Abstract
Background:
The peri-implant mucosa undergoes surgical and bacterial assaults in various stages of implant therapy, however, the literature on changes occurring in the peri-implant mucosa is minimal. This study was thus conducted to evaluate the change in the peri-implant mucosal thickness and its effect on the marginal bone levels around dental implants treated in a conventional two-stage implant therapy.
Materials and Methods:
A total of 36 implants were placed in 22 subjects. Two subjects dropped out. Thirty-three implants in 20 subjects were then evaluated. Initial mucosal thickness, marginal bone levels on radiographs, pain, and exudation were evaluated. All these parameters were recorded at the time of implant placement, at the time of cementation of final restoration, 6 months and 12 months post cementation/restoration.
Results:
The peri-implant mucosal thickness reduced from implant placement to second stage and till restorations and was statistically significant, in both the thick and thin biotypes, however, at 12 months there was a rebound of the tissue thickness, which was more in the thick biotype (P < 0.05). At 1-year follow-up, there was a reduction in the marginal bone levels, which was more in the thick biotype as compared to the thin biotype (P < 0.05).
Conclusion:
The mucosa at implant sites undergoes a reduction in thickness from the time of implant placement till the placement of final restorations. The placement of the final restorations and then end of active therapy leads to a rebound of the tissue thickness. Sites with thicker tissues preoperatively have a lesser bone loss and better rebound as compared to thinner tissues.
Keywords: Dental implants, marginal bone levels, peri-implant mucosa, tissue biotype, tissue thickness
INTRODUCTION
The survival and success of dental implants have been found to be influenced by various factors. Some factors influence osseointegration whereas some influence the long-term survival. The factors such as bone quality and quantity, primary stability of implant, implant surface characteristics are few of the factors, which influence osseointegration of the implant.[1] However, the long-term success of implants is determined by factors such as the bone quality, type of prosthesis, occlusal loading, oral hygiene, overlying soft tissue, and regularity of recall visits.[2,3,4,5,6,7]
The stability of the marginal bone levels has long been used as a determining factor while describing the survival and success of dental implants.[8] Bone loss of 0.5–1 mm in the 1st year postrestoration was considered as physiological and was attributed to the bone remodeling that happens after implant placement surgery, placement of restoration, and initial occlusal loading of the restoration along with the implant design and surface.[9]
The role of the peri-implant mucosa as one of the factors determining the stability of marginal bone levels has received little attention as compared to various other factors that have been studied. Cochran et al.[10] stated a need of 3 mm of peri-implant mucosa for a stable epithelial, connective tissue attachment. Lindhe et al.[11] stated that the biologic width around implants serves as a protective mechanism for the underlying bone. Hermann et al.[12] evaluated the formation of biologic width around dental implants and concluded that excision of overlying soft tissue leads to a compensatory bone resorption meaning that the amount of soft tissue thickness has to be re-established irrespective of sacrificing the bone levels. Most of the studies assessing the impact of the soft tissue on the marginal bone levels have evaluated the width of the attached mucosa, measured as the distance from the free mucosal margin to the muco-gingival junction.[13,14] However, the change in soft tissue thickness, which describes the bulk of the tissues as measured from the surface of the mucosa to the body of the implant/abutment has not been evaluated and so has been its effect on the marginal bone levels around implants. This study was thus conducted to evaluate the change in soft tissue thickness around the dental implants and co-relate the same with the marginal bone levels over a 1-year postrestorative period.
MATERIALS AND METHODS
The study was conducted in the Department of Oral Implantology and Periodontics after getting the approval from the Institutional Review Board. Patient inclusion criteria were: Patients willing to participate in the study and give a written informed consent, patients requiring placement of implants for replacement of missing teeth, nonsmokers, patients with good general health, patients’ not on any medications that would influence the gingival status. All the patients to have a well-healed partially edentulous site and a healthy overlying mucosa. Each site had to be single missing tooth site bound by natural teeth. The sample size was determined by setting the alpha value at 0.05. The single implant was treated as the statistical unit. Before the study onset, each variable was assessed to evaluate if the parameters were normally distributed and parametric statistical tests could be applied. As the variables appeared to be normally distributed, frequencies were calculated, and thus the sample size was determined.
A total of 22 patients (10 males and 12 females) were enrolled in this study and received a total of 36 implants.
All the implants were placed by the same operator (SK) and were in the range of 3.75–4.2 mm diameter. The implants were placed with a conventional protocol, that is, submerged placement at the marginal level in well-healed sites at a minimum of 3 months after extraction. The second stage procedure was done at 3 months after implant placement and 15 days later the final impressions were made. All the abutments had a finish line set at 1.5 mm from the implant mouth. The final restorations were cemented on an average 10–15 days after the final impressions.
The following parameters were evaluated: (1) Mucosal thickness, (2) radiographic bone level. These parameters were evaluated by a single examiner who was trained to measure the mucosal thickness and the radiographic bone levels.
Measurement of the mucosal thickness
At the implant site, local anesthetic was administered after which the tissue thickness was measured using an endodontic reamer (size 20-yellow) with a stopper [Figure 1]. The thickness of the tissues was determined by the depth of penetration of the reamer from the external surface of the mucosa to the point where bony resistance could be felt. The stopper was then adjusted, and the depth of penetration/thickness was measured in millimeters on a geometric scale/ruler.
Figure 1.
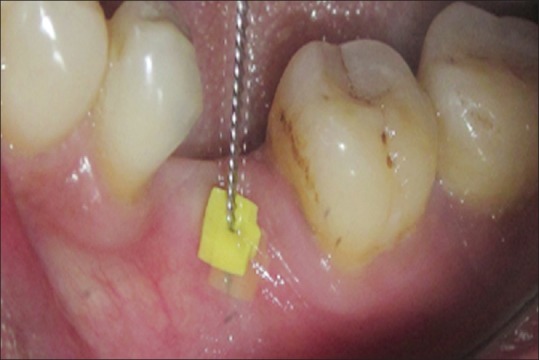
The measurement of soft tissue thickness
The measurements were done at three points on the crest of the edentulous ridge namely mesial, mid and distal, and on the buccal surface of the ridge at a point 3 mm apical the crestal measurements and 1 mm coronal to the muco-gingival junction. This point was kept as a standard throughout the study.
Sites with a mucosal thickness of 2.0 mm or more at the baseline, that is, at implant placement were categorized as a thick biotype case group and those <2.0 mm were categorized as the thin biotype case group.[15] All the measurements were repeated at the second stage, at the insertion of the prosthesis, at 6-month and 1-year recall visits. After the second stage, the only the buccal measurements were recorded.
The marginal bone changes were recorded on serial radiographs using the neck height and thread pitch of the implants [Figures 2 and 3] and probing pocket depth (in mm) was also evaluated.[16]
Figure 2.
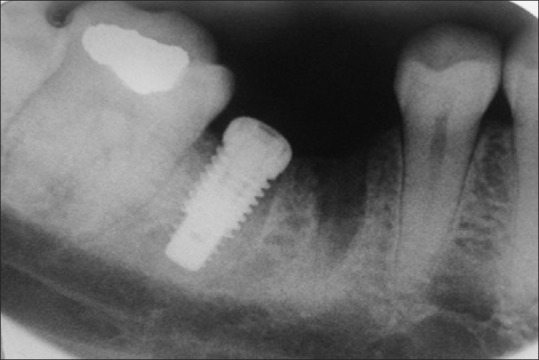
Radiographic evaluation of bone levels at baseline soon after implant placement
Figure 3.
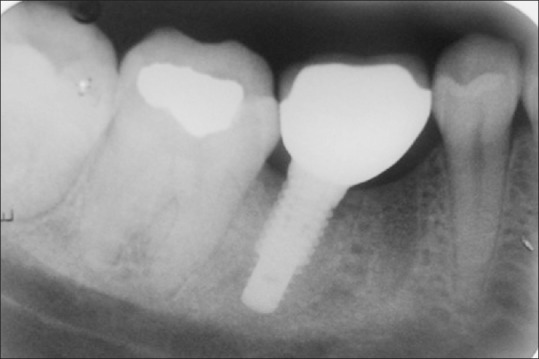
Radiographic evaluation of bone levels at cementation
Statistical analysis
The evaluation of soft tissue changes between the thick and the thin biotypes was done at various time intervals and their correlation with the crestal bone changes was done with the Student's paired t-test.
RESULTS
Twenty of the 22 patients completed the follow-up visits, 2 patients (1 male and 1 female) dropped out of the study, and a total of 33 implants were available for analysis. Of the 33 implants sites, 16 sites were classified as thick biotype, and the remaining 17 were sites and were thin biotype cases. Each implant was considered as 1 unit.
Clinical parameters
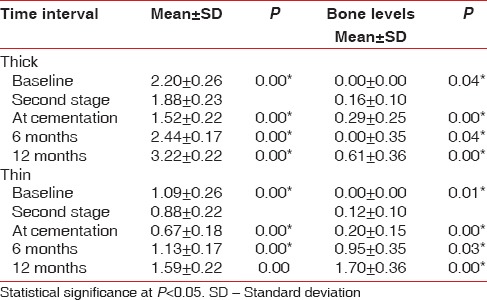
The mean mucosal thickness at baseline around the sites classified as thick biotype was 2.20 mm ± 0.26 mm and around the thin biotype, it was 1.09 mm ± 0.26 mm, it reduced to 1.88 mm ± 0.23 mm in the thick biotype and 0.88 ± 0.22 in the thin biotype cases at second stage and 1.52 ± 0.22 and 0.67 ± 0.18 in the thick and the thin biotypes, respectively, at cementation. The reduction was more pronounced in the thin biotype as compared to the thick.
Soft tissue rebound was detected at 6 and 12 months postcementation in both the biotypes. At 6 months and 12 months postrestoration the soft tissue thickness was 2.44 mm ± 0.17 mm and 3.22 mm ± 0.22 mm in the thick biotype, respectively, and in the same time duration the respective soft tissue thickness was 1.13 ± 0.17 and 1.59 ± 0.22 in the thin biotype, respectively, indicating an increase in the soft tissue thickness with respect to the baseline values [Table 1].
Table 1.
Correlation of soft tissue changes and marginal bone changes in thick and thin biotypes at different time intervals
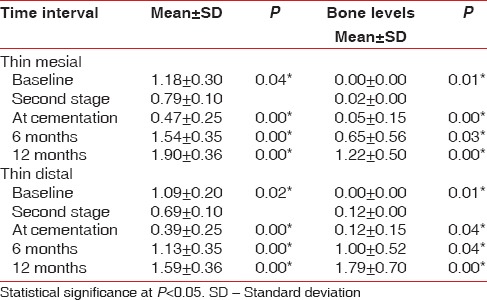
When a comparison was made to evaluate the soft tissue thickness on the mesial and distal sides in thick and thin biotypes, a statistically significant difference was observed [Tables 2 and 3].
Table 2.
Correlation of soft tissue changes and marginal bone changes in mesial and distal sides of thick biotype at different time intervals
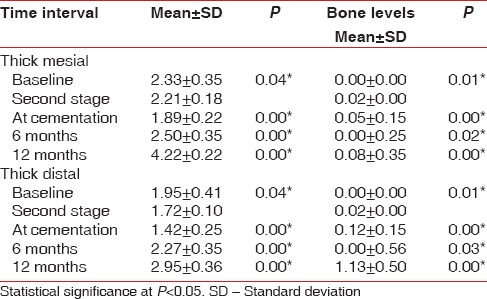
Table 3.
Correlation of soft tissue changes and marginal bone changes in mesial and distal sides of thin biotype at different time intervals
Radiographic parameters
There was a significant difference in the bone levels in both the groups. At the end of 1-year, a mean bone loss of 0.61 mm ± 0.36 mm was noted in the thick group and 1.70 mm ± 0.36 mm mean bone loss was noted in the thin group [Table 1]. The evaluation of vertical bone loss on the mesial and distal sides of the thick and the thin biotypes also revealed a statistically significant reduction from baseline to cementation.
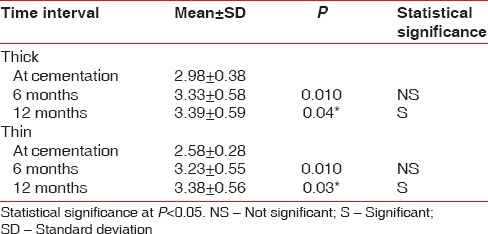
The probing pocket depths showed statistically significant difference in both the groups at cementation to 6 months (P = 0.01) and 12 months (P = 0.02) postcementation. However, there was no statistically significant difference found in the probing pocket depth between 6 months to 12 months postcementation [Table 4].
Table 4.
Comparison of probing pocket depth at different time intervals in the thick and the thin biotypes
DISCUSSION
The soft tissue in an edentulous site undergoes multiple surgical assaults, from the time of implant placement till restorations has been fitted. The current study was conducted to evaluate progressively the change in the soft tissue thickness from implant placement to 1-year postrestorative and also to evaluate its effect on the marginal bone levels.
The mucosal thickness was measured using, and endodontic reamer with a stopper and the depth was measured till the point the reamer made the first contact with the bone surface and for the same the intra examiner error (kappa value was set at κ >0.6) thus reproducibility of the measurements through the study was ensured.[17]
A value of 2 mm of soft tissue thickness at the time of implant placement was used as the benchmark to group the patients into thick and thin biotype cases.[15] In a study was done by Sharma et al.[18] on the population similar to the one used in the study had a soft tissue thickness around natural teeth ranged from 0.56 mm to 1.02 mm. As the edentulous ridges were evaluated in the study, mucosal thickness measured was thicker than around natural teeth.
A reduction in the tissue in both the thick and thin biotype patients from the time of implant placement to restoration stage and from the second stage to restoration was observed and a subsequent increase in the thickness was observed after the placement of the final restorations. The reduction in thickness till cementation and the concomitant increase later is attributable to the surgical intervention at implant placement as well at implant uncovering at second stage[19] and to the formation and organization of the supramarginal connective tissue, morphology of the peri-implant mucosa, and establishment of the biologic width around the implants, respectively.[20,21]
The thicker biotype cases showed a lesser reduction in thickness from placement to restoration and higher rebound from restoration to the 1-year follow-up, as compared to the thin biotype cases. This is due to the higher amount of the connective tissue and vascularity in thicker as compared to the thin biotype tissues, and thus the ability of these tissues to re-organize is better than thinner tissues.[21]
This study also evaluated the effect of tissue thickness change on the marginal bone levels as the stability of the marginal bone levels has been used as a benchmark for implant success. The bone levels were measured on serial radiographs taken throughout the course of study, where the measurements were carried out from the implant shoulder to the first thread exposed. Bone loss was observed in both the thick and the thin biotype cases, over the duration of the study. This bone loss happening around the implants can be divided into two parts, the first being the one occurring from the time of implant placement to time of restoration and second one from the time of restoration to the end of the follow-up period.
The bone loss from the baseline to the period of first 6 months of implant placement can be attributed to the surgical trauma and bone remodeling that happens soon after the implant placement.[19,22] The bone loss that was observed from 6 months to 12 months postcementation, can be attributed to various factors such as stress at the marginal bone which may cause micro-fracture or overload, or even the biomechanical adaptation of the bone to the occlusal load resulting in marginal bone loss in the 1st year in function.[23,24]
The amount of bone loss was more in thin biotype cases as compared to thick. The difference between the two biotypes can be attributed to the fact that the thick tissues formed the biologic width by proliferating laterally or coronally, which is unlike to that observed in thin biotype cases wherein the bone around the implants underwent remodeling to accommodate the soft tissue biologic width.
The present study was carried out only for a period of 1-year, and it can be hoped that marginal bone levels would stabilize at the end of 1-year post cementation and achieve a “steady-state phenomenon.”[13,22] However, it should be kept in mind that ongoing marginal bone loss is a factor affecting the outcome of implant treatment.
The peri-implant sulcus forms after the second stage procedure and is due to the formation and stabilization and organization and maturation of the connective tissue fibers of the overlying soft tissue, and depending on the nature of the overlying soft tissue, resorption of the bone at the crest may occur to accommodate the biologic width around the implants.[10] Though the probing depth around dental implants is influenced by various factors such as probing force and angulation, probe tip diameter, roughness of the implant type of abutment/restoration, inflammatory state of the periodontium and firmness of the marginal tissues, and access to probing,[16] deeper probing depth often indicate bone loss. An increase in the probing pocket depth in both the biotypes from the time of restoration to 1-year was observed in the study. This can also be related to the bone loss occurring to re-establish the biologic width, coronal movement of the marginal tissue due to an increase in the thickness or a combination of both.[12] In the present study, the probing pocket depth roughly co-related to the combined values of the mucosal thickness and bone loss in both the thick and thin biotype cases (thick biotype probing pocket depth of 3.39 mm ± 0.59 mm = soft tissue thickness of 3.22 mm ± 0.22 mm, + bone loss of 0.61 mm ± 0.36 mm, Thin biotype probing pocket depth of 3.38 ± 0.56 = soft tissue thickness of 1.59 mm ± 0.22 mm and bone loss of 1.70 mm ± 0.36 mm).
CONCLUSION
Thus, it can be concluded that the soft tissue undergoes a change in its thickness from implant placement to restoration and till 1-year postrestoration. The changes are more pronounced in thin biotype cases as compared to the thick biotype ones, and these changes also influence the marginal bone levels around the implants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 2001;10(Suppl 2):S96–101. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry PJ. Oral implant restoration for enhanced oral function. Clin Exp Pharmacol Physiol. 2005;32:123–7. doi: 10.1111/j.1440-1681.2005.04140.x. [DOI] [PubMed] [Google Scholar]
- 3.Oh TJ, Yoon J, Misch CE, Wang HL. The causes of early implant bone loss: Myth or science? J Periodontol. 2002;73:322–33. doi: 10.1902/jop.2002.73.3.322. [DOI] [PubMed] [Google Scholar]
- 4.Grunder U. Stability of the mucosal topography around single-tooth implants and adjacent teeth: 1-year results. Int J Periodontics Restorative Dent. 2000;20:11–7. [PubMed] [Google Scholar]
- 5.Misch CE, Perel ML, Wang HL, Sammartino G, Galindo-Moreno P, Trisi P, et al. Implant success, survival, and failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17:5–15. doi: 10.1097/ID.0b013e3181676059. [DOI] [PubMed] [Google Scholar]
- 6.Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 7.Chung DM, Oh TJ, Shotwell JL, Misch CE, Wang HL. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol. 2006;77:1410–20. doi: 10.1902/jop.2006.050393. [DOI] [PubMed] [Google Scholar]
- 8.Novaes AB, Jr, Papalexiou V, Muglia V, Taba M., Jr Influence of interimplant distance on gingival papilla formation and bone resorption: Clinical-radiographic study in dogs. Int J Oral Maxillofac Implants. 2006;21:45–51. [PubMed] [Google Scholar]
- 9.Misch CE, Dietsh-Misch F, Hoar J, Beck G, Hazen R, Misch CM. A bone quality-based implant system: First year of prosthetic loading. J Oral Implantol. 1999;25:185–97. doi: 10.1563/1548-1336(1999)025<0185:ABQISF>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Cochran DL, Hermann JS, Schenk RK, Higginbottom FL, Buser D. Biologic width around titanium implants. A histometric analysis of the implanto-gingival junction around unloaded and loaded nonsubmerged implants in the canine mandible. J Periodontol. 1997;68:186–98. doi: 10.1902/jop.1997.68.2.186. [DOI] [PubMed] [Google Scholar]
- 11.Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3:9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- 12.Hermann JS, Buser D, Schenk RK, Higginbottom FL, Cochran DL. Biologic width around titanium implants. A physiologically formed and stable dimension over time. Clin Oral Implants Res. 2000;11:1–11. doi: 10.1034/j.1600-0501.2000.011001001.x. [DOI] [PubMed] [Google Scholar]
- 13.Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416. doi: 10.1016/s0300-9785(81)80077-4. [DOI] [PubMed] [Google Scholar]
- 14.Jeong SM, Choi BH, Kim J, Lee DH. A 1-year prospective clinical study of soft tissue conditions and marginal bone changes around dental implants after flapless implant surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:41–46. doi: 10.1016/j.tripleo.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23:971–3. doi: 10.1111/j.1600-051x.1996.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 16.Salvi GE, Lang NP. Diagnostic parameters for monitoring peri-implant conditions. Int J Oral Maxillofac Implants. 2004;19(Suppl):116–27. [PubMed] [Google Scholar]
- 17.Andrade R, Espinoza M, Gómez EM, Espinoza JR, Cruz E. Intra- and inter-examiner reproducibility of manual probing depth. Braz Oral Res. 2012;26:57–63. doi: 10.1590/s1806-83242012000100010. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Thakur SL, Joshi SK, Kulkarni SS. Measurement of gingival thickness using digital vernier caliper and ultrasonographic method: A comparative study. J Investig Clin Dent. 2014;5:138–43. doi: 10.1111/jicd.12026. [DOI] [PubMed] [Google Scholar]
- 19.Brisman DL. The effect of speed, pressure, and time on bone temperature during the drilling of implant sites. Int J Oral Maxillofac Implants. 1996;11:35–7. [PubMed] [Google Scholar]
- 20.Nagpal S, Kamath S, Thakur S, Kulkarni S. Correlation between occlusal forces and marginal bone levels around implant-retained restorations: A clinico-radiological study. J Oral Implantol. 2012;38:261–9. doi: 10.1563/AAID-JOI-D-10-00024. [DOI] [PubMed] [Google Scholar]
- 21.Linkevicius T, Apse P, Grybauskas S, Puisys A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int J Oral Maxillofac Implants. 2009;24:712–9. [PubMed] [Google Scholar]
- 22.Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11–25. [PubMed] [Google Scholar]
- 23.Jemt T, Book K. Prosthesis misfit and marginal bone loss in edentulous implant patients. Int J Oral Maxillofac. 1996;11:620–5. [PubMed] [Google Scholar]
- 24.Akça K, Uysal S, Cehreli MC. Implant-tooth-supported fixed partial prostheses: Correlations between in vivo occlusal bite forces and marginal bone reactions. Clin Oral Implants Res. 2006;17:331–6. doi: 10.1111/j.1600-0501.2005.01169.x. [DOI] [PubMed] [Google Scholar]


