tested by administration of 2 × 106 MSCs or SOD3-transduced MSCs subcutaneously 24 h before and at day 6 of IMQ application. Control mice received subcutaneous injections of an equal volume of phosphate-buffered saline at the same time points.
Histological evaluation and IHC analysis
Sections from the mouse back skins were stained with H&E stain for histological evaluation. Immunohistochemical analysis was performed as previously described (24). Sections were incubated with primary anti-mouse antibodies; CD3 (Abcam), CD11c (Abcam), and Gr1 (Abcam), and then with diluted biotin-conjugated goat anti-rabbit IgG at 4°C following the development using 3,3′-diaminobenzidine. Sections stained with an antibody of the same isotype as the specific antibody, but of irrelevant specificity, served as control.
T-cell proliferation and differentiation
The CFSE-MLR assays were performed to determine the proliferation of CD4+ and CD8+ T cells (37). Similarly, CD4+ T-cell differentiation was induced under appropriate polarizing conditions as described in the Supplementary Materials and Methods section.
Flow cytometric analysis
Single-cell suspensions from skin, spleen, and lymph nodes were labeled with the following monoclonal antibodies and analyzed using BD FACSCanto™ II; PECY7 or fluorescein isothiocyanate-conjugated anti-mouse CD4, phycoerythrin (PE)-conjugated anti-mouse CD8, antigen-presenting cell (APC)-conjugated anti-mouse Gr1, PE-conjugated anti-mouse CD11C, APC-conjugated CD25, and PE-conjugated FOXP3. All of these antibodies were purchased either from Becton Dickinson, eBioscience, or Biolegend.
Reverse transcriptase-PCR and real-time quantitative PCR
First strand of cDNA was reverse transcribed from 1 μg of total RNA isolated from the mouse back skin using the QuantiTech reverse transcription kit (Qiagen). The primer sets for IL-1β, IL-6, IL-17, IL-20, IL-22, IL-23 TNF-α, IFN-γ, CXCL-1, CCL-17, CCL-20, PDE4A, and PDE4B were purchased from Qiagen. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an endogenous control. PCR was performed using Rotor-Gene 6000 (Corbett) and a QuantiTect SYBR Green PCR Kit (Qiagen). The amplification program consisted of 1 cycle at 95°C for 10 min, followed by 35 cycles at 95°C for 20 s, 55°C for 20 s, and 72°C for 20 s. The expression of adenosine receptor subtypes A1, A2A, A2B, A3, and GAPDH as a control was assessed by reverse transcriptase (RT)-PCR. Previously designed primers were used for RT-PCR (19).
Western blot
Total protein was extracted using the mouse back skin and Western blotting was performed. Equal amounts of protein were loaded per lane and the blotted membranes were incubated overnight with specific primary antibodies for target molecules and were then detected using an enhanced chemiluminescence system (GE Healthcare Life Sciences).
Statistical analysis
Statistical significance was assessed by comparing mean ± standard deviation values with Student's t-test or analysis of variance (ANOVA) for independent groups wherever appropriate. All determinations were performed in triplicate, and experiments were repeated thrice unless otherwise stated.
Supplementary Material
Abbreviations Used
- ANOVA
analysis of variance
- APC
antigen-presenting cell
- cAMP
cyclic adenosine monophosphate
- CCL
chemokine ligand
- CD
cluster of differentiation
- CFSE-MLR
carboxyfluorescein diacetate succinimidyl ester–mixed lymphocyte reaction
- CREB
cAMP response element-binding protein
- DETCA
sodium diethyldithiocarbamate trihydrate
- FBS
fetal bovine serum
- FOXP3
forkhead box P3
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GATA3
transacting T-cell-specific transcription factor
- H&E
hematoxylin and eosin
- HO-1
heme oxygenase-1
- icIL-1Ra
intracellular IL-1 receptor antagonist
- IDO-1
indoleamine-pyrrole 2,3-dioxygenase
- IFN-γ
interferon-gamma
- IHC
immunohistochemistry
- IL
interleukin
- IMQ
imiquimod
- JAK-STAT
Janus kinase-signal transducer and activator of transcription
- JNK
c-Jun NH (2)-terminal kinase
- MAP
mitogen-activated protein
- MSCs
mesenchymal stem cells
- NF-κB
nuclear factor-kappa B
- PDE4
phosphodiesterase 4
- PE
phycoerythrin
- PGE2
prostaglandin E2
- PKA
protein kinase A
- RORγt
retinoic acid-related orphan receptor gamma
- ROS
reactive oxygen species
- RPMI
Roswell Park Memorial Institute
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SD
standard deviation
- sIL-1Ra
soluble IL-1 receptor antagonist
- SOD3
extracellular superoxide dismutase
- TGF-β
transforming growth factor-beta
- Th
T helper
- TLR-7
toll-like receptor-7
- TNF-α
tumor necrosis factor-α
Acknowledgments
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (Grant No. NRF-2013M3A9A9050567), and the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant No. HI12C17990100).
Author Disclosure Statement
The authors state no conflicts of interest exist.
References
- 1.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, and Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A 102: 11474–11479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrés RM, Hald A, Johansen C, Kragballe K, and Iversen L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp Dermatol 22: 323–328, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Arbiser JL, Bips M, Seidler A, Bonner MY, and Kovach C. Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases. J Am Acad Dermatol 67: e81–e83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbiser JL, Govindarajan B, Battle TE, Lynch R, Frank DA, Ushio-Fukai M, Perry BN, Stern DF, Bowden GT, Liu A, Klein E, Kolodziejski PJ, Eissa NT, Hossain CF, and Nagle DG. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J Invest Dermatol 126: 1396–1402, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Augello A, Tasso R, Negrini S, Amateis A, Indiveri F, Cancedda R, and Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol 35: 1482–1490, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Baek JO, Byamba D, Wu WH, Kim TG, and Lee MG. Assessment of an imiquimod-induced psoriatic mouse model in relation to oxidative stress. Arch Dermatol Res 304: 699–706, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Baumer W, Hoppmann J, Rundfeldt C, and Kietzmann M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets 6: 17–26, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Campanati A, Orciani M, Gorbi S, Regoli F, Di Primio R, and Offidani A. Effect of biologic therapies targeting tumour necrosis factor-α on cutaneous mesenchymal stem cells in psoriasis. Br J Dermatol 167: 68–76, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, and Arbiser JL. Reactive oxygen signaling and MAPK activation distinguish Epstein-Barr virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proc Natl Acad Sci U S A 102: 175–179, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, and Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum 65: 1181–1193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, and Roh HJ. IFATS collection: immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells 27: 259–265, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, and Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99: 3838–3843, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Divya MS, Roshin GE, Divya TS, Rasheed VA, Santhoshkumar TR, Elizabeth KE, James J, and Pillai RM. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res Ther 3: 57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, and Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol 218: 74–86, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gieseke F, Böhringer J, Bussolari R, Dominici M, Handgretinger R, and Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood 116: 3770–3779, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, Kupper TS, Sehgal PB, and Gottlieb AB. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A 86: 6367–6371, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, and Chandel NS. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal 6: ra8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen C, Kragballe K, Westergaard M, Henningsen J, Kristiansen K, and Iversen L. The mitogen-activated protein kinases p38 and ERK1/2 are increased in lesional psoriatic skin. Br J Dermatol 152: 37–42, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Katebi M, Soleimani M, and Cronstein BN. Adenosine A2A receptors play an active role in mouse bone marrow-derived mesenchymal stem cell development. J Leukoc Biol 85: 438–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim BH, Na KM, Oh I, Song IH, Lee YS, Shin J, and Kim TY. Kurarinone regulates immune responses through regulation of the JAK/STAT and TCR-mediated signaling pathways. Biochem Pharmacol 85: 1134–1144, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Kim BH, Lee H, Jeon B, Lee YS, Kwon MJ, and Kim TY. Regulation of skin inflammation and angiogenesis by EC-SOD via HIF-1α and NF-κB pathways. Free Radic Biol Med 51: 1985–1995, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Kwon MJ, Han J, Kim BH, Lee YS, and Kim TY. Superoxide dismutase 3 suppresses hyaluronic acid fragments mediated skin inflammation by inhibition of toll-like receptor 4 signaling pathway: superoxide dismutase 3 inhibits reative oxygen species-induced trafficking of toll-like receptor 4 to lipid rafts. Antioxid Redox Signal 16: 297–313, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Kwon MJ, Jeon YJ, Lee KY, and Kim TY. Superoxide dismutase 3 controls adaptive immune responses and contributes to the inhibition of ovalbumin-induced allergic airway inflammation in mice. Antioxid Redox Signal 17: 1376–1392, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai Y, Li D, Li C, Muehleisen B, Radek KA, Park HJ, Jiang Z, Li Z, Lei H, Quan Y, Zhang T, Wu Y, Kotol P, Morizane S, Hata TR, Iwatsuki K, Tang C, and Gallo RL. The antimicrobial protein REG3A regulates keratinocyte proliferation and differentiation after skin injury. Immunity 37: 74–84, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, and Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363: 1439–1441, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Li ZJ, Sohn KC, Choi DK, Shi G, Hong D, Lee HE, Whang KU, Lee YH, Im M, Lee Y, Seo YJ, Kim CD, and Lee JH. Roles of TLR7 in activation of NF-κB signaling of keratinocytes by imiquimod. PLoS One 8: e77159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang D, Zuo A, Shao H, Chen M, Kaplan HJ, and Sun D. Anti-inflammatory or proinflammatory effect of an adenosine receptor agonist on the Th17 autoimmune response is inflammatory environment-dependent. J Immunol 193: pii: , 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim JY, Park MJ, Im KI, Kim N, Jeon EJ, Kim EJ, Cho ML, and Cho SG. Combination cell therapy using mesenchymal stem cells and regulatory T-cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of Th1/Th2 and Th17/Treg cells in a murine acute graft-versus-host disease model. Cell Transplant 23: 703–714, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Maley AM. and Arbiser JL. Gentian violet: a 19th century drug re-emerges in the 21st century. Exp Dermatol 22: 775–780, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto M, Sudo T, Saito T, Osada H, and Tsujimoto M. Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-kappa B ligand (RANKL). J Biol Chem 275: 31155–31161, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, and Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 103: 4619–4621, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Mosenden R. and Tasken K. Cyclic AMP-mediated immune regulation-overview of mechanisms of action in T cells. Cell Signal 23: 1009–1016, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Na K, Kim KE, Park ST, and Kim TY. EC-SOD suppresses contact hypersensitivity in mouse skin by impairing langerhans cells migration. J Invest Dermatol 127: 1930–1937, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Nakajima K, Kanda T, Takaishi M, Shiga T, Miyoshi K, Nakajima H, Kamijima R, Tarutani M, Benson JM, Elloso MM, Gutshall LL, Naso MF, Iwakura Y, DiGiovanni J, and Sano S. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol 186: 4481–4489, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Nestle FO, Kaplan DH, and Barker J. Mechanism of disease: psoriasis. N Engl J Med 361: 496–509, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Oger S, Mehats C, Dallot E, Cabrol D, and Leroy MJ. Evidence for a role of phosphodiesterase 4 in lipopolysaccharide-stimulated prostaglandin E2 production and matrix metalloproteinase-9 activity in human amniochorionic membranes. J Immunol 174: 8082–8089, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Oku M, Okumi M, Sahara H, Hirakata A, Onoe T, Griesemer AD, and Yamada K. Porcine CFSE mixed lymphocyte reaction and PKH-26 cell-mediated lympholysis assays. Transpl Immunol 20: 78–82, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Orciani M, Campanati A, Salvolini E, Lucarini G, Di Benedetto G, Offidani A, and Di Primio R. The mesenchymal stem cell profile in psoriasis. Br J Dermatol 165: 585–592, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, and Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 104: 11002–11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, Chung BH, Lee JW, Kim HY, and Cho SG. Transforming growth factor β-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum 63: 1668–1680, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Reddy NP, Vemuri MC, and Pallu R. Isolation of stem cells from human umbilical cord blood. Methods Mol Biol 407: 149–163, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, and Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141–150, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Ross AD, Banda NK, Muggli M, and Arend WP. Enhancement of collagen-induced arthritis in mice genetically deficient in extracellular superoxide dismutase. Arthritis Rheum 50: 3702–3711, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, and Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178: 2229–2240, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Salem HK. and Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28: 585–596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, and DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 11: 43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Schon MP. and Schon M. Imiquimod: mode of action. Br J Dermatol 157 Suppl 2: 8–13, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Schon MP, Schon M, and Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol 126: 1338–1347, 2006 [DOI] [PubMed] [Google Scholar]
- 49.St Hilaire C, Carroll SH, Chen H, and Ravid K. Mechanisms of induction of adenosine receptor genes and its functional significance. J Cell Physiol 218: 35–44, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su WR, Zhang QZ, Shi SH, Nguyen AL, and Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells 29: 1849–1860, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Tokura Y, Mori T, and Hino R. Psoriasis and other Th17-mediated skin diseases. J UOEH 32: 317–328, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, and Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol 182: 5836–5845, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Walter A, Schäfer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, Schönewolf N, Dummer R, Bloch W, Werner S, Beer HD, Knuth A, and van den Broek M. Aldara activates TLR7-independent immune defence. Nat Commun 4: 1560, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Uchi H, Hayashida S, Urabe K, Moroi Y, and Furue M. Differential expression of phosphorylated extracellular signal-regulated kinase 1/2, phosphorylated p38 mitogen-activated protein kinase and nuclear factor-kappaB p105/p50 in chronic inflammatory skin diseases. J Dermatol 36: 534–540, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, Vom Baur E, Witte K, Warszawska K, Philipp S, Johnson-Leger C, Volk HD, Sterry W, and Sabat R. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med (Berl) 87: 523–536, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Works MG, Yin F, Yin CC, Yiu Y, Shew K, Tran TT, Dunlap N, Lam J, Mitchell T, Reader J, Stein PL, and D'Andrea A. Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced pasoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL17 axis. J Immunol 193: 3278–3287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Q, Mrowietz U, and Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med 47: 891–905, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.