Abstract
Transcription factors (TFs) are DNA-binding proteins that play critical roles in regulating gene expression. These proteins control all major cellular processes, including growth, development, and homeostasis. Because of their pivotal role, cells depend on proper TF function. It is, therefore, not surprising that TF deregulation is linked to disease. The therapeutic drug targeting of TFs has been proposed as a frontier in medicine. RNA aptamers make interesting candidates for TF modulation because of their unique characteristics. The products of in vitro selection, aptamers are short nucleic acids (DNA or RNA) that bind their targets with high affinity and specificity. Aptamers can be expressed on demand from transgenes and are intrinsically amenable to recognition by nucleic acid-binding proteins such as TFs. In this study, we review several natural prokaryotic and eukaryotic examples of RNAs that modulate the activity of TFs. These examples include 5S RNA, 6S RNA, 7SK, hepatitis delta virus-RNA (HDV-RNA), neuron restrictive silencer element (NRSE)-RNA, growth arrest-specific 5 (Gas5), steroid receptor RNA activator (SRA), trophoblast STAT utron (TSU), the 3′ untranslated region of caudal mRNA, and heat shock RNA-1 (HSR1). We then review examples of unnatural RNA aptamers selected to inhibit TFs nuclear factor-kappaB (NF-κB), TATA-binding protein (TBP), heat shock factor 1 (HSF1), and runt-related transcription factor 1 (RUNX1). The field of RNA aptamers for DNA-binding proteins continues to show promise.
Introduction
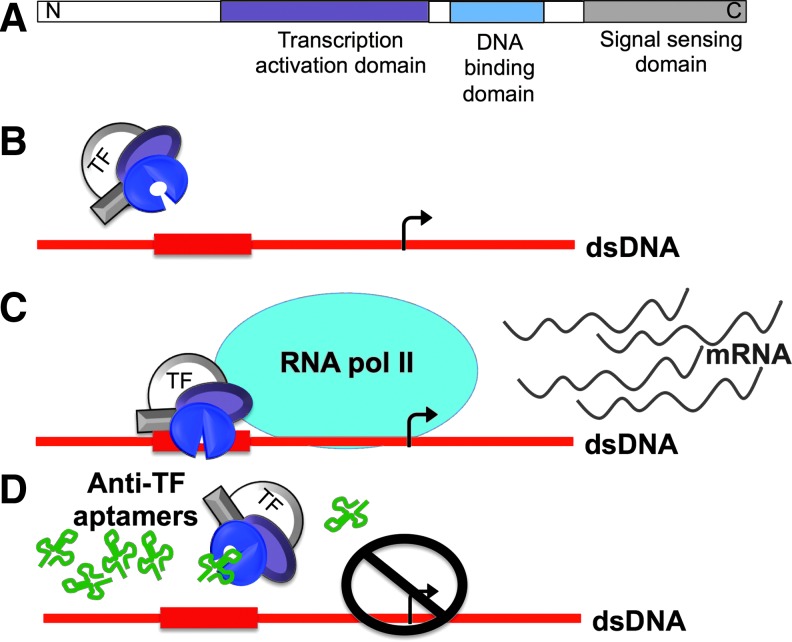
Regulation of gene expression is crucial for the development and survival of cells, resulting in exquisite control of the function and development of living organisms. Gene expression is regulated at many stages, but a dominant role is played by control of transcription initiation. Crucial in this process are sequence-specific DNA-binding proteins termed transcription factors (TFs). Possessing modular structures often including a DNA-binding domain and a transcriptional activation or repression domain, some TFs also contain signal-sensing domains (Fig. 1A) [1,2]. TFs can regulate transcription either positively or negatively [3,4]. Because of their specificity and role in controlling gene expression, TFs make compelling targets for therapeutic manipulation to control genes that are either deregulated due to derangement of signaling cascades, or due to the deregulation of the TF itself. While it is commonly recognized that TFs are attractive therapeutic targets for the next generation of drugs, there has been little progress toward this goal [5,6].
FIG. 1.
Example of transcription factor (TF) modular structure and proposed mechanism of anti-TF aptamers. (A) Schematic illustration of an example TF showing separate structural modules with different functions: transcription activation domain, DNA-binding domain, and signal-sensing domain. (B) Potential mechanism of anti-TF aptamers. TFs are activated and bind to promoter and enhancer consensus sequences. (C) Upon DNA binding TFs promote and regulate chromatin modification and recruitment of RNA polymerase. (D) Aptamers with high specificity and affinity against a TF might competitively bind the target and inhibit binding of TF to dsDNA, resulting in inhibition of gene expression.
Currently most marketed drugs are small molecules, less likely to compete with large charged molecular surfaces such as those involved in TF binding to DNA. In this study we review the intriguing cases of natural and selected RNA aptamers that bind and competitively inhibit TFs.
Aptamers are short RNA or DNA sequences that fold into complex three-dimensional structures and bind to their targets with high affinity and specificity. They are typically the product of the in vitro technique termed SELEX (systematic evolution of ligands by exponential enrichment) [7,8]. Several considerations make aptamers intriguing tools for TF inhibition. Target affinity can be comparable to antibodies (nanomolar to picomolar range), moderate molecular mass allows access to smaller biological compartments, targeting is flexible, the agents appear to be nonimmunogenic, and high specificity can be achieved. For example, an aptamer to growth factor fibroblast growth factor-2 (FGF-2) reportedly binds 20,000-fold more tightly to its target than to closely related FGF homologs [9], and synthetic aptamers can be modified to increase bioavailability while preserving ease of preparation and lack of toxicity [10,11]. Recent advances in high-throughput technology have improved aptamer selection [12–15]. While nucleic acid aptamers face the obvious challenge of cell penetration, RNA aptamers have the unique advantage that they can be encoded in transgenes for endogenous expression after gene delivery.
Anti-TF aptamers have been conceived as therapeutic agents, either to inhibit the expression of genes that are transactivated by the target TF, or to activate genes that are transcriptionally repressed by the target TF. In principle, appropriate RNA aptamers can be selected for binding to the DNA-binding domain of a target TF, blocking it from binding to its double-stranded DNA target site and thereby competitively inhibiting its activity (Fig. 1B–D). TF inhibition by double-stranded DNA copies of the TF-binding site represents the simplest implementation of this concept. Such an approach was applied to E2F-1 with the goal of preventing a common cardiovascular disorder [16,17]. In this study, we focus instead on the intriguing concept of RNA aptamers against DNA-binding TFs where the opportunity for therapeutic expression from transgenes can be considered and the fascinating problem of RNA mimicry of DNA comes into play. We review both natural and in vitro-selected anti-TF RNA aptamers.
Natural Occurring Anti-TF Aptamers
A fascinating class of RNAs appear to function in the modulation of protein activity by mimicking the structures of other DNAs or RNAs. These natural RNAs act in a manner reminiscent of the proposed TF inhibitors described above (Table 1) [18].
Table 1.
Natural RNAs That Regulate Transcription Factors
| RNA | TF target | Regulation |
|---|---|---|
| 5S RNA | TFIIIA | Its own transcription |
| 6S RNA | σ70-RNAp | Wide-range regulation of transcription of several genes |
| 3′ UTR of cad | Bicoid | Expression of caudal |
| TSU | STAT1 | Reduces nuclear translocation and suppression of MHC genes |
| NRSE-RNA | NRSF/REST | Inhibits REST from binding to dsDNA and acting as a repressor |
| HSR1 | HSF1 | Stimulates heat shock response by stabilizing HSF1 |
| HDV | RNAP II | Regulates its own transcription |
| 7SK | HMGA1 | Inhibits HMGA1 binding to dsDNA |
| ncRNA anti-p53 | p53 | Fine tunes p53 response |
| Gas5 | GR | Reduces GR response |
| SRA | SRA | Activates SRA response by acting as a scaffold |
Gas5, growth arrest-specific 5; GR, glucocorticoid receptor; HDV, hepatitis delta virus; HMGA1, high mobility group protein A1; HSF1, heat shock transcription factor 1; HSR1, heat shock RNA-1; MHC, major histocompatibility complex; NRSE, neuron restrictive silencer element; NRSF, neuron-restrictive silencer factor; REST, RE1-silencing transcription factor; RNAP II, RNA polymerase II; SRA, steroid receptor RNA activator; STAT1, signal transducers and activators of transcription; TF, transcription factor; TSU, trophoblast STAT utron; UTR, untranslated region.
5S rRNA
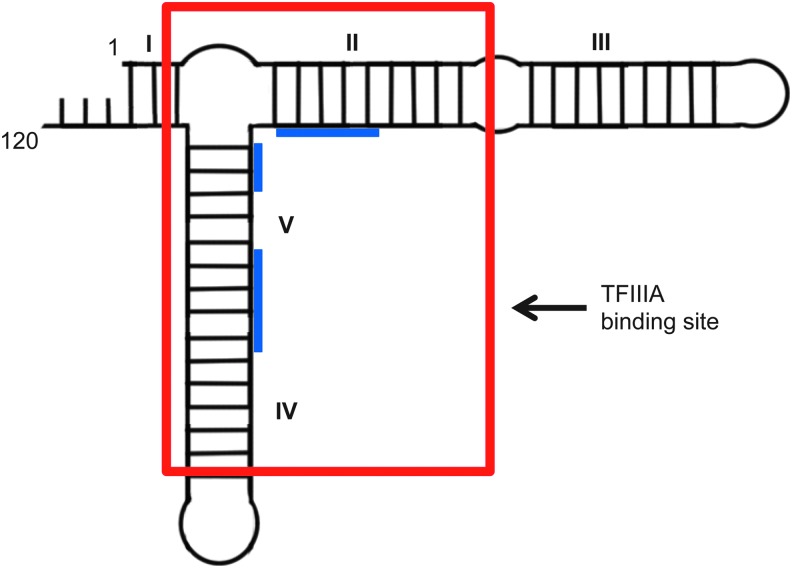
5S rRNA is a universal component of the large ribosomal subunit. Although it is essential for the activity of the ribosome, its precise role remains elusive. Transcription of 5S rRNA during oogenesis in Xenopus laevis is controlled by TFIIIA, a positive regulator that binds to an internal control region of the 5S rRNA gene [19,20]. TFIIIA is a zinc metalloprotein [21] composed of nine classical cys2-his2 zinc fingers arranged consecutively [22]. In the 1980s it was discovered that TFIIIA possesses the remarkable ability to bind to the transcript of the gene it controls, 5S rRNA, forming a storage ribonucleoprotein particle (7S RNAP) (Fig. 2). These particles accumulate to massive levels in the oocyte before ribosome assembly [19,23–26]. Thus, although TFIIIA is cataloged as a DNA-binding protein, it has the unusual ability to interact specifically with both double-stranded DNA and with RNA.
FIG. 2.
Xenopus laevis 5S RNA. Schematic secondary structure of X. laevis oocyte 5S rRNA. Helices and loops are numbered. The putative TFIIIA-binding site is boxed in red and physical interaction regions with zinc fingers are shown in blue. Adapted from Romby et al. [162].
The competitive nature of DNA versus RNA binding initially suggested that the same binding domain of TFIIIA interacts with both DNA and its RNA transcript. However, biochemical analysis and subsequent high-resolution structure determination elucidated the more surprising and complex recognition mechanism for these nucleic acids. In vitro analysis of a series of TFIIIA zinc finger deletions revealed that fingers 1–3 (numbered from N- to C-terminus) contributed most to the interaction with DNA, while fingers 4–6 where largely responsible for RNA binding [27].
These biochemical data were subsequently supported by high-resolution structural studies using X-ray crystallography [28] and nuclear magnetic resonance (NMR) [29]. Different sets of zinc fingers indeed dominate the different interactions between TFIIIA and its cognate DNA and RNA partners through induced fit interactions [29]. It is important to note that when TFIIIA binds 5S rRNA, the protein can no longer bind DNA, despite the involvement of different fingers. Thus, although 5S RNA is not acting as a perfect mimic of the TFIIIA target DNA, it effects competitive inhibition by a mechanism comparable to that proposed above.
6S RNA
Another striking natural example of an RNA that acts as a competitive inhibitor of a DNA-binding protein is provided by prokaryotic 6S RNA. This small RNA is important for the bacterial stress response upon nutrient deprivation [30]. Detected initially because of its high abundance in Escherichia coli [31], this RNA was subsequently shown to inhibit normal transcription by binding directly to the housekeeping holoenzyme form of RNA polymerase (σ70-RNAp), preventing its binding to gene promoters. Remarkably, 6S RNA forms a stable, long-lived complex with σ70-RNAp, but not with free polymerase or RNA polymerases containing alternative σ subunits [32–34].
6S RNA controls a large number of genes by downregulating the transcription of most σ70-dependent promoters. 6S RNA accumulates to high levels during late stationary phase and binds efficiently to σ70-RNAp. During this time, most 6S RNA is bound to the polymerase. This explains downregulation of transcription at σ70-dependent promoters [35–37]. During the exponential phase, in contrast, most σ70-RNAp is bound to DNA. 6S RNA inhibition of specific promoters leads to an altered program of gene expression, apparently adapting to the nutrient stress of stationary phase. When cells are moved to rich media, nucleotide triphosphate (NTP) concentrations rise and 6S RNA apparently becomes a template for RNA polymerase, generating a small RNA product (pRNA), and resulting in the release and degradation of 6S RNA and recycling RNA polymerase.
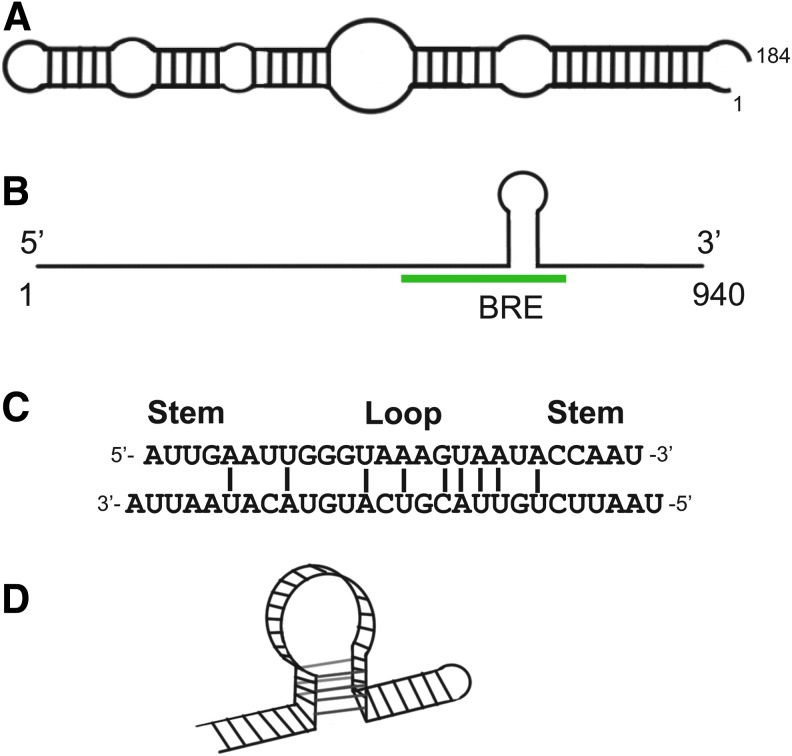
Computer predictions and structural mapping have shown that 6S RNA is largely double-stranded with a single-stranded central bulge (Fig. 3A). This conserved secondary structure is required for 6S RNA interactions with σ70-RNAp [32]. The resemblance of the 6S RNA secondary structure to the conformation of promoter DNA within an open complex led to speculation that the 6S RNA might interact with RNA polymerase as a mimic of promoter DNA [34]. This speculation was later confirmed by biochemical studies where 6S RNA was found to be engaged at the RNA polymerase active site, as it actually can serve as a functional template to generate pRNAs [38]. Thus, when 6S RNA is bound to the active site of the RNA polymerase in the presence of low NTP concentrations, the RNA inhibits transcription by a decoy function, sequestering RNA polymerase from binding DNA promoters.
FIG. 3.
Natural anti-TF RNA aptamers. (A) Escherichia coli 6S RNA. Schematic of 6S RNA secondary structure, adapted from Wassarman [33]. (B) Schematic of caudal (cad) mRNA showing 940-nucleotide transcript. Highlighted in green is the region found to interact with bicoid protein, termed the bcd recognition element (BRE). The hairpin depicted in this region is a hypothetical secondary structure proposed for the interaction with bcd. (C) Hypothetical trophoblast STAT utron (TSU) model involving base pairing of promoter-like motifs 1 and 8 in TSU. (D) Synthetic TSU model RNA based on GAS motifs in loop–loop bent helical structures. C and D adapted from Peyman [47].
3′ Untranslated region of caudal
Arthropods, such as the fruit fly Drosophila melanogaster, are composed of body segments. During early embryonic development, the segments of the embryo adopt their own identities. The primary determinant of anterior pattern in the embryo is the graded expression of bicoid (bcd), a gene encoding a homeodomain TF. Homeodomain proteins are transcriptional regulators that specify the body plan by controlling transcription of their target genes. Bcd mRNA is tightly localized to the anterior pole of the egg [39,40]. After fertilization, this mRNA is translated giving rise to an anterior-to-posterior gradient of bcd protein that then activates the transcription of target genes at distinct concentration thresholds [39–43]. Transcription activation by bcd is mediated by direct binding of the homeodomain to DNA targets. Shortly after the bcd gradient is established, a second homeodomain protein, caudal (cad), accumulates in an opposing posterior-to-anterior gradient under bcd control.
The regulation of cad by bcd is necessary for proper patterning. Inappropriate expression of cad causes deletions of head and thoracic segmentation [44]. Unexpectedly, it was found that regulation of cad by bcd occurs at the level of translation. The bcd homeodomain acts on the 3′ untranslated region (UTR) of the cad message [45,46]. Just as in the examples presented above, bcd binding to the 3′ UTR of cad mRNA (Fig. 3B) excludes bcd from binding to DNA. In this case, bcd simultaneously acts as a translational repressor of cad. Thus, it is a remarkable feature that a single protein executes both transcriptional and translational control. The detailed mechanism by which bcd binds both DNA and RNA remains speculative. However, the proposed model is that the bcd DNA recognition domain contacts RNA in a similar manner to the basic domain of another protein that recognizes RNA, the Rev protein of human immunodeficiency virus (HIV).
Other portions of the bcd homeodomain, such as the N-terminal arm, are also rich in arginine residues and may stabilize this interaction by making additional contacts with the RNA. The premise that the same protein residues involved in DNA recognition may also be involved in binding RNA is supported by the observation that amino acid substitutions in the bcd DNA recognition α-helix can block the translational repression of cad [45].
Trophoblast STAT utron
A repressor RNA termed trophoblast STAT utron (TSU) has been reported to bind TF signal transducers and activators of transcription (STAT1) [47], a sequence-specific TF involved in immune function and development. Complex formation between STAT1 and TSU appeared to reduce STAT1 nuclear translocation and repress major histocompatibility complex (MHC) class II expression in the early embryo [47]. Sequence-specific binding was suggested by results of experiments with RNA constructs carrying 80 nucleotides of the TSU transcript, including two predicted stem-loops and a central hairpin. Electrophoretic gel mobility shift studies showed that the loop–loop structures with the partially complementary motif 1 (5′GUAAAGUAA3′) and motif 8 (5′UUACGUCAU3′) formed complexes with STAT1, while controls did not (Fig. 3C, D). It has been proposed that STAT1 binds TSU using motifs similar to the specific STAT DNA-binding site, suggesting that the protein employs a similar mechanism in binding both DNA and RNA. Subsequent characterization of the TSU system has not been reported.
Neuron-restrictive silencer element-RNA (RE1-RNA)
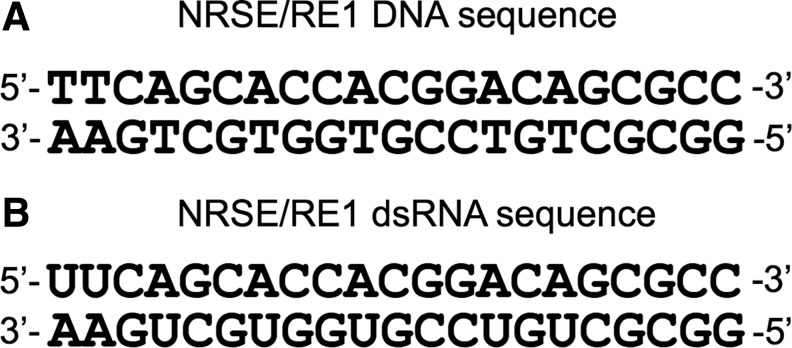
RNAs extracted from adult hippocampal neural stem cells included a double-stranded RNA containing a sequence corresponding to a 21 base pair DNA element termed neuron-restrictive silencer element (NRSE), also known as RE1 (Fig. 4) [48]. Further experiments suggested that this RNA interacts with the neuron-restrictive silencer factor (NRSF) [49], also known as RE1-silencing transcription factor (REST) [50].
FIG. 4.
Schematic diagram of neuron-restrictive silencer factor (NRSF)/RE1-silencing transcription factor (REST) sequence specificity. (A) Neuron-restrictive silencer element (NRSE/RE1) element found in dsDNA. (B) Identical NRSE/RE1 sequence found in NRSE-RNA.
NRSF/REST is a Krüppel family of zinc finger transcription repressor expressed at high levels in most non-neuronal cells and in undifferentiated neuronal progenitors. However, its expression is low in mature neurons [49–52]. REST binds the conserved NRSE/RE1 DNA element, where it acts as a modular scaffold for the assembly of diverse macromolecular complexes blocking transcription. REST represses hundreds of neuronal genes, including those encoding ion channels, neurotrophins, neurotransmitters, synaptic vesicle proteins, and a broad range of factors involved in neurite growth, axonal guidance, and transport [53,54].
It was shown that the noncoding NRSE-RNA binds transcription factor REST during a defined period of neuronal differentiation and effects changes in REST-dependent gene expression, thus modulating the function of REST between activation and repression of neurogenesis. Electrophoretic gel mobility shift assays revealed that the affinity of NRSF/REST for NRSE-RNA was much higher than its affinity for NRSE double-stranded DNA. Moreover, the expression of NRSE-RNA was shown to be necessary and sufficient to direct multipotent neuronal stem cells toward a neuronal fate, suggesting that this RNA can function as an endogenous inducer of neuronal differentiation [48]. Based on immunoprecipitation experiments and mutation analysis, it was inferred that there is a physical interaction between NRSE-RNA and REST protein [48].
A simple model was proposed in which NRSE-RNA-dependent gene activation is induced through the physical interaction of the RNA as a competitive decoy for REST, releasing the genome from repression. Although structural studies are lacking, it has been suggested that the interaction of NRSE-RNA with NRSF/REST involves one of the eight zinc fingers of the protein [48]. Zinc finger-containing proteins have the potential to bind either RNA or double-stranded DNA, as described above in the case of TFIIIA. Although NRSE-RNA competes for binding against the double-stranded DNA element RE1, it is possible that NRSF/REST binds through different zinc fingers than those involved in DNA binding.
It is important to note that in vivo studies from two different groups [48,55] showed stimulatory effects attributed to the sequestration of NRSF/REST when using vectors intended to express NRSE-RNA from NRSE/RE1 DNA sequences. However, Kuwabara et al. pointed out that these effects were probably due to sequestration of NRSF/REST by the plasmid DNA, and not by expressed NRSE-RNA. These false positive results point to the potential advantage of anti-REST RNA aptamers. It has previously been shown that RNA aptamers can bind DNA-binding proteins without encoding the same cognate DNA recognition sequences, avoiding the situation where both a DNA template and its RNA product both interact with a protein.
A broad range of neurological diseases, including glioma, stroke, and neurodegeneratation (including Huntington's disease), are characterized by deregulation of REST. In fact, most of these disorders are characterized by increased REST activity, leading to transcriptional repression. Thus, REST represents a candidate for therapeutic TF inhibition [56–58] for treatment of these diseases [59].
Heat shock RNA-1
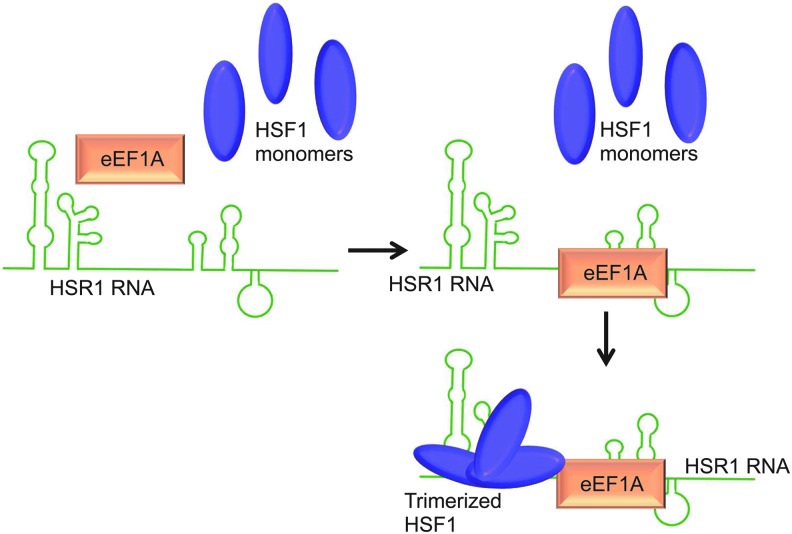
Heat shock RNA-1 (HSR1) is a noncoding RNA that stimulates the mammalian heat shock response. This response is a major cellular defense mechanism after cellular stress. During heat shock, rapid and substantial changes occur in the pattern of gene expression. HSR1 activates the heat shock transcription factor 1 (HSF1), which is essential for the induction of expression of heat shock proteins (HSPs) and other cytoprotective proteins [60]. HSR1 was identified in a screen seeking putative auxiliary factors involved in the activation of HSF1. A complex of HSF1 and elongation factor (eEF1A) coimmunoprecipitated from cell lysates. Initial in vitro studies seeking to activate HSF1 with eEF1A isolated from cells were unsuccessful.
Moreover, HSF1 binding to DNA was strongly inhibited in vitro by preincubation of the cell lysate with RNase A, which strongly pointed to the involvement of an RNA. HSR1 is ∼600 nucleotides in length, lacks a poly(A) tail, and acts together with eEF1A to activate transcription factor HSF1 [61]. Two domains near the 5′ terminus of HSR1 are essential for activation of HSF1 (Fig. 5). In vivo studies using vectors expressing siRNA against different domains of HSR1 supported previous findings: heat shock induction of HSF1 DNA-binding activity was severely impaired in siRNA-treated cells and not in controls [61]. It was hypothesized that the noncoding HSR1 RNA forms a complex with eEF1A that then binds and facilitates the assembly and stability of HSF1. HSR1 provides an example of a noncoding RNA that may be essential for the proper function of a TF. This makes HSR1 an interesting pharmacological target for various conditions associated with HSF1 activation, such as inflammation, ischemia/reperfusion, and cancer [61,62].
FIG. 5.
Heat shock RNA-1 (HSR1) mechanism of action. The HSR1-eEF1A complex is proposed to facilitate and stabilize the trimerization of HSF, which binds at the 5′ terminus of HSR1. HSR1-eEF1A complexes are necessary for activation of heat shock transcription factor 1 (HSF1).
Hepatitis delta virus
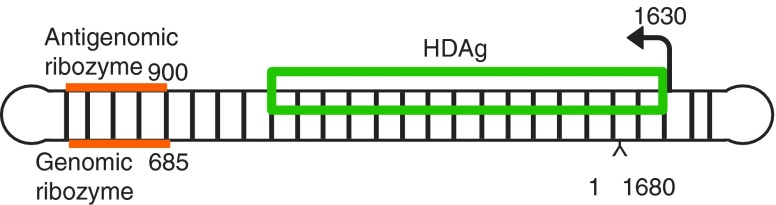
Hepatitis delta virus (HDV) provides a particularly remarkable example of double-stranded DNA mimicry by RNA. HDV is the smallest known human RNA pathogen. It is a defective virus that requires the hepatitis B virus envelop proteins for encapsidation and propagation [63–65]. This RNA folds on itself to form a rod-like structure that can be divided into two domains (Fig. 6) [64,66,67]. The left terminal domain includes both genomic and antigenomic self-cleaving RNA motifs. The right terminal domain contains a single open reading frame encoding two viral proteins, the small HDAg (HDAg-S) and the large HDAg (HDAg-L). Although these proteins are almost identical, each plays a distinct role. HDAg-S is essential for HDV replication, while the HDAg-L is necessary for virion assembly [66,68,69]. Replication of the HDV RNA apparently takes place in the nucleus of infected cells using a symmetrical rolling cycle mechanism, in which replication of the infectious circular RNA monomer produces linear, multimeric strands that are subsequently cleaved by endogenous ribozymes and ligated, yielding antigenomic circular monomers. These RNAs are then used as templates. The same three steps are repeated to generate genomic RNA progeny [70,71].
FIG. 6.
Schematic depiction of hepatitis delta virus genome. The delta ribozyme motifs (dRz, in orange) and their respective cleavage sites are indicated. The promoter on the genomic strand (indicated by the arrow) and the region decoding the HDAg mRNA is boxed (in green). This scheme was adapted from Kuo et al. [163].
As HDV does not encode its own RNA-dependent RNA polymerase (RNApol), a host DNA-dependent RNA polymerases (RNApol) must somehow be involved in the replication and transcription of HDV RNAs. In fact, RNA polymerase II (RNApol II) was reported to interact with HDV-derived RNAs at sites located within the terminal stem-loop domains [72,73]. Mutagenesis near the terminal loops of the rod affected both HDV accumulation in cells and RNApol II binding in vitro [73–75]. Furthermore, an RNA fragment derived from the right terminal stem-loop region of genomic HDV-RNA, including the site of HDAg mRNA transcription has been shown in several experiments to serve as a template for in vitro transcription. Using RNA affinity chromatography it was established that an RNAP II preinitiation complex forms on this promoter RNA, analogous to what is observed on double-stranded DNA promoters during transcription [75,76]. The crystal structure of purified RNApol II engaged in transcription of an HDV-RNA was solved. When superimposed with the structure of RNApol II engaging a DNA template, it was evident that both nucleic acids occupy the same site. These striking findings suggest that RNApol II recognizes the HDV RNA and normal DNA templates in a similar way [77]. RNApol has been found to engage RNA as template in other cases, including the peach latent mosaic viroid RNA genome [78,79] and endogenous bacterial 6S RNA [38]. Thus, certain RNA viruses have exploited the ability of special RNA structures to mimic double-stranded DNA with sufficient accuracy that they can recruit a host DNA-dependent RNA polymerase as their replicase.
7SK
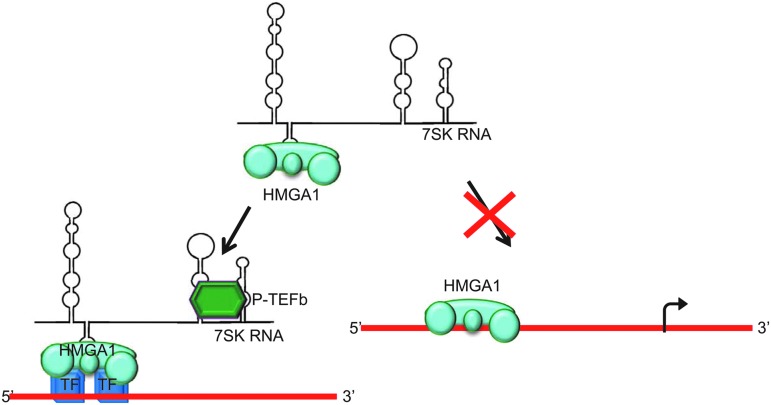
The nuclear noncoding 7SK RNA is a highly abundant RNA found in eukaryotic cells. 7SK RNA is believed to negatively regulate transcription elongation by inactivating the positive transcription elongation factor b (P-TEFb) [80–82]. In the past decade it has been reported that 7SK interacts with the high mobility group protein A1 (HMGA1) [83]. HMGA1 proteins are highly expressed during development, apparently influencing cell proliferation, embryonic cell growth, and cell differentiation [84–88].
HMGA proteins have three AT-hook DNA-binding motifs that allow them to preferentially bind in the minor groove of AT-rich, B-form DNA sequences [89]. HMGA1 proteins may facilitate gene transcription by strongly altering DNA structure, resulting in a more open chromatin state. These proteins also physically interact with many different TFs, orchestrating their assembly at promoter and enhancer regions [90–92]. The interaction of 7SK RNA and HMGA1 is reported to be highly specific. 7SK RNA interacts with the N-terminal domain of HMGA1, binding to an AT-hook motif through the second major hairpin of the RNA (loop 2) [83].
7SK RNA was shown to compete with DNA for HMGA1 binding, thus functioning as a competitive transcription regulator (Fig. 7) [83,93]. Overexpression of the 7SK loop 2 structure as a chimera with the Epstein–Barr virus EBER2 RNA was reported to alter gene expression in a manner similar to knockdown of HMGA1, supporting the apparent regulatory function of 7SK on HMGA1 [83].
FIG. 7.
Cellular function of 7SK-HMGA1 complex. 7SK RNA acts as a negative regulator of high mobility group protein A1 (HMGA1) DNA-binding, subsequently regulating HMGA1 target gene expression. Adapted from Benecke and Eilebrecht [93].
Mysteriously, HMGA1 proteins are frequently overexpressed in tumor cells, with HMGA1 expression levels often correlated with tumor malignancy. This has suggested that HMGA1 proteins might be targeted therapeutically [94,95]. The natural role of 7SK RNA as an HMGA1 antagonist suggests that this RNA might be manipulated for such therapeutic purposes. Finally, it has also been reported that HMGA1 proteins interact with other RNA molecules, including the transactivating response element in the nascent transcript of HIV-1 [96,97]. This observation emphasizes the promiscuity of HMGA1 as a dual DNA/RNA-binding protein, a recognized theme in biology [98].
p53
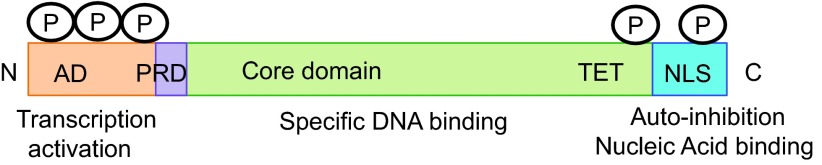
The p53 tumor suppressor protein plays a prominent role in cell growth, DNA repair, cell cycle arrest, and apoptosis. Mutation of p53 is among the most prevalent abnormalities in human cancer [99]. Although p53 is classified as a sequence-specific DNA-binding TF, it has also been reported to bind to RNA. Unlike many of the cases described above, interaction of p53 with RNA and DNA appears to be through two distinct binding domains [100]. The p53 core contains the DNA-binding domain, which recognizes the p53 consensus sequence in promoters of target genes (Fig. 8). RNA and single-stranded DNA, however, are recognized by the cationic C-terminus, which is involved in the regulation of p53 activity [100]. Posttranslational modification of the p53 C-terminus activates sequence-specific DNA binding by the DNA-binding domain, apparently by relieving autoinhibition [101]. Nucleic acid binding to the p53 C-terminus, therefore, has the potential to alter p53 function [102].
FIG. 8.
p53 protein biochemistry and putative regulatory interactions. p53 domains. AD, activation domain; PRD, proline-rich SH3-binding domain; TET, tetramerization domain; NLS, nuclear localization sequence; P, phosphorylation sites. Adapted from Cassiday and Maher [98].
The physiological significance of RNA-p53 interactions remains controversial. It has been shown that the unmodified cationic p53 C-terminus binds RNA strongly and with little sequence specificity both in vitro and in the yeast three-hybrid system [103]. In contrast, physiologically relevant posttranslational modification of the p53 C-terminal domain abrogates this nucleic acid binding [104,105]. Because different p53 isoforms are expressed under specific cell conditions, and posttranslational modifications are variable, it remains possible that RNA binding at the p53 C-terminus could have a rare physiological role. Emerging research into the function of long noncoding RNAs also suggests that these RNAs could play roles in fine-tuning p53 function [106].
RNAs That Regulate Activity of Nuclear Receptors
Nuclear receptor (NR) TFs respond to small molecule metabolites and fat-soluble compounds to regulate gene expression. They differ from other receptors in their unique ability to directly control gene expression through binding to genomic DNA, thus being classified as TFs [107,108]. NRs allow organisms to respond correctly to their environment by coordinating multicellular metabolism, development, reproduction, and homeostasis across diverse tissues [109]. NRs possess a conserved architecture and signaling mechanism. The DNA-binding domain contains two zinc fingers near the N-terminus, which contact the double-stranded DNA helix and form a dimerization interface. The ligand-binding domain resides at the C-terminus and engages cognate hormones as well as coactivators and corepressor complexes. NRs are classified based on their mechanism of action or homology. Class 1 receptors, such as the classical steroid receptors [eg, estrogen, androgen, and glucocorticoid receptors (GRs)], are activated by ligands, while the class 2 receptors [eg, peroxisome proliferator-activated receptors (PPARS), vitamin D receptor, and thyroid receptor] function as transcriptional activators in the presence of ligand, but as repressors in the absence of ligand [109,110].
In the past decade, an increasing number of noncoding RNAs with regulatory functions have been reported [111]. Two of these RNAs have been proposed as regulators of NRs, the growth arrest-specific 5 (Gas5) RNA, and the steroid receptor RNA activator (SRA).
Growth arrest-specific 5
Gas5 RNA was so named because it has been observed to accumulate in growth-arrested cells [112]. Surprisingly, Gas5 RNA has been reported to interact with the DNA-binding domain of the ligand-activated GR and suppress GR-induced transcription of endogenous glucocorticoid-responsive genes by competitive inhibition of GR binding to target glucocorticoid response elements (GREs) [113]. Glucocorticoids, a class of steroid hormones, serve as intracellular mediators that link systemic physiology to cellular activities [114].
Gas5 RNA apparently functions as a starvation-linked or growth arrest-linked riborepressor of GR. Gas5 RNA was identified by a LexA-based yeast two-hybrid screen with the Jurkat cell complementary DNA (cDNA) library, using the GR DNA binding domain (DBD) as bait. Increased association of GR and Gas5 RNA was observed by coimmunoprecipitation when HeLa cells were treated with dexamethasone, a GR agonist. Evidence of Gas5 RNA association with the GR DNA-binding domain came from results of experiments in which a GR chimera, G-gal-G, in which the DBD was replaced with that of the bacterial transcription factor GAL4. This chimera showed no interaction with Gas5 RNA [113]. Gas5 RNA is detected in the cytoplasm, but is more prominent in the nucleus [113,115].
Studies thus suggest that Gas5 RNA accumulates in growth-arrested cells in response to serum withdrawal, acting as a negative regulator of GR-induced transcription. Gas5 RNA is encoded by the Gas5 gene, which produces two mature, spliced forms of Gas5 RNA. The full-length Gas5b RNA is 630 nucleotides long and forms hairpin structures (Fig. 9A), apparently interacting with GR through a particular 3′ sequence. Full-length RNA and fragments containing nucleotides 400 to 598 reportedly bound GR in response to suppressed GR-induced transcriptional activity [113]. This region of Gas5 RNA contains two GRE-like sequences predicted to be base-paired within a hairpin structure (containing a G540 in the 5′ strand and a C554 in the 3′ strand). These RNA sequences resemble the consensus DNA GRE sequence and define affinity to the GR DNA-binding domain [113,116]. These Gas5 RNA GRE-like sequences were found to be necessary for binding to GR, with equilibrium dissociation constant estimates of ∼30 nM, tighter than those reported for GR binding to its cognate DNA (∼60 nM) [117]. Gas5 is thus mechanistically reminiscent of the bacterial 6S RNA, which has been documented to bind RNA polymerase and inhibit transcription by mimicking the open promoter to which RNA polymerase binds [118,119]. Gas5 is, therefore, another putative competitive inhibitor of a TF, apparently acting through RNA mimicry of double-stranded DNA. Particularly mysterious in this case is how the GRE-like sequences are recognized by GR in the context of the A-form helical structure of duplex RNA.
FIG. 9.
RNAs that regulate nuclear receptors. (A) Schematic secondary structure of growth arrest-specific 5 (Gas5) nucleotides 400–598. Gas5 (400–598) consists of six hairpin structures. Hairpin 5 is proposed to contain two glucocorticoid response element (GRE) sequences at nucleotides 539–544 and 553–559 (shown in red), which form a double-stranded hairpin structure that may mimic a GRE in dsDNA. Gas5 hairpin 3 is proposed to contain an MRE mimic (highlighted in purple at nucleotides 540–554). These residues are conserved among the consensus DNA GREs and are thought to be critical for interaction with residues of the glucocorticoid receptor DNA binding domain. Adapted from Kino [113]. (B) Three SRA1 complementary DNAs (cDNAs) sharing a central core region, but different in their 5′ and 3′ extremities and below a schematic representation of the proposed steroid receptor RNA activator (SRA) RNA secondary structures and assigned motifs. Adapted from Refs. [164,165].
Steroid receptor RNA activator
The steroid receptor coactivator 1 (SRC-1), as well as an RNA that increased the transcription activation of steroid receptors, were found when searching for coactivators of NRs using a yeast two-hybrid screening assay normally used to identify protein–protein interactions [120]. The unexpected RNA activator, termed SRA, appears to be transcriptional coactivator that acts in a manner selective for the amino-terminal transcription activation function (AF-1) of steroid receptors. It is expressed as multiple isoforms in a cell-specific manner [120]. The apparent transcriptional regulatory activity of SRA has been confirmed by overexpression in mammalian cells, where it is reported to enhance steroid receptor-mediated transactivation without significantly enhancing the levels of basal transcription from other promoters.
Treatment of cells with antisense oligodeoxyribonucleotides against SRA reportedly induced ∼70% reduction of the steroid-dependent transcription [120]. SRA differs from eukaryotic transcriptional activators in its ability to function as an RNA transcript that selectively regulates the activity of a family of transcriptional activators, existing in distinct ribonucleoprotein complexes. Thus SRA is apparently expressed in steroid target tissues and functions as a component of a large multiprotein complex to selectively enhance transcriptional activation in this context. SRA has several isoforms, all of them containing an identical core region of 687 nucleotides, but diverging in length and sequence in 5′ and 3′ regions. It has been proposed that SRA can serve as an organizing platform upon which relevant molecular components are assembled (Fig. 9B) [121]. In this regard, SRA is more a natural organizer of protein–protein interactions on DNA than an inhibitor of DNA–protein interactions.
Synthetic Anti-TF Aptamers
Natural examples of RNAs that function as mimics of DNA and inhibit TFs have inspired several research groups to use random RNA libraries and in vitro selection to seek engineered competitive TF inhibitors of this type. This work has led to the description of several unnatural RNA aptamers with potential for therapeutic control of gene expression (Table 2).
Table 2.
Selected RNAs That Regulate Transcription Factors
| RNA aptamer | Target | Potential application |
|---|---|---|
| Anti-NF-κB | Homodimer p50, heterodimer p50/p65 | Inhibitors of NF-κB |
| Anti-TBP | TBP, TBP•TATA | Tool to study protein–protein interactions and inhibition |
| Anti-HSF1 | HSF1 | Tool to understand transcriptional mechanisms and inhibitors |
| Anti-RUNX1 | RHD-CBFβ, Runt | Inhibitors |
CBF, core-binding factor; NF-κB, nuclear factor-kappaB; RHD, runt homology domain; RUNX1, runt-related transcription factor 1; TBP, TATA-binding protein.
Anti-nuclear factor-kappaB aptamers
In vitro selection was used to identify a high-affinity RNA aptamer specific for p50-containing forms of TF nuclear factor-kappaB (NF-κB). This TF is an important activator of genes involved in diverse biological activities, including cell proliferation, cell growth, resistance to apoptosis, and immune functions, such as inflammation and the synthesis of chemokines, interferons, MHC proteins, growth factors, and cell adhesion molecules [122]. NF-κB also plays a key role in the expression of HIV-1 genes after lymphocyte activation [123]. Inactive NF-κB protein is localized in the cytoplasm bound to inhibitor of kappaB (I-κB). Upon activation by I-κB phosphorylation, I-κB is ubiquitinated and degraded, releasing NF-κB to be translocated to the nucleus for activation of target gene expression.
With aims of generating a potential candidate for inhibition of NF-κB-dependent gene activation, a high-affinity 31-nucleotide RNA hairpin aptamer was identified as a subdomain of a larger RNA developed through in vitro selection [124]. In vitro and in vivo selections were then used to improve the affinity of anti-NF-κB for p502 and heterodimer p50/p65 [124–126]. Other selections have identified different RNA aptamers against p65 NF-κB subunits [127,128].
Anti-p50 RNA aptamers were found to bind with nanomolar affinity (Kd ∼ 1 nM) in a 1:2 RNA-to-p50 ratio in solution [129]. The RNA was found competent to strongly bind NF-κB p50 protein in vivo using the yeast three-hybrid system [125,126]. Interestingly, when the 2.45 Å resolution cocrystal structure of the NF-κB p50 Rel homology domain homodimer bound to a 29-nuclotide form of the anti-NF-κB RNA aptamer was solved, it was revealed that one RNA molecule binds identically to each of the p50 monomers of the homodimer, forming a RNA2:p502 complex [130]. Each RNA hairpin is folded into an irregular helix characterized by a series of unpredicted noncanonical base pairing and stacking interactions. The result is a wide major groove that perfectly complements the surface of the protein in size and shape. The most striking feature of the complex is that RNA mimics the DNA B form such that the chemistry of the core RNA/p50 RHR complex interface is essentially identical to that of the κB-DNA/p50 Rel homology domain interface. Thus, although the RNA aptamer bears no obvious sequence homology to κB-DNA (Fig. 10), it binds p50 with striking similarity to κB-DNA. It remains unknown whether there are natural RNA partners for NF-κB TFs. What is clear from the study of selected anti-NF-κB RNA aptamers is that there is no obstacle to identifying RNA ligands whose affinity for DNA-binding proteins rivals or exceeds that of the natural DNA partner.
FIG. 10.
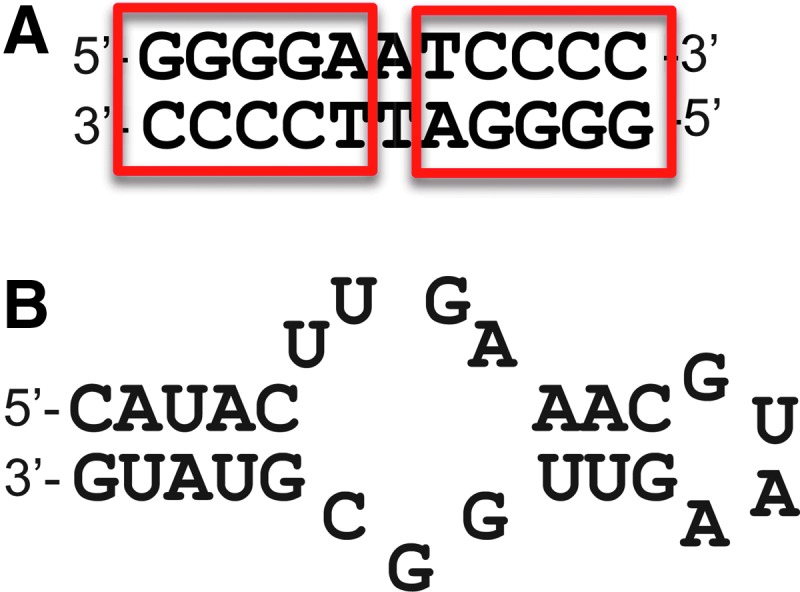
Nuclear factor-kappaB (NF-κB) interacting nucleic acids. (A) Example κB DNA sequence from the MHC1 class I gene promoter (red boxes illustrate DNA half-sites). (B) Sequence and secondary structure of the in vitro selected anti-NF-κB RNA aptamer that binds the NF-κB p50 subunit. Adapted from Ghosh et al. [166]. MHC1, major histocompatibility complex class 1.
Anti-TATA-binding protein
RNA aptamers against TATA-binding protein (TBP) have been described in two reports [131,132]. TBP is a critical basal TF that associates with the core promoter and acts with other factors to initiate gene transcription. TBP recruitment is the first and generally the rate-limiting step of transcription initiation by all three types of eukaryotic RNA polymerases [133,134]. TBP binds to the minor groove of the TATA box sequence [135,136].
In the first study [132], RNA aptamers against yeast TBP were identified by in vitro selection. After 11 selection cycles, no dominant consensus sequence was observed. Nonetheless, subsequent validation experiments suggested that the recovered RNAs specifically bound to TBP. Three aptamers were chosen for further affinity characterization by electrophoretic gel mobility shift assay. Equilibrium dissociation constants ranged between ∼3 and 10 nM. The aptamers were shown to compete with TBP for a TATA-binding sequence in double-stranded DNA, suggesting that the aptamers target the DNA-binding surface of TBP. The RNA aptamers were then incubated with multiple TBP•TATA-containing complexes to explore their disruptive activity. These data provided insight into the dynamics of TBP interactions during transcription reinitiation on a relevant kinetic time scale, and it was proposed that RNA aptamers could be used as tools to dissect transcription initiation mechanisms.
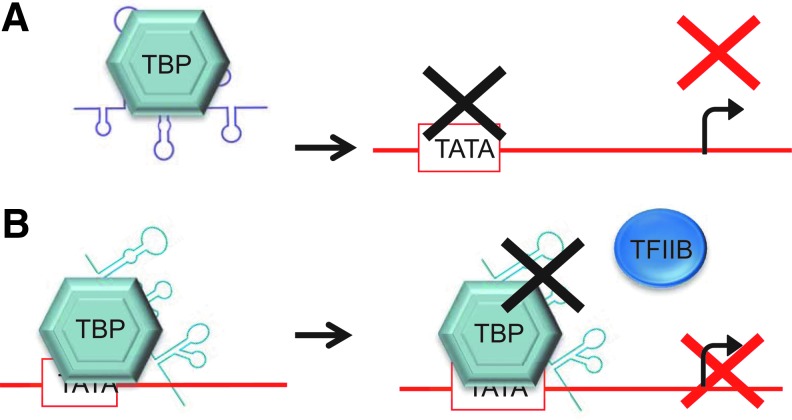
The second study [131] involved in vitro selections against Drosophila TBP in free and TATA-bound forms. Using RNA libraries from 4 cycles of selection against TBP (prior study), 12 further parallel selection rounds were performed against TBP or a TATA•TBP complex. Interestingly, no sequence was found in common between either the selected pools or the pool created in the first study. Aptamers from both selections were studied in transcription initiation assays in vitro. It was proposed that aptamers from selections against free TBP target the DNA-binding surface of TBP and inhibit TBP binding to TATA box. In contrast, aptamers from the TATA•TBP selection were proposed to bind other surfaces and disrupt TBP interactions with other factors, thus preventing formation of the transcription initiation complex (Fig. 11). No further characterization of anti-TBP aptamers has been reported. If issues of delivery or endogenous production of such agents could be resolved, it would be interesting to compare effects of aptamer inhibition of TBP with those obtained with small molecules such as tallimustine, which bind TA-rich DNA and inhibits its interaction with TBP [137].
FIG. 11.
Proposed inhibition mechanism for two anti-TATA-binding protein (TBP) RNA aptamers. (A) Aptamer selected against TBP DNA-binding site acts as a competitive inhibitor preventing transcription by sequestering TBP. (B) Aptamers selected against a TBP-TATA complex are proposed to bind the TBP surface, preventing its interaction with other factors, for example, TFIIB, hence suppressing transcription.
Anti-heat shock factor
RNA aptamers have been selected against heat shock factor (HSF) with the goal of dissecting transcription activation mechanisms in vivo and in vitro [138]. HSF is a highly conserved TF crucial for the stress response [139] and aging [140] of eukaryotic cells. HSF regulates genes involved in energy generation, signal transduction, vesicular transport, and chaperone function [141]. HSF functions as a homotrimer and has a highly conserved DNA-binding domain.
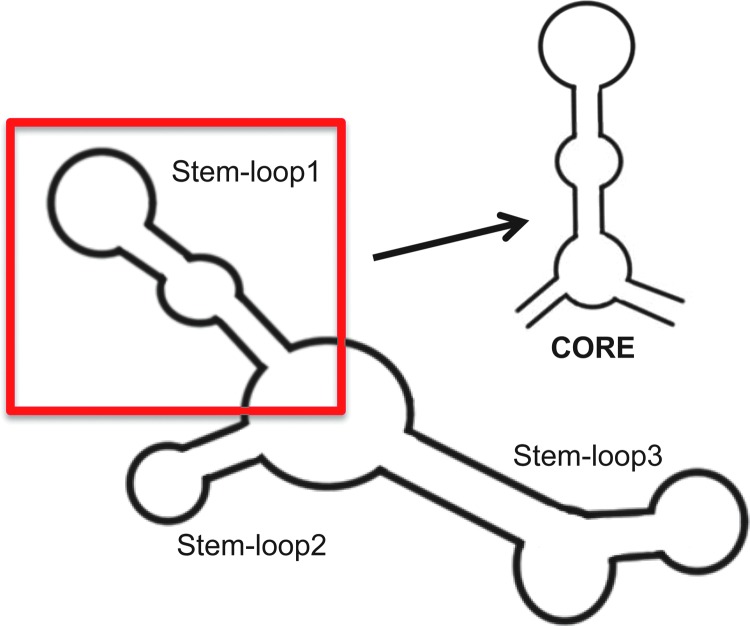
A dominant anti-HSF RNA aptamer was 90 nucleotides in length and secondary structure prediction suggested a three-way junction radiating three different stem-loops (Fig. 12). It was found that the minimal structure required for binding and inhibition was a 45-nucleotide RNA core. Full-length and core aptamers were characterized by equilibrium dissociation constants of 20–40 and 40–80 nM, respectively. Aptamer specificity was assessed by testing binding to other TF such as TBP, GAGA, and Gal4. Binding was not observed even at high protein concentrations. When exposed to lysates of SF9 cells that did and did not express HSF, aptamer complexes were only detected when HSF was present [138].
FIG. 12.
Schematic of anti-HSF1 secondary structure (in red) minimal structure required for binding and inhibition.
Clever in vivo studies were then designed using an aptamer expression system to rapidly generate high concentrations of a divalent version of anti-HSF, with a higher affinity for HSF (Kd∼8 nM). Endogenous expression during Drosophila development produced phenotypes that closely resembled abnormalities that occur when HSP activity is reduced, particularly a notched wing phenotype (observed in ∼90% of flies expressing the divalent anti-HSF1 aptamer construct). It was further shown that abnormal phenotypes caused by aptamer inhibition of HSF1 could be suppressed upon HSF1 overexpression. Thus, these experiments convincingly demonstrated that anti-HSF1 aptamers can prevent HSF1 from activating gene expression under normal and stress conditions [142]. Since it has been noted that downregulation of HSF activity sensitizes cancer cells to anticancer drugs [143], anti-HSF1 aptamers could have a potential value in cancer therapy.
Anti-runt-related transcription factor 1
RNA aptamers against the runt-related transcription factor 1 (RUNX1) TF have been reported. RUNX1 also known as acute myeloid leukemia 1 protein (AML1) is the α subunit of the core-binding factor (CBF) [144]. RUNX1 is one of the most important regulators of hematopoiesis, regulating transcription of a range of blood cell-specific genes [145,146]. RUNX1-deficient mice do not generate definitive hematopoietic cells and embryos die at developmental day 12 [147]. RUNX1 interacts with DNA through a 128 amino acid runt homology domain (RHD) localized at its N-terminus.
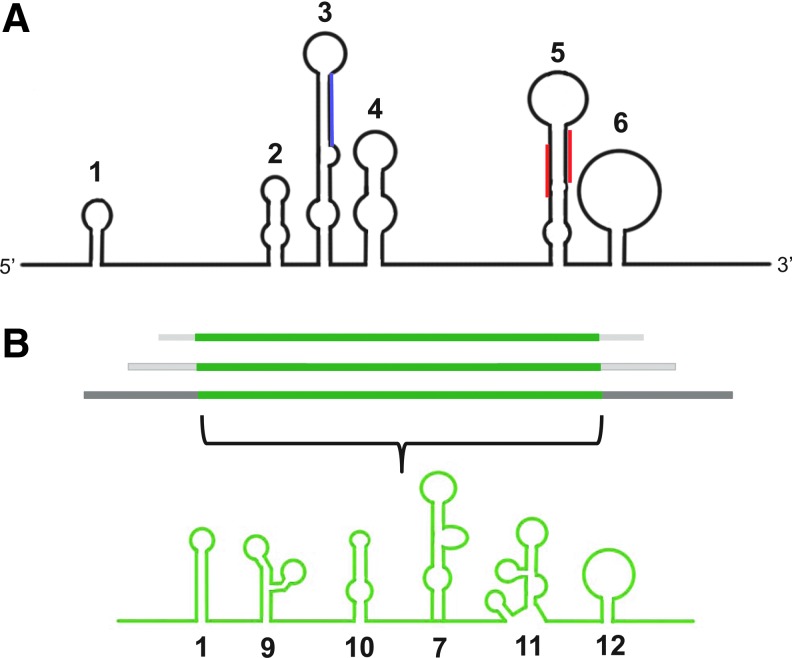
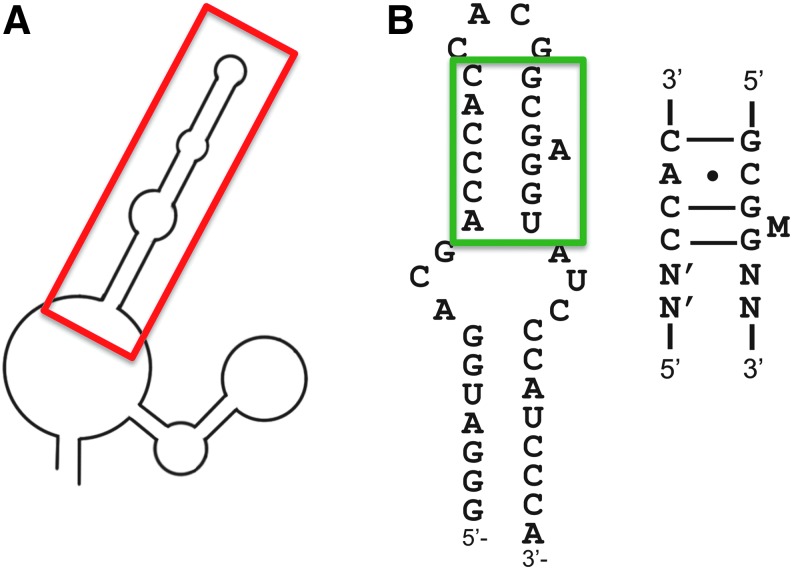
A 2009 report describes 2′-fluoro-pyrimidine-modified RNA aptamers selected against a recombinant RHD-CBFβ complex using a random library containing 50 random nucleotides [148]. After 10 cycles of selection, the authors found aptamers with affinity for their target (Kd ∼ 100 nM). Surprisingly, when the authors tested aptamers with or without 2′-fluoro-modified pyrimidines, similar affinities were observed, suggesting that these modified nucleotides did not contribute to aptamer structure or sequence recognition by the protein. Competition experiments confirmed that anti-RHD-CBFβ RNA aptamers interfere with the formation of DNA-RHD-CBFβ complexes. Secondary structure prediction for these aptamers suggested that they contain a 5′ stem-loop that is strongly protected by RHD-CBFβ in footprint assays [148]. This stem-loop structure proved to be sufficient to inhibit RHD-CBFβ binding to RNA (Fig. 13A). Further experiments demonstrated that the aptamer was specific for RHD by using antibodies against the N- and C-terminal regions of RUNX1 and demonstrating a super shift of the aptamer complex in electrophoretic gel mobility shift assays.
FIG. 13.
Anti-runt-related transcription factor 1 (RUNX1) aptamers. (A) Schematic of the secondary structure of a representative aptamer (minimal region for RUNX1 inhibition boxed in red). Adapted from Barton et al. [148]. (B) These proposed secondary structure of the minimal region hairpin (38 nucleotides, boxed in green) thought to mimic the Runt-binding double-stranded DNA element (RDE). Adapted from Fukunaga et al. [149].
RNA aptamers against Runt domain of RUNX1 were also described in a 2013 publication describing two distinct RNA libraries with 30 or 40 random positions [149]. After nine rounds of selection, all aptamers contained the conserved sequence motifs 5′CCAC3′ and 5′GCGMG3′ spaced by four to six nucleotides. The predicted secondary structures of these aptamers show a hairpin structure with internal loops. Enzymatic probing using single-strand-specific RNases corroborated this structure (Fig. 13B). A minimal structure of the main hairpin structure bound Runt with affinity similar to the full-length aptamer (equilibrium dissociation constant values of 1–3 nM). The NMR solution structure of a minimal aptamer of 22 nucleotides showed that the hairpin loop is contorted such that the motif contains an AH+-C mismatch and a base triple to adopt an unusual backbone structure that mimics the double-stranded DNA structure of the Runt recognition sequence [150]. A comparative study of RUNX1 bound to the aptamer motif and the runt domain corroborated these findings. The anti-RUNX1 RNA aptamer thus provides another remarkable example of the growing list of RNAs that mimic DNA architecture when binding to DNA-binding proteins.
Conclusion
TFs are often deranged in disease, making them attractive targets for therapy. TFs occur in lower concentrations than other targets and form focal points in deregulated pathways [6]. Inhibition of deregulated TFs might eventually be achieved utilizing RNA aptamer inhibitors.
In this study we have reviewed several examples of RNAs that modulate the activity of TFs in various contexts. Our survey began with natural RNAs that modulate TF activity either resulting in inhibition of transcription (5S RNA, 6S RNA, 3′ UTR of cad, TSU, 7SK, anti-p53 RNA, and Gas5) or in modulation of transcription by binding to TFs or polymerases (NRSE-RNA, HDV, and SRA). Although unrecognized initially, the increasing inventory of natural RNA partners for DNA-binding proteins, including TFs and polymerases, suggests that there may be another level of gene expression regulation yet to be characterized. It is interesting to note that several of the known TFs modulated by RNA are zinc finger proteins (TFIIIA, REST, STAT1, TRA1, WT1) [98]. The DNA-binding domains of these proteins are structurally conserved [151–155], and seem to have evolved variants selective for RNA or DNA recognition, with opportunity for promiscuity. Thus, we believe that it is likely that many coding and noncoding RNAs may play roles as TF decoys or adapters [156].
We next reviewed examples of several artificially selected RNA aptamers against TFs. Such aptamers have typically been selected with the goal of creating tools for understanding protein–protein and protein–DNA interactions, and with an eye toward potential therapeutic use (anti-NF-κB, anti-TBP, anti-HSF1, anti-RUNX). The reported aptamers typically have high target affinity, often higher than their affinity for a cognate DNA-binding sequence. The aptamers are also typically characterized by high specificity and distinguish between isoforms and other related proteins. Most anti-TF aptamers engage in mimicry of double-stranded DNA. These characteristics together with low immunogenicity, small size, ease of synthesis and purification, and facile modification have made RNA aptamers attractive leads for therapeutic concepts [157,158].
Although RNA aptamers present obvious challenges in drug delivery, they can be encoded by synthetic genes whose expression can be controlled. Maximizing aptamer expression from transgenes has been overcome in two ways. Expression can be increased by creating expression systems in which aptamer multimer transcripts self-cleave using punctuating ribozyme sequences [142,159], or by inserting self-splicing introns with aptamer sequences into every copy of the ∼150 highly expressed rRNA genes using a specific homing endonuclease. This approach may help deliver high concentrations of desired aptamer with minimal collateral disruption [160], but requires transgenic technology.
Therapeutic aptamer drugs have been slow to develop. While a number of aptamers have completed various stages of preclinical development, only one aptamer (targeting vascular endothelial growth factor) completed phase III clinical trials and is now marketed for the treatment of age-related macular degeneration [10,161]. The existence of natural RNA aptamers for TF and other DNA-binding proteins points the way to a potential future for unnatural RNA aptamers for research and therapy.
Acknowledgments
This work was supported by the Mayo Graduate School and the Mayo Foundation. E.M. was supported by a Mayo Graduate School Harry and Debra Stonecipher Predoctoral Fellowship.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Travers A. (1993). DNA-Protein Interactions. Chapman & Hall, London, p. 136 [Google Scholar]
- 2.Mitchell PJ. and Tjian R. (1989). Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 245:371–378 [DOI] [PubMed] [Google Scholar]
- 3.Hanna-Rose W. and Hansen U. (1996). Active repression mechanisms of eukaryotic transcription repressors. Trends Genet 12:229–234 [DOI] [PubMed] [Google Scholar]
- 4.Latchman DS. (1996). Inhibitory transcription factors. Int J Biochem Cell Biol 28:965–974 [DOI] [PubMed] [Google Scholar]
- 5.Latchman DS. (2000). Transcription factors as potential targets for therapeutic drugs. Curr Pharm Biotechnol 1:57–61 [DOI] [PubMed] [Google Scholar]
- 6.Darnell JE., Jr. (2002). Transcription factors as targets for cancer therapy. Nat Rev Cancer 2:740–749 [DOI] [PubMed] [Google Scholar]
- 7.Tuerk C. and Gold L. (1990). Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510 [DOI] [PubMed] [Google Scholar]
- 8.Ellington AD. and Szostak JW. (1990). In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822 [DOI] [PubMed] [Google Scholar]
- 9.Jellinek D, Green LS, Bell C, Lynott CK, Gill N, Vargeese C, Kirschenheuter G, McGee DP, Abesinghe P, et al. (1995). Potent 2′-amino-2′-deoxypyrimidine RNA inhibitors of basic fibroblast growth factor. Biochemistry 34:11363–11372 [DOI] [PubMed] [Google Scholar]
- 10.Pendergrast PS, Marsh HN, Grate D, Healy JM. and Stanton M. (2005). Nucleic acid aptamers for target validation and therapeutic applications. J Biomol Tech 16:224–234 [PMC free article] [PubMed] [Google Scholar]
- 11.Keefe AD, Pai S. and Ellington A. (2010). Aptamers as therapeutics. Nat Rev Drug Discov 9:537–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drolet DW, Jenison RD, Smith DE, Pratt D. and Hicke BJ. (1999). A high throughput platform for systematic evolution of ligands by exponential enrichment (SELEX). Comb Chem High Throughput Screen 2:271–278 [PubMed] [Google Scholar]
- 13.Roulet E, Busso S, Camargo AA, Simpson AJ, Mermod N. and Bucher P. (2002). High-throughput SELEX SAGE method for quantitative modeling of transcription-factor binding sites. Nat Biotechnol 20:831–835 [DOI] [PubMed] [Google Scholar]
- 14.Jolma A, Kivioja T, Toivonen J, Cheng L, Wei G, Enge M, Taipale M, Vaquerizas JM, Yan J, et al. (2010). Multiplexed massively parallel SELEX for characterization of human transcription factor binding specificities. Genome Res 20:861–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoon S, Zhou B, Janda KD, Brenner S. and Scolnick J. (2011). Aptamer selection by high-throughput sequencing and informatic analysis. Biotechniques 51:413–416 [DOI] [PubMed] [Google Scholar]
- 16.Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakama M, Zhang L, Kaneda Y, Ogihara T. and Dzau VJ. (1995). A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci U S A 92:5855–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mann MJ. and Dzau VJ. (2000). Therapeutic applications of transcription factor decoy oligonucleotides. J Clin Invest 106:1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storz G, Opdyke JA. and Wassarman KM. (2006). Regulating bacterial transcription with small RNAs. Cold Spring Harb Symp Quant Biol 71:269–273 [DOI] [PubMed] [Google Scholar]
- 19.Bogenhagen DF, Sakonju S. and Brown DD. (1980). A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3′ border of the region. Cell 19:27–35 [DOI] [PubMed] [Google Scholar]
- 20.Sakonju S, Bogenhagen DF. and Brown DD. (1980). A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5′ border of the region. Cell 19:13–25 [DOI] [PubMed] [Google Scholar]
- 21.Mattaj IW, Lienhard S, Zeller R. and DeRobertis EM. (1983). Nuclear exclusion of transcription factor IIIA and the 42s particle transfer RNA-binding protein in Xenopus oocytes: a possible mechanism for gene control? J Cell Biol 97:1261–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrana KE, Churchill ME, Tullius TD. and Brown DD. (1988). Mapping functional regions of transcription factor TFIIIA. Mol Cell Biol 8:1684–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picard B. and Wegnez M. (1979). Isolation of a 7S particle from Xenopus laevis oocytes: a 5S RNA-protein complex. Proc Natl Acad Sci U S A 76:241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelke DR, Ng SY, Shastry BS. and Roeder RG. (1980). Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell 19:717–728 [DOI] [PubMed] [Google Scholar]
- 25.Pelham HR. and Brown DD. (1980). A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A 77:4170–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honda BM. and Roeder RG. (1980). Association of a 5S gene transcription factor with 5S RNA and altered levels of the factor during cell differentiation. Cell 22:119–126 [DOI] [PubMed] [Google Scholar]
- 27.Clemens KR, Wolf V, McBryant SJ, Zhang P, Liao X, Wright PE. and Gottesfeld JM. (1993). Molecular basis for specific recognition of both RNA and DNA by a zinc finger protein. Science 260:530–533 [DOI] [PubMed] [Google Scholar]
- 28.Lu D, Searles MA. and Klug A. (2003). Crystal structure of a zinc-finger-RNA complex reveals two modes of molecular recognition. Nature 426:96–100 [DOI] [PubMed] [Google Scholar]
- 29.Lee HK, Choi YS, Park YA. and Jeong S. (2006). Modulation of oncogenic transcription and alternative splicing by beta-catenin and an RNA aptamer in colon cancer cells. Cancer Res 66:10560–10566 [DOI] [PubMed] [Google Scholar]
- 30.Storz G, Vogel J. and Wassarman KM. (2011). Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hindley J. (1967). Fractionation of 32P-labelled ribonucleic acids on polyacrylamide gels and their characterization by fingerprinting. J Mol Biol 30:125–136 [DOI] [PubMed] [Google Scholar]
- 32.Trotochaud AE. and Wassarman KM. (2005). A highly conserved 6S RNA structure is required for regulation of transcription. Nat Struct Mol Biol 12:313–319 [DOI] [PubMed] [Google Scholar]
- 33.Wassarman KM. (2007). 6S RNA: a small RNA regulator of transcription. Curr Opin Microbiol 10:164–168 [DOI] [PubMed] [Google Scholar]
- 34.Wassarman KM. and Storz G. (2000). 6S RNA regulates E. coli RNA polymerase activity. Cell 101:613–623 [DOI] [PubMed] [Google Scholar]
- 35.Neusser T, Gildehaus N, Wurm R. and Wagner R. (2008). Studies on the expression of 6S RNA from E. coli: involvement of regulators important for stress and growth adaptation. Biol Chem 389:285–297 [DOI] [PubMed] [Google Scholar]
- 36.Cavanagh AT, Klocko AD, Liu X. and Wassarman KM. (2008). Promoter specificity for 6S RNA regulation of transcription is determined by core promoter sequences and competition for region 4.2 of sigma70. Mol Microbiol 67:1242–1256 [DOI] [PubMed] [Google Scholar]
- 37.Gildehaus N, Neusser T, Wurm R. and Wagner R. (2007). Studies on the function of the riboregulator 6S RNA from E. coli: RNA polymerase binding, inhibition of in vitro transcription and synthesis of RNA-directed de novo transcripts. Nucleic Acids Res 35:1885–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassarman KM. and Saecker RM. (2006). Synthesis-mediated release of a small RNA inhibitor of RNA polymerase. Science 314:1601–1603 [DOI] [PubMed] [Google Scholar]
- 39.Berleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M. and Nusslein-Volhard C. (1988). The role of localization of bicoid RNA in organizing the anterior pattern of the Drosophila embryo. EMBO J 7:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Driever W. and Nusslein-Volhard C. (1988). The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54:95–104 [DOI] [PubMed] [Google Scholar]
- 41.Driever W. and Nusslein-Volhard C. (1988). A gradient of bicoid protein in Drosophila embryos. Cell 54:83–93 [DOI] [PubMed] [Google Scholar]
- 42.Driever W. and Nusslein-Volhard C. (1989). The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature 337:138–143 [DOI] [PubMed] [Google Scholar]
- 43.Struhl G, Struhl K. and Macdonald PM. (1989). The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell 57:1259–1273 [DOI] [PubMed] [Google Scholar]
- 44.Mlodzik M. and Gehring WJ. (1987). Expression of the caudal gene in the germ line of Drosophila: formation of an RNA and protein gradient during early embryogenesis. Cell 48:465–478 [DOI] [PubMed] [Google Scholar]
- 45.Dubnau J. and Struhl G. (1996). RNA recognition and translational regulation by a homeodomain protein. Nature 379:694–699 [DOI] [PubMed] [Google Scholar]
- 46.Rivera-Pomar R, Niessing D, Schmidt-Ott U, Gehring WJ. and Jackle H. (1996). RNA binding and translational suppression by bicoid. Nature 379:746–749 [DOI] [PubMed] [Google Scholar]
- 47.Peyman JA. (1999). Repression of major histocompatibility complex genes by a human trophoblast ribonucleic acid. Biol Reprod 60:23–31 [DOI] [PubMed] [Google Scholar]
- 48.Kuwabara T, Hsieh J, Nakashima K, Taira K. and Gage FH. (2004). A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 116:779–793 [DOI] [PubMed] [Google Scholar]
- 49.Schoenherr CJ. and Anderson DJ. (1995). The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267:1360–1363 [DOI] [PubMed] [Google Scholar]
- 50.Chong JA, Tapia-Ramirez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD. and Mandel G. (1995). REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80:949–957 [DOI] [PubMed] [Google Scholar]
- 51.Scholl T, Stevens MB, Mahanta S. and Strominger JL. (1996). A zinc finger protein that represses transcription of the human MHC class II gene, DPA. J Immunol 156:1448–1457 [PubMed] [Google Scholar]
- 52.Palm K, Belluardo N, Metsis M. and Timmusk T. (1998). Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci 18:1280–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson R, Teh CH, Kunarso G, Wong KY, Srinivasan G, Cooper ML, Volta M, Chan SS, Lipovich L, et al. (2008). REST regulates distinct transcriptional networks in embryonic and neural stem cells. PLoS Biol 6:e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B. and Buckley NJ. (2004). Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci U S A 101:10458–10463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koch JC, Barski E, Lingor P, Bahr M. and Michel U. (2011). Plasmids containing NRSE/RE1 sites enhance neurite outgrowth of retinal ganglion cells via sequestration of REST independent of NRSE dsRNA expression. FEBS J 278:3472–3483 [DOI] [PubMed] [Google Scholar]
- 56.Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV. and Zukin RS. (2003). Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci 23:2112–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burgess R, Jenkins R. and Zhang Z. (2008). Epigenetic changes in gliomas. Cancer Biol Ther 7:1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Formisano L, Noh KM, Miyawaki T, Mashiko T, Bennett MV. and Zukin RS. (2007). Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A 104:4170–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buckley NJ, Johnson R, Zuccato C, Bithell A. and Cattaneo E. (2010). The role of REST in transcriptional and epigenetic dysregulation in Huntington's disease. Neurobiol Dis 39:28–39 [DOI] [PubMed] [Google Scholar]
- 60.Sarge KD, Zimarino V, Holm K, Wu C. and Morimoto RI. (1991). Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev 5:1902–1911 [DOI] [PubMed] [Google Scholar]
- 61.Shamovsky I, Ivannikov M, Kandel ES, Gershon D. and Nudler E. (2006). RNA-mediated response to heat shock in mammalian cells. Nature 440:556–560 [DOI] [PubMed] [Google Scholar]
- 62.Westerheide SD. and Morimoto RI. (2005). Heat shock response modulators as therapeutic tools for diseases of protein conformation. J Biol Chem 280:33097–33100 [DOI] [PubMed] [Google Scholar]
- 63.Rizzetto M, Canese MG, Arico S, Crivelli O, Trepo C, Bonino F. and Verme G. (1977). Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 18:997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai MM. (2005). RNA replication without RNA-dependent RNA polymerase: surprises from hepatitis delta virus. J Virol 79:7951–7958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor JM. (2006). Hepatitis delta virus. Virology 344:71–76 [DOI] [PubMed] [Google Scholar]
- 66.Chen PJ, Kalpana G, Goldberg J, Mason W, Werner B, Gerin J. and Taylor J. (1986). Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A 83:8774–8778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tseng CH. and Lai MM. (2009). Hepatitis delta virus RNA replication. Viruses 1:818–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chao M, Hsieh SY. and Taylor J. (1990). Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol 64:5066–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee CZ, Chen PJ. and Chen DS. (1995). Large hepatitis delta antigen in packaging and replication inhibition: role of the carboxyl-terminal 19 amino acids and amino-terminal sequences. J Virol 69:5332–5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor JM. (2009). Chapter 3. Replication of the hepatitis delta virus RNA genome. Adv Virus Res 74:103–121 [DOI] [PubMed] [Google Scholar]
- 71.Yasnee B. and Pelchat M. (2009). Subversion of RNA processing pathways by the Hepatitis delta Virus. In: RNA Processing. Grabowski Paula, ed. pp. 101–106. http://cdn.intechopen.com/pdfs-wm/18458.pdf
- 72.Chang FL, Chen PJ, Tu SJ, Wang CJ. and Chen DS. (1991). The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc Natl Acad Sci U S A 88:8490–8494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greco-Stewart VS, Miron P, Abrahem A. and Pelchat M. (2007). The human RNA polymerase II interacts with the terminal stem-loop regions of the hepatitis delta virus RNA genome. Virology 357:68–78 [DOI] [PubMed] [Google Scholar]
- 74.Beard MR, MacNaughton TB. and Gowans EJ. (1996). Identification and characterization of a hepatitis delta virus RNA transcriptional promoter. J Virol 70:4986–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abrahem A. and Pelchat M. (2008). Formation of an RNA polymerase II preinitiation complex on an RNA promoter derived from the hepatitis delta virus RNA genome. Nucleic Acids Res 36:5201–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yudkovsky N, Ranish JA. and Hahn S. (2000). A transcription reinitiation intermediate that is stabilized by activator. Nature 408:225–229 [DOI] [PubMed] [Google Scholar]
- 77.Lehmann E, Brueckner F. and Cramer P. (2007). Molecular basis of RNA-dependent RNA polymerase II activity. Nature 450:445–449 [DOI] [PubMed] [Google Scholar]
- 78.Pelchat M, Grenier C. and Perreault JP. (2002). Characterization of a viroid-derived RNA promoter for the DNA-dependent RNA polymerase from Escherichia coli. Biochemistry 41:6561–6571 [DOI] [PubMed] [Google Scholar]
- 79.Pelchat M. and Perreault JP. (2004). Binding site of Escherichia coli RNA polymerase to an RNA promoter. Biochem Biophys Res Commun 319:636–642 [DOI] [PubMed] [Google Scholar]
- 80.Zhou Q, Li T. and Price DH. (2012). RNA polymerase II elongation control. Annu Rev Biochem 81:119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Z, Zhu Q, Luo K. and Zhou Q. (2001). The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317–322 [DOI] [PubMed] [Google Scholar]
- 82.Nguyen VT, Kiss T, Michels AA. and Bensaude O. (2001). 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414:322–325 [DOI] [PubMed] [Google Scholar]
- 83.Eilebrecht S, Brysbaert G, Wegert T, Urlaub H, Benecke BJ. and Benecke A. (2011). 7SK small nuclear RNA directly affects HMGA1 function in transcription regulation. Nucleic Acids Res 39:2057–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melillo RM, Pierantoni GM, Scala S, Battista S, Fedele M, Stella A, De Biasio MC, Chiappetta G, Fidanza V, et al. (2001). Critical role of the HMGI(Y) proteins in adipocytic cell growth and differentiation. Mol Cell Biol 21:2485–2495 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Lundberg K, Karlson JR, Ingebrigtsen K, Holtlund J, Lund T. and Laland SG. (1989). On the presence of the chromosomal proteins HMG I and HMG Y in rat organs. Biochim Biophys Acta 1009:277–279 [DOI] [PubMed] [Google Scholar]
- 86.Chiappetta G, Avantaggiato V, Visconti R, Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti V, et al. (1996). High level expression of the HMGI (Y) gene during embryonic development. Oncogene 13:2439–2446 [PubMed] [Google Scholar]
- 87.Bustin M. and Reeves R. (1996). High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acid Res Mol Biol 54:35–100 [DOI] [PubMed] [Google Scholar]
- 88.Beaujean N, Bouniol-Baly C, Monod C, Kissa K, Jullien D, Aulner N, Amirand C, Debey P. and Kas E. (2000). Induction of early transcription in one-cell mouse embryos by microinjection of the nonhistone chromosomal protein HMG-I. Dev Biol 221:337–354 [DOI] [PubMed] [Google Scholar]
- 89.Huth JR, Bewley CA, Nissen MS, Evans JN, Reeves R, Gronenborn AM. and Clore GM. (1997). The solution structure of an HMG-I(Y)-DNA complex defines a new architectural minor groove binding motif. Nat Struct Biol 4:657–665 [DOI] [PubMed] [Google Scholar]
- 90.Zhang XM. and Verdine GL. (1999). A small region in HMG I(Y) is critical for cooperation with NF-kappaB on DNA. J Biol Chem 274:20235–20243 [DOI] [PubMed] [Google Scholar]
- 91.Yie J, Liang S, Merika M. and Thanos D. (1997). Intra- and intermolecular cooperative binding of high-mobility-group protein I(Y) to the beta-interferon promoter. Mol Cell Biol 17:3649–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Currie RA. (1997). Functional interaction between the DNA binding subunit trimerization domain of NF-Y and the high mobility group protein HMG-I(Y). J Biol Chem 272:30880–30888 [DOI] [PubMed] [Google Scholar]
- 93.Benecke AG. and Eilebrecht S. (2015). RNA-mediated regulation of HMGA1 function. Biomolecules 5:943–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wood LJ, Mukherjee M, Dolde CE, Xu Y, Maher JF, Bunton TE, Williams JB. and Resar LM. (2000). HMG-I/Y, a new c-Myc target gene and potential oncogene. Mol Cell Biol 20:5490–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huso TH. and Resar LM. (2014). The high mobility group A1 molecular switch: turning on cancer—can we turn it off? Expert Opin Ther Targets 18:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eilebrecht S, Wilhelm E, Benecke BJ, Bell B. and Benecke AG. (2013). HMGA1 directly interacts with TAR to modulate basal and Tat-dependent HIV transcription. RNA Biol 10:436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eilebrecht S, Becavin C, Leger H, Benecke BJ. and Benecke A. (2011). HMGA1-dependent and independent 7SK RNA gene regulatory activity. RNA Biol 8:143–157 [DOI] [PubMed] [Google Scholar]
- 98.Cassiday LA. and Maher LJ., III (2002). Having it both ways: transcription factors that bind DNA and RNA. Nucleic Acids Res 30:4118–4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prives C. and Hall PA. (1999). The p53 pathway. J Pathol 187:112–126 [DOI] [PubMed] [Google Scholar]
- 100.Ahn J. and Prives C. (2001). The C-terminus of p53: the more you learn the less you know. Nat Struct Biol 8:730–732 [DOI] [PubMed] [Google Scholar]
- 101.Hupp TR, Meek DW, Midgley CA. and Lane DP. (1992). Regulation of the specific DNA binding function of p53. Cell 71:875–886 [DOI] [PubMed] [Google Scholar]
- 102.Anderson ME, Woelker B, Reed M, Wang P. and Tegtmeyer P. (1997). Reciprocal interference between the sequence-specific core and nonspecific C-terminal DNA binding domains of p53: implications for regulation. Mol Cell Biol 17:6255–6264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riley KJ, Cassiday LA, Kumar and A.Maher LJ., III (2006). Recognition of RNA by the p53 tumor suppressor protein in the yeast three-hybrid system. RNA 12:620–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Riley KJ, Ramirez-Alvarado M. andMaher LJ., III (2007). RNA-p53 interactions in vitro. Biochemistry 46:2480–2487 [DOI] [PubMed] [Google Scholar]
- 105.Riley KJ. and Maher LJ., III (2007). p53 RNA interactions: new clues in an old mystery. RNA 13:1825–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grossi E, Sanchez Y. and Huarte. M. (2015). Expanding the p53 regulatory network: lncRNAs take up the challenge. Biochim Biophys Acta DOI: 10.1016/j.bbagrm.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 107.Evans RM. (1988). The steroid and thyroid hormone receptor superfamily. Science 240:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olefsky JM. (2001). Nuclear receptor minireview series. J Biol Chem 276:36863–36864 [DOI] [PubMed] [Google Scholar]
- 109.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, et al. (1995). The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Novac N. and Heinzel T. (2004). Nuclear receptors: overview and classification. Curr Drug Targets Inflamm Allergy 3:335–346 [DOI] [PubMed] [Google Scholar]
- 111.Mattick JS. (2005). The functional genomics of noncoding RNA. Science 309:1527–1528 [DOI] [PubMed] [Google Scholar]
- 112.Schneider C, King RM. and Philipson L. (1988). Genes specifically expressed at growth arrest of mammalian cells. Cell 54:787–793 [DOI] [PubMed] [Google Scholar]
- 113.Kino T, Hurt DE, Ichijo T, Nader N. and Chrousos. GP. (2010). Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal 3:ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Charmandari E, Kino T. and Chrousos GP. (2004). Glucocorticoids and their actions: an introduction. Ann N Y Acad Sci 1024:1–8 [DOI] [PubMed] [Google Scholar]
- 115.Coccia EM, Cicala C, Charlesworth A, Ciccarelli C, Rossi GB, Philipson L. and Sorrentino V. (1992). Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol 12:3514–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dimitrov RA. and Zuker M. (2004). Prediction of hybridization and melting for double-stranded nucleic acids. Biophys J 87:215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rundlett SE. and Miesfeld RL. (1995). Quantitative differences in androgen and glucocorticoid receptor DNA binding properties contribute to receptor-selective transcriptional regulation. Mol Cell Endocrinol 109:1–10 [DOI] [PubMed] [Google Scholar]
- 118.Barrandon C, Spiluttini B. and Bensaude O. (2008). Non-coding RNAs regulating the transcriptional machinery. Biol Cell 100:83–95 [DOI] [PubMed] [Google Scholar]
- 119.Willkomm DK. and Hartmann RK. (2005). 6S RNA—an ancient regulator of bacterial RNA polymerase rediscovered. Biol Chem 386:1273–1277 [DOI] [PubMed] [Google Scholar]
- 120.Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, Tsai MJ. and O'Malley BW. (1999). A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 97:17–27 [DOI] [PubMed] [Google Scholar]
- 121.Beato M. and Vicent GP. (2013). A new role for an old player: steroid receptor RNA activator (SRA) represses hormone inducible genes. Transcription 4:167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D. and Miyamoto S. (1995). Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev 9:2723–2735 [DOI] [PubMed] [Google Scholar]
- 123.Nabel G. and Baltimore D. (1987). An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711–713 [DOI] [PubMed] [Google Scholar]
- 124.Lebruska LL. and Maher LJ., III (1999). Selection and characterization of an RNA decoy for transcription factor NF-kappa B. Biochemistry 38:3168–3174 [DOI] [PubMed] [Google Scholar]
- 125.Cassiday LA. and Maher LJ., III (2001). In vivo recognition of an RNA aptamer by its transcription factor target. Biochemistry 40:2433–2438 [DOI] [PubMed] [Google Scholar]
- 126.Cassiday LA. and Maher LJ., III (2003). Yeast genetic selections to optimize RNA decoys for transcription factor NF-kappa B. Proc Natl Acad Sci U S A 100:3930–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wurster SE, Bida JP, Her YF. and Maher LJ., III (2009). Characterization of anti-NF-kappaB RNA aptamer-binding specificity in vitro and in the yeast three-hybrid system. Nucleic Acids Res 37:6214–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wurster SE. and Maher LJ., III (2008). Selection and characterization of anti-NF-kappaB p65 RNA aptamers. RNA 14:1037–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cassiday LA, Lebruska LL, Benson LM, Naylor S, Owen WG. and Maher LJ., III (2002). Binding stoichiometry of an RNA aptamer and its transcription factor target. Anal Biochem 306:290–297 [DOI] [PubMed] [Google Scholar]
- 130.Huang DB, Vu D, Cassiday LA, Zimmerman JM, Maher LJ, III, and Ghosh G. (2003). Crystal structure of NF-kappaB (p50)2 complexed to a high-affinity RNA aptamer. Proc Natl Acad Sci U S A 100:9268–9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hohmura KI, Shi H. and Hirayoshi K. (2013). Perturbation of discrete sites on a single protein domain with RNA aptamers: targeting of different sides of the TATA-binding protein (TBP). Biosci Biotechnol Biochem 77:1739–1746 [DOI] [PubMed] [Google Scholar]
- 132.Fan X, Shi H, Adelman K. and Lis JT. (2004). Probing TBP interactions in transcription initiation and reinitiation with RNA aptamers that act in distinct modes. Proc Natl Acad Sci U S A 101:6934–6939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buratowski S, Hahn S, Guarente L. and Sharp PA. (1989). Five intermediate complexes in transcription initiation by RNA polymerase II. Cell 56:549–561 [DOI] [PubMed] [Google Scholar]
- 134.Pugh BF. (2000). Control of gene expression through regulation of the TATA-binding protein. Gene 255:1–14 [DOI] [PubMed] [Google Scholar]
- 135.Nikolov DB, Hu SH, Lin J, Gasch A, Hoffmann A, Horikoshi M, Chua NH, Roeder RG. and Burley SK. (1992). Crystal structure of TFIID TATA-box binding protein. Nature 360:40–46 [DOI] [PubMed] [Google Scholar]
- 136.Chasman DI, Flaherty KM, Sharp PA. and Kornberg RD. (1993). Crystal structure of yeast TATA-binding protein and model for interaction with DNA. Proc Natl Acad Sci U S A 90:8174–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cozzi P. (2003). The discovery of a new potential anticancer drug: a case history. Farmaco 58:213–220 [DOI] [PubMed] [Google Scholar]
- 138.Zhao X, Shi H, Sevilimedu A, Liachko N, Nelson HC. and Lis JT. (2006). An RNA aptamer that interferes with the DNA binding of the HSF transcription activator. Nucleic Acids Res 34:3755–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sorger PK. and Pelham HR. (1988). Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54:855–864 [DOI] [PubMed] [Google Scholar]
- 140.Verbeke P, Fonager J, Clark BF. and Rattan SI. (2001). Heat shock response and ageing: mechanisms and applications. Cell Biol Int 25:845–857 [DOI] [PubMed] [Google Scholar]
- 141.Hahn JS, Hu Z, Thiele DJ. and Iyer VR. (2004). Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol 24:5249–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salamanca HH, Fuda N, Shi H. and Lis JT. (2011). An RNA aptamer perturbs heat shock transcription factor activity in Drosophila melanogaster. Nucleic Acids Res 39:6729–6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zaarur N, Gabai VL, Porco JA, Jr., Calderwood S. and Sherman MY. (2006). Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res 66:1783–1791 [DOI] [PubMed] [Google Scholar]
- 144.de Bruijn MF. and Speck NA. (2004). Core-binding factors in hematopoiesis and immune function. Oncogene 23:4238–4248 [DOI] [PubMed] [Google Scholar]
- 145.Speck NA. (2001). Core binding factor and its role in normal hematopoietic development. Curr Opin Hematol 8:192–196 [DOI] [PubMed] [Google Scholar]
- 146.Mikhail FM, Sinha KK, Saunthararajah Y. and Nucifora G. (2006). Normal and transforming functions of RUNX1: a perspective. J Cell Physiol 207:582–593 [DOI] [PubMed] [Google Scholar]
- 147.Okuda T, van Deursen J, Hiebert SW, Grosveld G. and Downing JR. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84:321–330 [DOI] [PubMed] [Google Scholar]
- 148.Barton JL, Bunka DH, Knowling SE, Lefevre P, Warren AJ, Bonifer C. and Stockley PG. (2009). Characterization of RNA aptamers that disrupt the RUNX1-CBFbeta/DNA complex. Nucleic Acids Res 37:6818–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fukunaga J, Nomura Y, Tanaka Y, Amano R, Tanaka T, Nakamura Y, Kawai G, Sakamoto T. and Kozu T. (2013). The Runt domain of AML1 (RUNX1) binds a sequence-conserved RNA motif that mimics a DNA element. RNA 19:927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nomura Y, Tanaka Y, Fukunaga J, Fujiwara K, Chiba M, Iibuchi H, Tanaka T, Nakamura Y, Kawai G, et al. (2013). Solution structure of a DNA mimicking motif of an RNA aptamer against transcription factor AML1 Runt domain. J Biochem 154:513–519 [DOI] [PubMed] [Google Scholar]
- 151.Wolfe SA, Zhou P, Dotsch V, Chen L, You A, Ho SN, Crabtree GR, Wagner G. and Verdine GL. (1997). Unusual Rel-like architecture in the DNA-binding domain of the transcription factor NFATc. Nature 385:172–176 [DOI] [PubMed] [Google Scholar]
- 152.Jin L, Sliz P, Chen L, Macian F, Rao A, Hogan PG. and Harrison SC. (2003). An asymmetric NFAT1 dimer on a pseudo-palindromic kappa B-like DNA site. Nat Struct Biol 10:807–811 [DOI] [PubMed] [Google Scholar]
- 153.Cho Y, Gorina S, Jeffrey PD. and Pavletich NP. (1994). Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science 265:346–355 [DOI] [PubMed] [Google Scholar]
- 154.Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell JE, Jr., and Kuriyan J. (1998). Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell 93:827–839 [DOI] [PubMed] [Google Scholar]
- 155.Becker S, Groner B. and Muller CW. (1998). Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature 394:145–151 [DOI] [PubMed] [Google Scholar]
- 156.Wang KC. and Chang HY. (2011). Molecular mechanisms of long noncoding RNAs. Mol Cell 43:904–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wlotzka B, Leva S, Eschgfaller B, Burmeister J, Kleinjung F, Kaduk C, Muhn P, Hess-Stumpp H. and Klussmann S. (2002). In vivo properties of an anti-GnRH Spiegelmer: an example of an oligonucleotide-based therapeutic substance class. Proc Natl Acad Sci U S A 99:8898–8902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.White RR, Sullenger BA. and Rusconi CP. (2000). Developing aptamers into therapeutics. J Clin Invest 106:929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shi H, Hoffman BE. and Lis JT. (1999). RNA aptamers as effective protein antagonists in a multicellular organism. Proc Natl Acad Sci U S A 96:10033–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang S, Zhao X, Suran R, Vogt VM, Lis JT. and Shi H. (2010). Knocking down gene function with an RNA aptamer expressed as part of an intron. Nucleic Acids Res 38:e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ng EW, Shima DT, Calias P, Cunningham ET, Jr., Guyer DR. and Adamis AP. (2006). Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov 5:123–132 [DOI] [PubMed] [Google Scholar]
- 162.Romby P, Baudin F, Brunel C, Leal de Stevenson I, Westhof E, Romaniuk PJ, Ehresmann C. and Ehresmann B. (1990). Ribosomal 5S RNA from Xenopus laevis oocytes: conformation and interaction with transcription factor IIIA. Biochimie 72:437–452 [DOI] [PubMed] [Google Scholar]
- 163.Kuo MY, Sharmeen L, Dinter-Gottlieb G. and Taylor J. (1988). Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol 62:4439–4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hube F, Guo J, Chooniedass-Kothari S, Cooper C, Hamedani MK, Dibrov AA, Blanchard AA, Wang X, Deng G, et al. (2006). Alternative splicing of the first intron of the steroid receptor RNA activator (SRA) participates in the generation of coding and noncoding RNA isoforms in breast cancer cell lines. DNA Cell Biol 25:418–428 [DOI] [PubMed] [Google Scholar]
- 165.Lanz RB, Razani B, Goldberg AD. and O'Malley BW. (2002). Distinct RNA motifs are important for coactivation of steroid hormone receptors by steroid receptor RNA activator (SRA). Proc Natl Acad Sci U S A 99:16081–16086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ghosh G, Huang DB. and Huxford T. (2004). Molecular mimicry of the NF-kappaB DNA target site by a selected RNA aptamer. Curr Opin Struct Biol 14:21–27 [DOI] [PubMed] [Google Scholar]