Summary
Background
The zoonotic parasite Plasmodium knowlesi has become the most common cause of human malaria in Malaysia and is present throughout much of southeast Asia. No randomised controlled trials have been done to identify the optimum treatment for this emerging infection. We aimed to compare artesunate–mefloquine with chloroquine to define the optimum treatment for uncomplicated P knowlesi malaria in adults and children.
Methods
We did this open-label, randomised controlled trial at three district hospitals in Sabah, Malaysia. Patients aged 1 year or older with uncomplicated P knowlesi malaria were randomly assigned, via computer-generated block randomisation (block sizes of 20), to receive oral artesunate–mefloquine (target dose 12 mg/kg artesunate and 25 mg/kg mefloquine) or chloroquine (target dose 25 mg/kg). Research nursing staff were aware of group allocation, but allocation was concealed from the microscopists responsible for determination of the primary endpoint, and study participants were not aware of drug allocation. The primary endpoint was parasite clearance at 24 h. Analysis was by modified intention to treat. This study is registered with ClinicalTrials.gov, number NCT01708876.
Findings
Between Oct 16, 2012, and Dec 13, 2014, we randomly assigned 252 patients to receive either artesunate–mefloquine (n=127) or chloroquine (n=125); 226 (90%) patients comprised the modified intention-to-treat population. 24 h after treatment, we recorded parasite clearance in 97 (84% [95% CI 76–91]) of 115 patients in the artesunate–mefloquine group versus 61 (55% [45–64]) of 111 patients in the chloroquine group (difference in proportion 29% [95% CI 18·0–40·8]; p<0·0001). Parasite clearance was faster in patients given artesunate–mefloquine than in those given chloroquine (18·0 h [range 6·0–48·0] vs 24·0 h [6·0–60·0]; p<0·0001), with faster clearance of ring stages in the artesunate–mefloquine group (mean time to 50% clearance of baseline parasites 8·6 h [95% CI 7·9–9·4] vs 13·8 h [12·1–15·4]; p<0·0001). Risk of anaemia within 28 days was lower in patients in the artesunate–mefloquine group (71 [62%; 95% CI 52·2–70·6]) than in those in the chloroquine group (83 [75%; 65·6–82·5]; p=0·035). Gametocytaemia as detected by PCR for pks25 was present in 44 (86%) of 51 patients in the artesunate–mefloquine group and 41 (84%) of 49 patients in the chloroquine group at baseline, and in three (6%) of 49 patients and two (4%) of 48 patients, respectively, at day 7. Fever clearance was faster in the artesunate–mefloquine group (mean 11·5 h [95% CI 8·3–14·6]) than in the chloroquine group (14·8 h [11·7–17·8]; p=0·034). Bed occupancy was 2426 days per 1000 patients in the artesunate–mefloquine group versus 2828 days per 1000 patients in the chloroquine group (incidence rate ratio 0·858 [95% CI 0·812–0·906]; p<0·0001). One (<1%) patient in the artesunate–mefloquine group had a serious neuropsychiatric event regarded as probably related to study drug.
Interpretation
Artesunate–mefloquine is highly efficacious for treatment of uncomplicated P knowlesi malaria. The rapid therapeutic response of the drug offers significant advantages compared with chloroquine monotherapy and supports a unified treatment policy for artemisinin-based combination therapy for all Plasmodium species in co-endemic areas.
Funding
Malaysian Ministry of Health, Australian National Health and Medical Research Council, and Asia Pacific Malaria Elimination Network.
Introduction
Zoonotic infection with the simian parasite Plasmodium knowlesi has become the most common cause of human malaria in Malaysia,1,2 with cases increasingly being reported throughout most of southeast Asia.3 Microscopic diagnosis remains problematic, with P knowlesi indistinguishable from Plasmodium malariae and frequently confused with Plasmodium falciparum because of morphological similarities in ring stages;3 misreporting as Plasmodium vivax is also an issue.1,4,5 The implications of misdiagnosis are serious, because, unlike P malariae, knowlesi malaria has a 24 h lifecycle and hyperparasitaemia can occur rapidly with severe complications and fatal outcomes.6,7 Chloroquine-resistant P falciparum8 and P vivax9 are co-endemic in Malaysia, further complicating the choice of antimalarial drugs, with potential serious complications associated with inadvertent chloroquine use for presumed P knowlesi malaria.
Findings from non-randomised studies suggest that both chloroquine and artemisinin-based combination therapy (ACT) are effective treatments for uncomplicated P knowlesi malaria,6,10 consistent with in-vitro antimalarial susceptibility testing.11 Furthermore, case reports in returned travellers treated with other antimalarial drugs, including atovaquone–proguanil and quinine, do not describe any treatment failures.3 However, no prospective randomised trials have been done to compare the rapidity of parasite clearance with these drugs, or to identify the optimum treatment for uncomplicated P knowlesi malaria. Additionally, there are no data comparing haematological recovery or transmission potential, for which longacting ACTs have an advantage in the treatment of other Plasmodium species.12,13
ACTs are recommended by both WHO and the Malaysian Ministry of Health as first-line therapy for P falciparum,14,15 and are increasingly being used for chloroquine-resistant P vivax in Malaysia and elsewhere in southeast Asia.16 A change to a unified ACT protocol would avoid suboptimum treatment with chloroquine in the event of P falciparum or P vivax being misdiagnosed as P knowlesi. The WHO 2015 malaria treatment guidelines for the first time include provisions for uncomplicated P knowlesi, recommending ACT as first-line treatment only in regions where chloroquine resistance to P vivax is present.14 In 2013, during the conduct of the present study, Malaysia’s national guidelines changed to recommend artemether–lumefantrine for uncomplicated knowlesi malaria, with alternative treatments of artesunate–mefloquine or chloroquine.15 However, the evidence base supporting both these recommendations is derived from prospective observational studies and not clinical efficacy trials. In Malaysia, where all patients with malaria are admitted to hospital, achievement of a faster early therapeutic response would allow for earlier hospital discharge with associated significant health-cost benefits.6,15
We did the ACT KNOW study to compare the fixed combination of artesunate–mefloquine—one of the two ACTs included in Malaysian Ministry of Health guidelines and commonly used for treatment of P knowlesi—with chloroquine to identify the optimum treatment for uncomplicated P knowlesi malaria in adults and children.
Methods
Study design and participants
We did this open-label, randomised controlled trial at three district hospitals in Sabah, Malaysia: Kudat, Kota Marudu, and Pitas hospitals. The Pitas study site was added in February, 2014, after the start of the study. Full details of the study rationale, design, methods, and statistical analysis plan are available in the published protocol.17
Patients who presented to the study hospital sites with uncomplicated P knowlesi malaria were eligible for inclusion if they were at least 1 year of age and weighed more than 10 kg, and had a microscopic diagnosis of P knowlesi monoinfection (including microscopy result of P malariae), a negative P falciparum malaria rapid diagnostic test (histidine-rich protein 2-based), and fever (temperature ≥37·5°C) or history of fever in the last 48 h. We excluded patients with clinical or laboratory criteria for severe malaria or warning signs according to modified WHO 2010 criteria,6 parasitaemia greater than 20 000 parasites per μL (a threshold associated with increased risk of severe malaria in P knowlesi infection6), pregnancy or lactation, known hypersensitivity or allergy to study drugs, serious underlying disease (cardiac, renal, or hepatic), any antimalarial drug use in the previous 2 months, and any contradindications to mefloquine use (history of psychiatric illness, epilepsy, or cerebral malaria).
This study was approved by the relevant human medical research ethics committees of the Ministry of Health, Malaysia, and Menzies School of Health Research, Australia. All participants provided written informed consent.
Randomisation and masking
Patients were randomly assigned (1:1), via computer-generated block randomisation (block sizes of 20), to receive artesunate–mefloquine or chloroquine. Treatment allocations were provided within sealed opaque envelopes, which were opened by a study nurse once the participant met all the study enrolment criteria and informed consent had been signed. Masking of research nursing staff to group allocation was not logistically possible with the standard commercially sourced drugs; however, assignment was concealed from the microscopists responsible for determination of the primary endpoint. Study participants were not informed of the name of the study drug they received. Research blood slides were labelled with patients’ study codes (non-identifiable for both demographic data and drug allocation), and microscopy results were transferred into the study database by a dedicated data entry clerk at the end of the patient’s follow-up period. Biochemical assays were done by hospital staff and PCR assays by research laboratory staff who were unaware of microscopy result and treatment group.
Procedures
Artesunate–mefloquine (Mepha, Aesch, Switzerland) consisted of three different fixed-dose oral formulations: 600 mg and 1500 mg, 300 mg and 750 mg, or 50 mg and 125 mg of artesunate and mefloquine, respectively. Participants received doses at enrolment and at 24 h and 48 h after treatment (target total dose 12 mg/kg artesunate and 25 mg/kg mefloquine). Chloroquine (Kotra Pharma, Melaka, Malaysia) consisted of 155 mg base tablets, with doses given at enrolment and at 6 h, 24 h, and 48 h after treatment (target total dose 25 mg/kg). Doses of artesunate–mefloquine and chloroquine were based on standard weight-dependent calculations consistent with WHO14 and Malaysian Ministry of Health treatment guidelines.15 Administration of all doses was supervised by a study team member (MJG, CSW, BEB, or TWY); all patients were monitored for 1 h after treatment, with re-administration of the full dose if vomiting occurred.
Venous blood was taken before enrolment to assess enrolment criteria, including haematology, biochemistry, and rapid diagnostic tests, and was used for subsequent PCR confirmation of species by use of previously described methods.18,19 Patients had finger-prick blood sampling every 6 h for assessment of microscopic parasite clearance and vital signs documented during admission and at each follow-up visit. Venous blood was taken for haematological investigations daily during admission and then at follow-up visits. Patients were discharged after completion of study drug administration and after obtaining two consecutive blood slides negative for asexual parasites. Daily symptoms were recorded in addition to separate adverse events.
We quantified the level of P knowlesi gametocytes in blood with RT-PCR by measuring the level of pks25, an mRNA transcript that is expressed in mature gametocytes. The assay is based on a previously described assay for the P vivax ortholog pvs25,20 and is described in detail in the appendix. Nucleic acid extraction was done as described above, and genomic DNA was removed with RNA-free DNase treatment (Ambion, Life Technologies, Carlsbad, CA, USA). Quantification was done in triplicate and the average recorded. To control for potential DNA contamination, an additional PCR reaction was run after heat inactivation of the reverse transcriptase enzyme. RNA standards were prepared with the Riboprobe Transcription System (Promega, Fitchburg, WI, USA) from a 267 base-pair fragment of the pks25 mRNA sequence cloned into the pGEM-T Easy plasmid vector (Promega) that had been linearised by digestion with Not1 (New England BioLabs, Ipswich, MA, USA). Tenfold serial dilutions were PCR-amplified in each test run and used to generate a standard curve. All positive tests at day 7 were validated by a PCR test targeting the conserved 18S ribosomal RNA of plasmodia.
Outcomes
The primary endpoint was parasite clearance at 24 h, defined as the difference between treatment groups in the proportion of patients with negative microscopy for P knowlesi asexual parasites at 24 h. Secondary endpoints included microscopic parasite clearance, as measured by time to the first of two negative blood films; the proportion of patients who were aparasitaemic at 48 h and 72 h; and the linear slope constant of the loge parasite–time profile, as defined by parasite clearance methodology from the WorldWide Antimalarial Resistance Network (WWARN).21 Other a-priori secondary endpoints were the early and late treatment outcomes at days 28 and 42, as defined by WHO criteria;14 haematological outcomes (risk of anaemia as per WHO age-based criteria22 at day 28, fractional fall in haemoglobin at day 3, and haemoglobin nadir); proportion of patients with P knowlesi gametocytes at day 7 after treatment; the risk of adverse events and serious adverse events and the relation to study drugs; and the length of hospital inpatient stay.
Statistical analysis
A sample size of 114 participants in each group would provide 90% power to falsify the null hypothesis (ie, to show no difference in parasite clearance between artesunate–mefloquine and chloroquine at 24 h) with an α level of 0·05, assuming 55% of participants given chloroquine10 and 33% of those given artesunate–mefloquine23 would remain parasitaemic 24 h after the start of treatment.
We assessed overall treatment outcomes by survival analysis, with cumulative incidences calculated with the Kaplan-Meier method and compared with the Mantel–Haenszel log-rank test. We compared between-group differences with Student’s t test or the Wilcoxon–Mann–Whitney test for continuous variables, and χ2 or Fisher’s exact test for categorical variables. We used Pearson or Spearman correlation coefficients to assess other associations between variables. We derived 95% CIs with the Wilson method. Microscopic asexual parasite and gametocyte counts were calculated on thick blood smear by use of a formula: parasite count per μL blood=parasites×total white blood cell count (from patient’s daily haemotology result)/200 white blood cells counted. We calculated parasite reduction ratios as 100 minus the percentage reduction from the baseline level. We used best-fit linear or tobit polynomial regression models to estimate the curve of loge parasite counts versus time after exclusion of the lag phase, tail, and outliers using WWARN parasite clearance methodology.21 Patients were excluded from model fitting if they had a baseline parasite count of either less than 1000 parasites per μL or of more than 1000 parasites per μL directly preceding the first negative slide.21
Analysis of the primary endpoint was by modified intention to treat. Data were double-entered by separate individuals into Epidata (version 3.1) and analysed with Stata (version 12).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
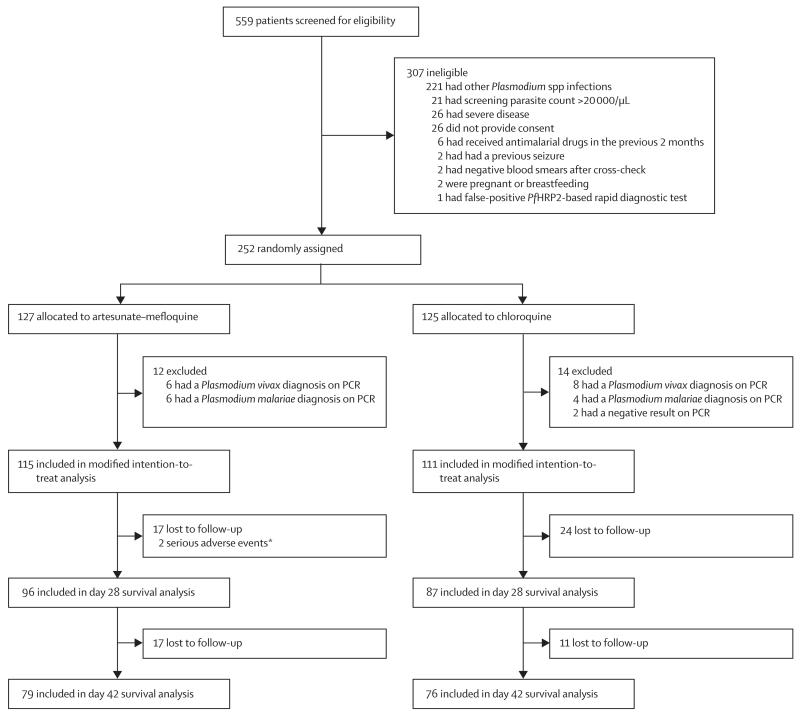
Figure 1 shows the trial profile. Enrolment began in Oct 16, 2012, at the Kudat study site; in Jan 9, 2013, at Kota Marudu; and in March 2, 2014, at Pitas, with all sites continuing until the end of the study period on Dec 13, 2014. Of the 559 malaria admissions at the combined study sites, 336 (60%) patients were microscopically diagnosed as P knowlesi monoinfection, of whom 252 (75%) were enrolled in the study and randomly assigned to receive artesunate–mefloquine (n=127) or chloroquine (n=125; figure 1). 26 (11%) of the initially randomised patients were subsequently excluded from the analysis because of either a PCR diagnosis of P vivax or P malariae or a negative result, leaving 115 patients in the artesunate–mefloquine group and 111 patients in the chloroquine group for the primary modified intention-to-treat analysis (figure 1).
Figure 1. Trial profile.
PfHRP2=Plasmodium falciparum histidine-rich protein 2. *One death, one neuropsychiatric event.
Baseline demographic and clinical characteristics were similar between groups (table 1). The median age of patients was 32·5 years (range 3–85), with 20 (9%) patients younger than 12 years. The geometric mean parasite count at presentation was 1457 parasites per μL in the artesunate–mefloquine group and 1329 parasites per μL in the chloroquine group (table 1); ten (4%) patients had parasitaemia of less than 100 parasites per μL. Subsequent cross-check of enrolment hospital slides by research microscopists showed that nine patients with a parasite count of more than 20 000 parasites per μL and no severe criteria were included in the final modified intention-to-treat analysis; the highest count was 35 873 parasites per μL. Six (67%) of these patients were assigned to artesunate–mefloquine and three (33%) to chloroquine. Overall, 206 (91%) patients had blood cultures done, of which nine (4%) were positive for bacterial skin contaminants only.
Table 1. Baseline demographic and clinical characteristics.
| Artesunate–mefloquine group (n=115) |
Chloroquine group (n=111) | |
|---|---|---|
| Median age (years) | 33 | 32 |
| IQR | 21–49 | 21–50 |
| Range | 3–82 | 7–85 |
| Children (aged ≤12 years) | 12 (10%) | 8 (7%) |
| Sex | ||
| Male | 93 (81%) | 83 (75%) |
| Female | 22 (19%) | 28 (25%) |
| Bodyweight (kg) | 57 (50–68) | 56 (49–64) |
| Fever on admission* | 60 (52%) | 51 (46%) |
| Median duration of fever (days) | 5 | 4 |
| IQR | 3–7 | 3–7 |
| Range | 1–14 | 0–21 |
| Geometric mean parasite count (per μL) | 1457 | 1329 |
| 95% CI | 1061–2002 | 972–1817 |
| Range | 36–35008 | 33–35873 |
| Synchronous infections† | ||
| Rings | 31 (30%) | 18 (16%) |
| Trophozoites | 27 (23%) | 35 (32%) |
| Gametocytes present | 16 (14%) | 14 (13%) |
| Haemoglobin (g/L) | 136 | 131 |
| IQR | 114–146 | 118–141 |
| Range | 71–172 | 93–172] |
| Creatinine (μmol/L) | 88·5 (75·0–101·0) | 83·0 (72·0–95·5) |
| Median total dose administered (mg/kg) | ||
| Artesunate; mefloquine | 10·0; 25·0 | · · |
| IQR | 8·6–11·8; 15·7–41·7 | · · |
| Range | 6·3–16·7; 21·4–29·4 | · · |
| Chloroquine | · · | 27·2 |
| IQR | · · | 24·2–31·0 |
| Range | · · | 15·8–40·1 |
Data are n (%) or median (IQR), unless otherwise indicated.
Temperature of 37·5°C or higher.
One lifecycle stage comprising more than 90% of all asexual parasites.
96 (83%) patients in the artesunate–mefloquine group and 87 (78%) patients in the chloroquine group completed follow-up to day 28 and were included in the secondary survival analysis of the treatment outcome (figure 1). No patient in either treatment group had either early or late treatment failure.
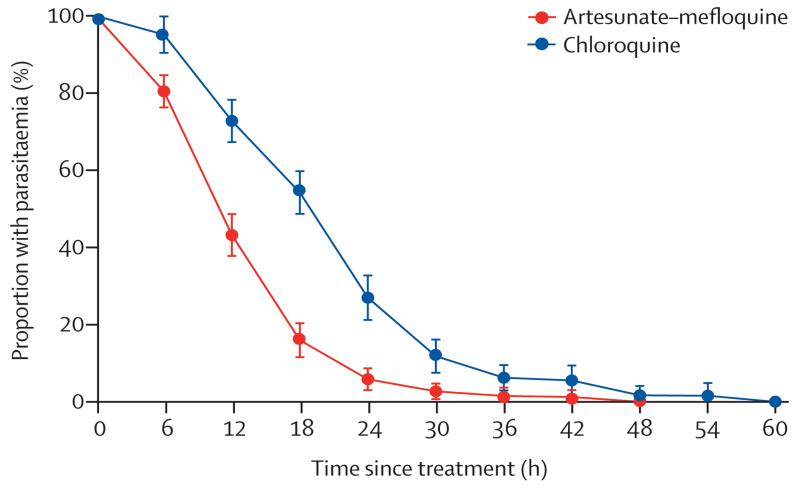
At 24 h, 97 (84%) patients in the artesunate–mefloquine group and 61 (55%) patients in the chloroquine group were aparasitaemic (difference in proportion 29%, 95% CI 18·0–40·8; p<0·0001; table 2). This difference was apparent in both children (67%, 95% CI 31·5–100; p=0·0009) and adults (26%, 14·1–38·3; p<0·0001). At 48 h, aparasitaemia was achieved in all patients given artesunate–mefloquine and 109 (98%) patients given chloroquine, although the difference between groups was not significant (table 2). All patients were negative by microscopy at 72 h (table 2), with the slowest time to clearance (60 h) reported in a patient in the chloroquine group who had a baseline parasite count of 12 968 parasites per μL. Overall, parasite clearance was faster in patients in the artesunate–mefloquine group than in those in the chloroquine group (table 2, figure 2). After exclusion of patients with an initial parasite count of less than 1000 parasites per μL (table 2), the slopes of the clearance curves were 0·301 in the artesunate–mefloquine group and 0·207 in the chloroquine group (p<0·0001; table 2). Fever clearance time was correlated positively with parasite clearance time (rs=0·231; p=0·005), with significantly faster clearance in patients given artesunate–mefloquine (mean 11·5 h [95% CI 8·3–14·6]) than in those given chloroquine (14·8 h [11·7–17·8]; p=0·034).
Table 2. Parasite clearance.
| Artesunate–mefloquine group (n=115) |
Chloroquine group (n=111) | p value | |
|---|---|---|---|
| Parasitological response (aparasitaemia) | |||
| 24 h | 97 (84%; 76·4 to 90·5) | 61 (55%; 45·2 to 64·4) | <0·0001 |
| Odds ratio (95% CI) | 4·4 (2·4 to 8·3) | · · | · · |
| 48 h | 115 (100%; 96·8 to 100) | 109 (98%; 93·6 to 99·8) | 0·148 |
| 72 h | 115 (100%; 96·8 to 100) | 111 (100%; 96·7 to 100) | · · |
| Median parasite clearance time (h) | 18·0 | 24·0 | <0·0001 |
| IQR | 12 to 21 | 24 to 30 | · · |
| Range | 6 to 48 | 6 to 60 | · · |
| Mean slope of curve (k) for log10 normalised parasite clearance* |
0·301 (0·280 to 0·322) | 0·207 (0·192 to 0·223) | <0·0001 |
| Lag phase present | 6 (9%) | 15 (24%) | 0·021 |
| Mean time to clearance of baseline parasites | |||
| 50% clearance (h) | 3·4 (2·9 to 4·0) | 6·3 (5·1 to 7·5) | <0·0001 |
| 90% clearance (h) | 8·9 (8·2 to 9·6) | 14·8 (13·4 to 16·1) | <0·0001 |
| 95% clearance (h) | 11·2 (10·4 to 12·1) | 18·5 (17·0 to 19·9) | <0·0001 |
| 99% clearance (h) | 17·1 (16·0 to 18·3) | 27·0 (25·0 to 29·1) | <0·0001 |
| Parasite reduction ratios | |||
| 6 h | 46·0 (31·6 to 60·4) | −164·0 (−405·4 to −77·3) | <0·001 |
| 12 h | 93·8 (91·8 to 95·9) | 55·9 (41·6 to 70·2) | <0·001 |
| 18 h | 99·3 (98·9 to 99·7) | 86·8 (82·0 to 91·6) | <0·001 |
| 24 h | 99·8 (99·7 to 100) | 97·5 (96·6 to 98·4) | <0·001 |
| Mean gametocyte parasite clearance time (microscopic; h) | 7·2 (6·8 to 7·6) | 7·9 (7·3 to 8·6) | 0·715 |
| Gametocyte pks25 assay | |||
| Positive day 0 | 41/49 (84%; 70·3 to 92·7) | 44/51 (86%; 73·7 to 94·3) | 0·716 |
| Positive day 7 | 2/48 (4%; 0·5 to 14·3) | 3/49 (6%; 1·3 to 16·9) | 0·663 |
Data are n (%; 95% CI), n (%), mean (95% CI), or n/N (%; 95% CI), unless otherwise specified.
48 patients excluded from the artesunate–mefloquine group because of baseline parasite counts of 1000 parasites per μL or less (N=67); 47 patients in the chloroquine group excluded because of baseline parasite counts of 1000 parasites per μL or less and one patient excluded because of a final parasite count more than 1000 parasites per μL before negative slide (N=63).
Figure 2. Parasite clearance.
Error bars show 95% CIs.
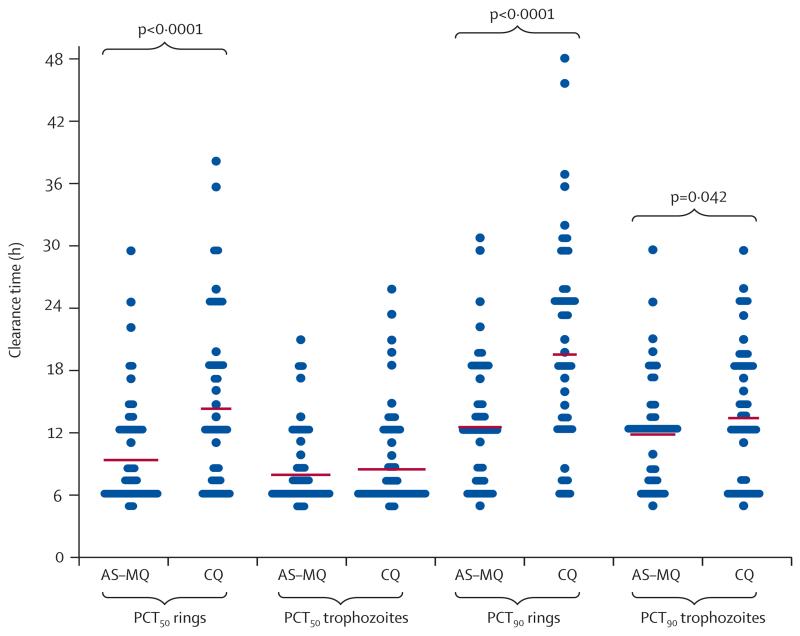
Patients in the artesunate–mefloquine group had faster ring-stage clearance than did those in the chloroquine group, with a mean time to 50% clearance of baseline parasites (PCT50) of 8·6 h (95% CI 7·9–9·4) versus 13·8 h (12·1–15·4; p<0·001), and a mean time to 90% clearance (PCT90) of 11·7 h (10·7–12·7) versus 18·9 h (17·1–20·7; p<0·0001). Trophozoite-stage clearance was similar between the artesunate–mefloquine and chloroquine groups based on mean PCT50 (7·6 h [95% CI 7·0–8·2] vs 7·9 h [7·2–8·6]; p=0.689), but was higher in the artesunate–mefloquine group at PCT90 (11·3 h [10·4–12·2] vs 12·8 h [11·7–14·0]; p=0·042; figure 3).
Figure 3. Stage-specific parasite clearance time.
Horizontal lines show mean values. AS–MQ=artesunate–mefloquine. CQ=chloroquine. PCT50=mean time to 50% clearance of baseline parasites. PCT90=mean time to 90% clearance of baseline parasites.
At baseline, gametocyte carriage, as detected by RT-PCR amplification of pks25 mRNA, was present in 44 (86%) of 51 patients in the artesunate–mefloquine group and 41 (84%) of 49 patients in the chloroquine group (p=0·716). At day 7, gametocytaemia persisted at low levels in three (6%) of 49 patients in the artesunate–mefloquine group and two (4%) of 48 patients in the chloroquine group (p=0·663). All patients with gametocytes present at day 7 had also been positive for gametocytes at baseline. In patients who were gametocytaemic by PCR at baseline, 16 (19%) of 85 patients had gametocytes detected by microscopy at baseline; no patients were positive for gametocytes by microscopy at any time during follow-up after hospital discharge.
Throughout 28 day follow-up, roughly 60% of patients had anaemia in the artesunate–mefloquine group and almost 75% had anaemia in the chloroquine group (table 3); this difference remained after controlling for confounding factors (adjusted odds ratio 2·5 [95% CI 1·2–5·1]; p=0·011). In the 145 (64%) patients without anaemia at baseline, none of 26 patients was anaemic at day 28 in the artesunate–mefloquine group compared with seven (26%) of 27 patients in the chloroquine group (p=0·005). A higher parasite clearance rate constant (k) was associated with a lower risk of anaemia during the 28 days, including after controlling for confounders (coefficient −2·49 [95% CI −0·74 to −4·24]; p=0·005).
Table 3. Haematological outcomes.
| Artesunate–mefloquine group (n=115) | Chloroquine group (n=111) | p value | |
|---|---|---|---|
| Mean fractional fall in haemoglobin at day 3 (%) | 12·2 (9·9–14·5) | 12·4 (10·7–14·1) | 0·862 |
| Haemoglobin nadir (g/L) | 120 | 117 | 0·407 |
| IQR | 101–130 | 103–125 | · · |
| Range | 67–154 | 78–152 | · · |
| Time to haemoglobin nadir (days) | 2 | 2 | 0·049 |
| IQR | 2–7 | 2–3 | · · |
| Range | 0–42 | 0–42 | · · |
| Prevalence of anaemia at day 28* | 26/95 (27%; 18·7–37·5) | 27/82 (33%; 22·9–44·1) | 0·421 |
| Prevalence of anaemia throughout day 28 follow-up | 71/115 (62%; 52·2–70·6) | 83/111 (75%; 65·6–82·5) | 0·045 |
Data are % (95% CI) or n/N (%; 95% CI), unless otherwise specified.
Anaemia calculated as per WHO age-based criteria.
Median duration of hospital stay was similar between treatment groups (4 days [3–4] in the artesunate–mefloquine group vs 4 days [3–4] in the chloroquine group; p=0·061). These results were confounded by the fact that all enrolled patients had to remain in hospital until treatment completion. With application of the national hospital discharge policy of two negative blood slides on consecutive days with study drug to be completed after discharge, bed occupancy for patients in the artesunate–mefloquine group was 2426 days per 1000 patients compared with 2828 days per 1000 patients in the chloroquine group (incidence rate ratio 0·858 [95% CI 0·812–0·906]; p<0·0001).
Rates of adverse events did not differ significantly between the study groups, including in grouped system analyses (table 4). The most frequent events were headache, dizziness, cough, abdominal pain, and vomiting (table 4). No patients needed rescue treatment.
Table 4. Adverse events.
| Artesunate–mefloquine group (n=115) |
Chloroquine group (n=111) |
p value | |
|---|---|---|---|
| Gastrointestinal | |||
| Vomiting | 25 (22%) | 35 (32%) | 0·103 |
| Abdominal pain | 30 (26%) | 31 (28%) | 0·786 |
| Diarrhoea | 6 (5%) | 9 (8%) | 0·392 |
| Any of above | 51 (44%) | 72 (65%) | 0·056 |
| Neurological | |||
| Dizziness | 75 (65%) | 75 (68%) | 0·777 |
| Headache | 98 (85%) | 95 (86%) | 0·935 |
| Vision or hearing problems | 9 (8%) | 13 (12%) | 0·335 |
| Ataxia | 4 (3%) | 0 | 0·122 |
| Psychosis | 1 (1%) | 0 | 0·509 |
| Any of above | 108 (94%) | 102 (92%) | 0·554 |
| Skin | |||
| Rash or itch | 4 (3%) | 7 (6%) | 0·331 |
| Respiratory | |||
| Cough | 37 (32%) | 35 (32%) | 0·882 |
| Shortness of breath | 13 (11%) | 16 (14%) | 0·500 |
| Any of above | 43 (37%) | 41 (37%) | 0·903 |
Data are n (%) of patients in the modified intention-to-treat population.
Two (<1%) patients had serious adverse events (table 4). The first was in a 41-year-old man in the artesunate–mefloquine group who developed acute psychosis on day 3, with auditory hallucinations, nausea, dizziness, and subsequent minor attempts at self-harm. The patient was transferred to a tertiary hospital, where investigations including a normal CT head scan excluded other common organic causes. The symptoms resolved over a period of 2 weeks, with no further recurrences during outpatient follow-up over the next 6 months. Causality was graded according to reporting of serious adverse events as probably related to mefloquine. The second serious adverse event was in a 55-year-old man with a heavy smoking history in the artesunate–mefloquine group who died after being readmitted to hospital 24 days after enrolment with negative blood slides for malaria and a diagnosis of severe community-onset hospital-associated bacterial pneumonia regarded as unrelated to study drug.
Discussion
Our findings show that both artesunate–mefloquine and chloroquine are highly effective for the treatment of uncomplicated P knowlesi malaria, with no treatment failures in either group during 42 days of follow-up. However, the early therapeutic response, as gauged by both parasite and fever clearance, was significantly faster in patients who received artesunate–mefloquine. The faster in-vivo parasite clearance of artesunate–mefloquine compared with chloroquine for knowlesi malaria is consistent with a previous in-vitro study11 of P knowlesi drug sensitivity, which showed a lower half maximum inhibitory concentration for artemisinin derivatives than for chloroquine. The broad lifecycle-stage action of artemisinin derivatives reported for both P falciparum24 and P vivax25 was also evident in the present study for P knowlesi, with a shorter PCT50 for both ring-stage and trophozoite-stage clearance with artesunate–mefloquine than with chloroquine. The lower activity of chloroquine versus artesunate–mefloquine against the early ring stage probably contributed to the continued rise in median parasitaemia from baseline recorded at 6 h in patients given chloroquine.
The sustained efficacy of chloroquine is consistent with P knowlesi being a zoonotic infection, with minimum selection of resistance from intense antimalarial treatment for all detected cases of malaria. This finding is also supported by studies reporting the successful use of either oral chloroquine or artemisinin-based combination therapy in east Malaysia,6,10 and case reports in returned travellers using chloroquine26 or artemether–lumefantrine.27 There was no in-vivo evidence to support a suggested lower innate efficacy of mefloquine against P knowlesi,11 although co-administration of artesunate–mefloquine precluded definitive assessment.
Artemisinin combinations might also minimise other morbidity associated with P knowlesi malaria, with a decreased risk of anaemia after administration of artesunate–mefloquine. Although prevalence of anaemia did not differ between groups at days 28 or 42 after treatment, only patients with pre-existing anaemia at enrolment still had persistent anaemia in the artesunate–mefloquine group, by contrast with a subset of patients in the chloroquine group who developed anaemia from a normal haemoglobin concentration at baseline. This finding was associated with the slower parasite clearance rate in the chloroquine group than in the artesunate–mefloquine group, with a higher cumulative number of red blood cells infected (particularly considering the fast 24 h replication of P knowlesi). We did not formally assess post-artesunate delayed haemolysis in view of the low parasite count cutoff for inclusion in the study. Although mild or transient decreases in haemoglobin might have taken place, this was not evident at the study follow-up timepoints and did not seem to have any clinical effect on risk of anaemia at day 28.
Most patients cleared their gametocytes with treatment; however, a few patients in both groups had persistent gametocytaemia at day 7, which suggests that the effect of both drugs might be mainly on the development of younger gametocytes, as shown with P falciparum.28 Whether persistent P knowlesi gametocytes are transmissible to other human or monkey hosts has not been assessed, although non-drug-exposed gametocytes in human beings have shown transmission competency in experimental conditions.29
Our findings are likely to be generalisable to elsewhere in southeast Asia where P knowlesi causes human malaria, because, due to the currently reported low endemicity and zoonotic transmission of P knowlesi in this region,3 these cases are also likely to be from drug-naive and hence highly sensitive parasites. Any generalisability might be somewhat restricted by the incomplete follow-up at day 42; however, a high proportion of patients remained assessable, with the absence of treatment failure in both groups likely to be valid. In the present study, patients with un-complicated knowlesi malaria with parasite counts up to 35 000 parasites per μL were treated safely with both artesunate–mefloquine and chloroquine; however, the small number of patients with parasite counts greater than 20 000 per μL does not allow for certainty regarding efficacy of either drug at this level of parasitaemia. Although a serious adverse event related to mefloquine use occurred in this study, mefloquine has been used extensively for uncomplicated P falciparum and P vivax malaria since the 1980s, and its adverse event profile has been well documented, with the risk of neuropsychiatric events occurring in one per 1217 patients of Asian origin, and increasing by seven times if re-treatment happens within 1 month.30 Mefloquine is currently recommended as a partner drug in ACT in the 2015 WHO malaria treatment guidelines,14 and artesunate–mefloquine is approved by the Malaysian Ministry of Health as one of two ACT drugs for uncomplicated malaria in Malaysia.15
Our findings support the use of ACT as a first-line treatment for uncomplicated P knowlesi malaria. Although chloroquine was also safe and efficacious, the use of ACT resulted in faster parasite clearance, with associated earlier fever clearance and hospital discharge and decreased morbidity due to anaemia. The absence of treatment failures in the artesunate–mefloquine group supports its use by the Malaysian Ministry of Health as an efficacious ACT treatment for knowlesi malaria. However, the serious neuropsychiatric adverse event probably related to mefloquine suggests that other ACTs with a better safety profile, such as artemether–lumefantrine, might be more suitable. Artemether–lumefantrine has proven efficacious in observational studies,6,23 and randomised trials of artemether–lumefantrine for P knowlesi are warranted. The cost of routine treatment of P knowlesi with the more expensive ACT drugs would be offset by earlier hospital discharge in pre-elimination settings, such as Malaysia, with mandatory hospital admission for malaria cases. P knowlesi is co-endemic with P falciparum and P vivax across southeast Asia. The presence of chloroquine resistance in P falciparum and P vivax, and the frequency of misdiagnosis of P knowlesi with these species,3,4 is an important factor in supporting change to a unified ACT treatment guideline for blood-stage treatment of all Plasmodium species in areas such as Malaysia where P knowlesi exists.
Supplementary Material
Panel: Research in context.
Evidence before this study
We searched PubMed between Aug 13, 2008, and July 8, 2015, with the terms “knowlesi” and “treatment” combined with “efficacy”, “randomised controlled trial”, “chloroquine”, “artemisinin-combination therapy”, “artesunate-mefloquine”, “mefloquine”, or “clinical trial”, with no date restrictions for published English-language reports. We reviewed all studies identified as including treatment of uncomplicated knowlesi malaria. Although non-randomised prospective studies showed efficacy of chloroquine and artemisinin-based combination therapies (ACT) for Plasmodium knowlesi malaria, no randomised controlled comparative trials were identified.
Added value of this study
To our knowledge, this is the first randomised controlled trial of uncomplicated P knowlesi malaria. Artesunate–mefloquine had a better early therapeutic response than did chloroquine, including faster parasite and fever clearance, and earlier hospital discharge. Although there were no treatment failures with either drug during day 42 follow-up, there were fewer cases of anaemia in the artesunate–mefloquine group. Gametocytes persisted to day 7 after treatment in both groups.
Implications of all the available evidence
Artesunate–mefloquine is rapidly efficacious and potentially more cost effective for treatment of uncomplicated P knowlesi malaria in Malaysia. Findings support a unified ACT treatment guideline for all Plasmodium species in P knowlesi co-endemic areas, where microscopic misdiagnosis of P knowlesi and chloroquine-resistant Plasmodium falciparum and Plasmodium vivax is common, and resistance to artemisinin has not been reported.
Acknowledgments
This study was funded by the Malaysian Ministry of Health (grant number BP00500420), the Asia Pacific Malaria Elimination Network (108-07), and the Australian National Health and Medical Research Council (NHMRC; 1037304 and 1045156; fellowships to NMA [1042072], TWY [605831], BEB [1088738]; and a scholarship to MJG [1074795]). RNP is a Wellcome Trust Senior Fellowship in Clinical Science (091625). JSM is an NHMRC Practitioner Fellow and is supported by a Government of Queensland Health Research Fellowship. We thank the participants in this study, the clinical and laboratory research staff, the hospital directors at the study sites, the study monitor (Phaik Yeong Cheah), the Director General of Health, the Malaysian Ministry of Health, the head of Clinical Research Centre Malaysia (Goh Pik Pin), and colleagues and staff of the UK Medical Research Council Monkeybar Project. We would also like to acknowledge Rebecca Rockett, Stacey Llewellyn, and Ryan Farid for assistance in developing the pks25 RT-PCR assay, and to Kim Piera and Ammar Aziz for doing the diagnostic Plasmodium species PCR and other logistical support.
Contributors
MJG, TW, TWY, NMA, LvS, and RNP designed the study. MJG, TWY, TW, CSW, and GSR supervised patient enrolment and data collection. MJG did the statistical analysis with input from TWY, NMA, and RNP. JSM and CP designed and undertook the gametocyte assay and interpreted the results. MJG wrote the first draft of the manuscript; TWY, NMA, and RNP edited the initial drafts; and all authors reviewed the final manuscript.
Footnotes
See Online/Comment http://dx.doi.org/10.1016/S1473-3099(15)00475-2
Declaration of interests
We declare no competing interests.
References
- 1.William T, Jelip J, Menon J, et al. Changing epidemiology of malaria in Sabah, Malaysia: increasing incidence of Plasmodium knowlesi. Malar J. 2014;13:390. doi: 10.1186/1475-2875-13-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusof R, Lau Y-L, Mahmud R, et al. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar J. 2014;13:1–9. doi: 10.1186/1475-2875-13-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber BE, William T, Grigg MJ, Yeo TW, Anstey NM. Limitations of microscopy to differentiate Plasmodium species in a region co-endemic for Plasmodium falciparum, Plasmodium vivax and Plasmodium knowlesi. Malar J. 2013;12:8. doi: 10.1186/1475-2875-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox-Singh J, Davis TM, Lee KS, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber BE, William T, Grigg MJ. A prospective comparative study of knowlesi, falciparum, and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and Plasmodium vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis. 2013;56:383–97. doi: 10.1093/cid/cis902. [DOI] [PubMed] [Google Scholar]
- 7.Rajahram GS, Barber BE, William T, Menon J, Anstey NM, Yeo TW. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J. 2012;11:284. doi: 10.1186/1475-2875-11-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdrager J. Epidemiology of the emergence and spread of drug-resistant falciparum malaria in South-East Asia and Australasia. J Trop Med Hyg. 1986;89:277–89. [PubMed] [Google Scholar]
- 9.Price RN, Seidlein von L, Valecha N, Nosten F, Baird KJ, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–91. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daneshvar C, Davis TM, Cox-Singh J, et al. Clinical and parasitological response to oral chloroquine and primaquine in uncomplicated human Plasmodium knowlesi infections. Malar J. 2010;9:238. doi: 10.1186/1475-2875-9-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatih FA, Staines HM, Siner A, et al. Susceptibility of human Plasmodium knowlesi infections to anti-malarials. Malar J. 2013;12:425. doi: 10.1186/1475-2875-12-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douglas NM, Simpson JA, Phyo AP, et al. Gametocyte dynamics and the role of drugs in reducing the transmission potential of Plasmodium vivax. J Infect Dis. 2013;208:801–12. doi: 10.1093/infdis/jit261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smithuis F, Kyaw MK, Phe O, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–81. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO . Guidelines for the treatment of malaria. Third Edition World Health Organization; Geneva: 2015. [Google Scholar]
- 15.Malaysian Ministry of Health . Management guidelines of malaria in Malaysia. Vector Borne Disease Sector, Disease Control Division, Ministry of Health; Malaysia: 2013. in Cooperation with Health Education Division. [Google Scholar]
- 16.Douglas NM, Anstey NM, Angus BJ, Nosten F, Price RN. Artemisinin combination therapy for vivax malaria. Lancet Infect Dis. 2010;10:405–16. doi: 10.1016/S1473-3099(10)70079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grigg MJ, William T, Dhanaraj P, et al. A study protocol for a randomised open-label clinical trial of artesunate-mefloquine versus chloroquine in patients with non-severe Plasmodium knowlesi malaria in Sabah, Malaysia (ACT KNOW trial) BMJ Open. 2014;4:e006005. doi: 10.1136/bmjopen-2014-006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WWARN [accessed Dec 24, 2014];Parasite clearance estimator. 2012 http://www.wwarn.org/tools-resources/toolkit/analyse/parasite-clearance-estimator-pce.
- 19.WHO [accessed June 10, 2015];Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011 http://www.who.int/vmnis/indicators/haemoglobin.pdf.
- 20.William T, Menon J, Rajahram G, et al. Severe Plasmodium knowlesi malaria in a tertiary care hospital, Sabah, Malaysia. Emerg Infect Dis. 2011;17:1248–55. doi: 10.3201/eid.1707.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P knowlesi. J Clin Microbiol. 2009;47:4173–75. doi: 10.1128/JCM.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padley D, Moody AH, Chiodini PL, Saldanha J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol. 2003;97:131–37. doi: 10.1179/000349803125002977. [DOI] [PubMed] [Google Scholar]
- 23.McCarthy JS, Griffin PM, Sekuloski S, et al. Experimentally induced blood-stage Plasmodium vivax infection in healthy volunteers. J Infect Dis. 2013;208:1688–94. doi: 10.1093/infdis/jit394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terkuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76:85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 25.Russell B, Chalfein F, Prasetyorini B, et al. Determinants of in vitro drug susceptibility testing of Plasmodium vivax. Antimicrob Agents Chemother. 2008;52:1040–45. doi: 10.1128/AAC.01334-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry A, Iriart X, Wilhelm N, et al. Imported Plasmodium knowlesi malaria in a French tourist returning from Thailand. Am J Trop Med Hyg. 2011;84:535–38. doi: 10.4269/ajtmh.2011.10-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoosen A, Shaw MTM. Plasmodium knowlesi in a traveller returning to New Zealand. Travel Med Infect Dis. 2011;9:144–48. doi: 10.1016/j.tmaid.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Peatey CL, Skinner-Adams TS, Dixon MWA, McCarthy JS, Gardiner DL, Trenholme KR. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis. 2009;200:1518–21. doi: 10.1086/644645. [DOI] [PubMed] [Google Scholar]
- 29.Chin W, Contacos PG, Collins WE, Jeter MH, Alpert E. Experimental mosquito-transmission of Plasmodium knowlesi to man and monkey. Am J Trop Med Hyg. 1968;17:355–58. doi: 10.4269/ajtmh.1968.17.355. [DOI] [PubMed] [Google Scholar]
- 30.Phillips-Howard PA, Kuile Ter FO. CNS adverse events associated with antimalarial agents. Fact or fiction? Drug Saf. 1995;12:370–83. doi: 10.2165/00002018-199512060-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.