Abstract
Background:
Furcation defects represent one of the most demanding therapeutic challenges for periodontal therapy. Various treatment modalities have been tried with different success rates. The present study was undertaken to evaluate the efficacy of freeze-dried bone allograft (FDBA) with and without bioabsorbable guided tissue regeneration (GTR) membrane Healiguide® in the treatment of Grade II furcation defects.
Materials and Methods:
Ten patients with bilateral Grade II furcation defects were selected for the study. After phase I therapy, subjects were divided into two arms and treated in a split-mouth design. Ten defects were treated with FDBA alone in the control arm. Ten defects were treated with FDBA in conjunction with bioabsorbable GTR membrane Healiguide® in test arm. Clinical parameters like plaque index, gingival index, vertical probing depth, horizontal probing depth, and relative attachment level (RAL) were assessed at baseline, 3 months, and 6 months postoperatively.
Results:
At 6 months, clinical improvement was seen in both the arms with mean pocket depth reduction of 1.2 ± 1.032 mm and 1.7 ± 0.948 mm and mean horizontal probing depth reduction being 2.1 ± 1.969 mm and 1.6 ± 1.264 mm in control and test arm, respectively. Both surgical procedures resulted in a statistically significant reduction in vertical and horizontal probing depths.
Conclusion:
Both the arms demonstrated a significant improvement in the probing depth, horizontal furcation depth, and RAL at 6 months postsurgery in the treatment of Grade II furcation defects. However, on the intergroup comparison, there was no statistically significant difference in the results achieved between two arms.
Keywords: Collagen membrane, freeze-dried bone allograft, Grade II furcation defects, guided tissue regeneration
INTRODUCTION
Periodontal diseases are among the most prevalent and widely spread diseases worldwide contributing to tooth morbidity and mortality. The extension of inflammation from existing periodontal pockets leads to attachment and bone loss on the furcal surfaces of molars leading to furcation involvement.[1] The ideal goal of furcation therapy is to retain tooth intact and to completely close the furcation thereby returning the local condition to anatomic normalcy facilitating long-term maintenance therapy, and improving likelihood of tooth retention.
Over a period of time, histological and clinical studies both in animals and humans have greatly improved the knowledge regarding regeneration of the periodontium and much attention has been given to guided tissue regeneration (GTR), bone grafting or their combination. Gottlow et al.[2] first reported that GTR could be favorably employed to regenerate the lost periodontal tissues in Grade II furcation involvement. In the last decade, bioabsorbable barriers have gained popularity in GTR therapy.[3]
A variety of bone grafting materials is available for use in conjunction with GTR therapy. The addition of a bone graft in combination with a barrier has demonstrated improved bone density, probing depth reduction, and clinical attachment gain versus barrier alone in human Grade II furcations.[4] Freeze-dried bone allografts (FDBA) were first used in periodontal therapy in early 1970's and since 1950 have been used clinically in orthopedic therapy.[5]
The purpose of this study was to compare and evaluate the efficacy of FDBA with and without bioabsorbable GTR membrane (Healiguide®, Advanced biotech, Chennai, India) in the treatment of Grade II furcation defects.
MATERIALS AND METHODS
Sample size calculation
The sample size was calculated on the basis of results of the pilot study. The pilot study was conducted on four subjects which were not included in the final study sample. The sample size was calculated using the N Master software (Department of Biostatistics, Christian Medical College, Vellore, India). The mean difference in horizontal probing depth from baseline to 6 months among test and control arms was taken for calculation of the sample size.
The mean difference in horizontal probing depth among test arm was 1.90 ± 0.98 and control arm was 0.70 ± 0.67. The alpha error was taken at 5% and the power of the study was taken at 80%. The sample size was estimated to be 8/arm. To compensate for attrition, two extra samples were included per arm. A final sample size of 10/arm was taken.
Data collection
Subjects were selected from patients visiting regular outpatient department, Department of Periodontology, Subharti Dental College and Hospital, Meerut, Uttar Pradesh. An assessment was made by history of the subjects, clinical examination, and routine investigations. Patients diagnosed with moderate to advanced chronic periodontitis were selected for the study. Informed consent was taken from all the patients who participated in the study. Institutional ethical committee approved the study design and protocol (No. SDC/2008/07/03).
Ten patients of age group 30–55 years, with bilateral Grade II furcation involvement in mandibular molars, who fulfilled the inclusion criteria, were included in the study and treated with split mouth design. Control arm included ten defects treated with FDBA® alone and test arm included ten defects treated with FDBA® and Healiguide® membrane.
Inclusion criteria
Patients diagnosed with Grade II furcation defects
Horizontal probing depth ≥4 mm
Vertical probing depth ≥4 mm
Gingival margin coronal to or at the level of the roof of the furcation.
Exclusion criteria
Patients with systemic diseases
Patients with compromised immune system
Smokers
Patients with history of any recent periodontal surgery in past 6 months
Known allergic responses to collagen products
Uncooperative patients.
All patients underwent phase I therapy which included scaling and root planning in two sessions by a single operator (P6 Piezo electric scaler, BONART®, Taiwan R.O.C and Gracey Currettes, Hu Freidy®, Chicago, IL, USA), followed by oral hygiene instructions. Four weeks after the phase I therapy patients were recalled and evaluation was done to confirm the criteria for selection. Patients who fulfilled the criteria were selected for the study and randomly allocated to the control and test arms in which the surgical procedure was carried out.
The following clinical parameters were recorded: Gingival index,[6] Plaque index,[7] Vertical Probing depth-A customized acrylic stent with an occluso-apical groove was prepared to standardize the insertion of the probe. UNC-15 probe was used for the measurement. The stent was stored on the cast. Horizontal probing depth was measured using a Nabers Probe no. 2 calibrated in millimeters. Relative attachment level (RAL) was calculated by measuring the distance from the base of the pocket to the fixed point on the stent (metal wire). Radiographs of the sites were recorded pre-operatively and post-operatively [Figure 1] at 3 months and 6 months.
Figure 1.
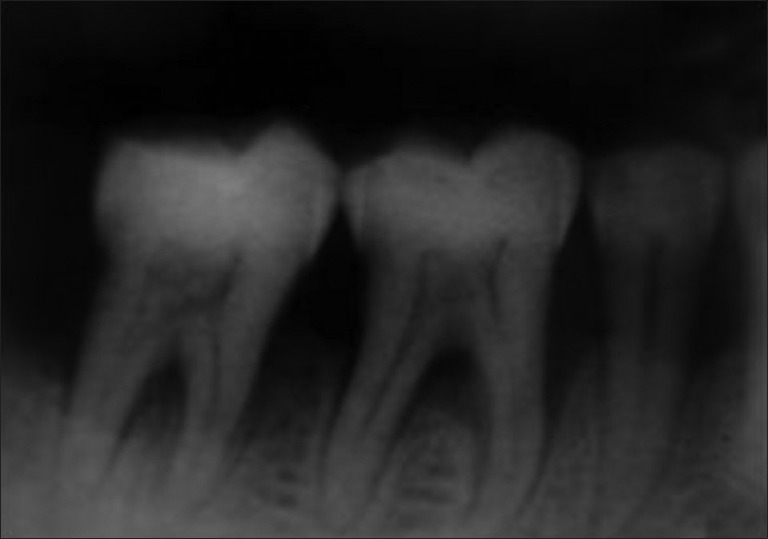
Radiograph at baseline
SURGICAL PROCEDURE
Control arm
Patients were scheduled for periodontal surgery after phase-I therapy. After injecting local anesthesia (2% Xylocaine® Dental with 1:80,000 adrenaline, Novocol Pharmaceutical of Canada, Cambridge, ON, Canada), at the site of surgery, sulcular incisions were given and full thickness flaps were raised to retain sufficient tissue and to obtain primary closure. Furcation defects were thoroughly debrided and root planed with hand and ultrasonic scalers [Figure 2]. Enamel projections, if noted were removed using a bur with a high-speed rotary instrument. This was followed by condensation of FDBA® graft material (By Tissue bank, Tata Memorial Hospital, Mumbai, India) [Figure 3] into the furcation defect. Flaps were then repositioned and sutured back (Mersilk®, Ethicon, Sommerville, NJ, USA). Periodontal dressing was applied over the site (Coe Pak®, GC America, Alsip, IL, USA).
Figure 2.

Grade II furcation in relation to 46 after flap reflection and debridement
Figure 3.
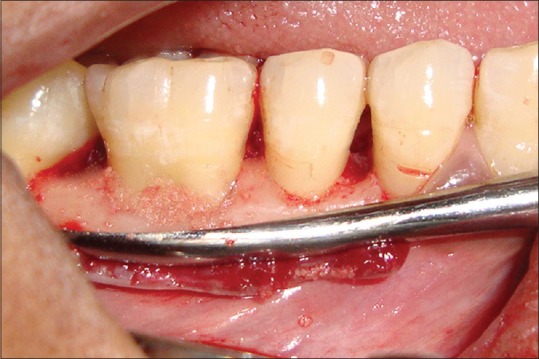
Freeze-dried bone allograft placed in Grade II furcation defect
Test arm
The subjects after phase I therapy underwent periodontal flap surgery as mentioned above. After thorough debridement of furcation defect, FDBA was condensed and covered by Healiguide® bioabsorbable GTR membrane [Figure 4] (Healiguide®, Advanced Biotech, Chennai, India). The membrane was stabilized with the help of a resorbable suture (Chromic Gut®, Ethicon, Sommerville, NJ, USA), and then the flap was sutured back in position. Periodontal dressing was applied over the site.
Figure 4.
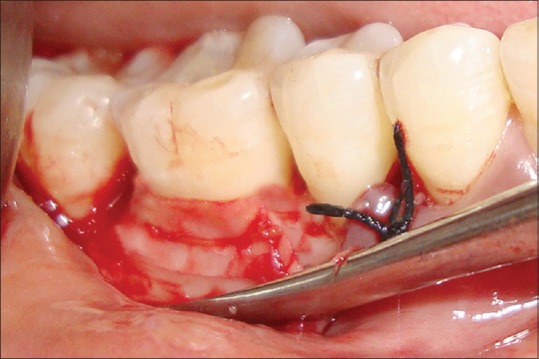
Guided tissue regeneration membrane Heliguide placed and sutured
Patients in both the surgical arms were subjected to a postoperative regimen of amoxicillin 500 mg tds for 5 days and ibuprofen 400 mg tds for 5 days and postoperative instructions were given. Patients were recalled for periodontal dressing and suture removal after 1 week. Patients were recalled at 1 month, 3 months, and 6 months post-operatively and clinical parameters and radiographs were recorded at 3 months [Figure 5] and 6 months [Figure 6] interval. Oral prophylaxis was performed in subjects if required at recall visits and reinforced the oral hygiene instructions.
Figure 5.
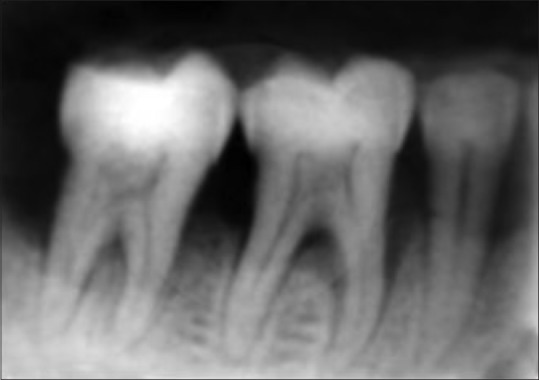
Radiograph at 3 months
Figure 6.
Radiograph at 6 months
Statistical analysis
The data included gingival index, plaque index, gingival bleeding index, probing depth (vertical and horizontal), RAL for both the treatment arms recorded at baseline, 3 months, and 6 months were tabulated and subjected to statistical analysis. The statistical analysis was carried out using the SPSS Version 16.0 (IBM Software Inc., Chicago, IL, USA). The mean and the standard deviation of all the clinical parameters at baseline, 3 months, and 6 months were calculated for both test and the control arms. The various parameters measured as the continuous variables were compared between the test and control arm at baseline, 3 months, and 6 months using the paired t-test. The comparison of various parameters within the test and control arm at the various intervals was done using the repeated measures ANOVA test with post-hoc Bonferroni test for the inter-interval comparison. The significance level was kept at 0.05 level.
RESULTS
No patient taking part in the study reported any local or systemic complication at any given point of time. It was observed not a single patient reported with any pain or swelling at the examination period of 6 months.
GINGIVAL INDEX
The mean gingival index score among Control arm at baseline was 1.52 ± 0.18 [Table 1] which decreased to 1.32 ± 0.18 [Table 1] at 6 months. The mean gingival index score among Test arm at baseline was 1.55 ± 0.15 [Table 1] which decreased to 1.25 ± 0.20 at 6 months. The mean gingival index score between the two arms were compared using the paired t-test at baseline, 3 months, and 6 months. The difference between both arms was found to be statistically not significant at baseline (P = 0.605), 3 months (P = 0.777), and 6 months (P = 0.418). Within control and test arms, the comparison at the different intervals was done using the repeated measure ANOVA test with post-hoc Bonferroni test. Within the control arm, there was a significant (P = 0.029) change in mean gingival score from baseline to 3 months and baseline to 6 months (P = 0.018). Whereas in the test arm, there was a significant change in mean gingival score from baseline to both 3 months (P = 0.019) and 6 months (P = 0.005) [Table 1].
Table 1.
Comparison of mean values of parameters at baseline, 3 months and 6 months among the control and test arm

PLAQUE INDEX
The mean plaque index at baseline in the control arm was 1.38 ± 0.13 [Table 1]. At 6 months, the plaque index decreased to 1.02 ± 0.04 [Table 1]. The mean plaque index at baseline in test arm was 1.38 ± 0.13 [Table 1]. At 6 months, the plaque index further decreased to 1.02 ± 0.04 [Table 1]. On comparison of the mean plaque scores between the two arms, differences were found to be statistically not significant at baseline (P = 1.000), 3 months (P = 0.876), and 6 months (P = 0.876). On comparison of the scores at different intervals in both the arms, it was observed that both control and test arm showed a significant change in mean plaque score from baseline to both 3 months (P = 0.001) and 6 months (P = 0.000) [Table 1].
PROBING DEPTH
The mean value for probing depth recorded at baseline in the control arm was 3.1 ± 1.10 mm [Table 1] which decreased to 1.9 ± 1.03 mm [Table 1] at 6 months.
The mean value for probing depth at baseline in test arm was 3.6 ± 1.07 mm [Table 1]. The value decreased to 1.9 ± 0.31 mm [Table 1] at 6 months. On comparison of the probing depth between the two arms, the difference between the two arms were found to be statistically not significant at baseline (P = 0.318), 3 months (P = 0.331), and 6 months (P = 1.000). In the test arm, significant change in mean probing depth from baseline to 3 months (P = 0.010) and 6 months (P = 0.001) months was seen. Similarly, results were seen in control arm with significant change from baseline to 3 months (P = 0.012) and 6 months (P = 0.015) [Table 1].
HORIZONTAL FURCATION DEPTH
The mean value for horizontal furcation depth recorded at baseline in the control arm was 4.6 ± 2.11 mm [Table 1] which decreased to 2.5 ± 0.52 mm [Table 1] at 6 months.
The mean value for horizontal furcation depth in test arm recorded at baseline was 4.6 ± 1.64 mm [Table 1] which decreased to 3.0 ± 0.66 mm [Table 1] at 6 months. On comparison of the scores at different intervals between both the arms, it was observed that the difference was statistically insignificant at baseline (P = 1.000), 3 months (P = 0.587), and 6 months (0.079). Both control and test arm showed a significant change in mean horizontal probing depth from baseline to 3 months and 6 months.
RELATIVE ATTACHMENT LEVEL
The mean value of RAL recorded at baseline in the control arm was 11.6 ± 2.22 mm [Table 1] which decreased to 9.5 ± 1.64 mm [Table 1] at 6 months.
The mean value for RAL in the test arm recorded at baseline was 11.8 ± 1.68 mm [Table 1] which decreased to 9.9 ± 1.85 mm [Table 1] at 6 months. On comparison of the mean RAL between the two arms, the result was statistically insignificant at baseline (P = 0.823), 3 months (P = 0.560), and 6 months (P = 0.616). On comparison of the scores at different intervals in both the arms, it was observed that test arm showed a significant (P = 0.010) change in mean RAL from baseline to 6 months, whereas the control arm showed significant change from baseline to both 3 months (P = 0.001) and 6 months (P = 0.001) [Table 1].
DISCUSSION
Freeze-dried bone allografts are prepared from cortical bone of donors. Cortical bone is preferred over cancellous bone because it has a higher concentration of bone inductive proteins and has less antigenicity. This antigenicity is further reduced by the process of freeze drying as it removes more than 95% of water content from the bone. FDBA are osteoconductive that is, they act as a trellis or scaffold over which new host bone can form.[8]
Several commercially available resorbable membranes have been developed with type I collagen as their main component. Collagen materials also possess additional advantages such as hemostasis and chemotaxis for periodontal ligament fibroblasts and gingival fibroblasts, reduced immunogenicity, easy manipulation, and ability to augment tissue thickness. Hence, collagen membranes are ideal for resorbable GTR membranes.[9] Healiguide® is a commercially available resorbable membrane with type I collagen as a major component which is of ovine origin.
It is believed that bone grafts not only provide osteoinductive/osteoconductive capacity for regeneration, but also maintain the space. Khanna et al.[10] reported that the combination of bone graft with GTR membrane yielded better results when compared to open flap debridement in the management of Grade II furcation defects. Prathap et al.[11] also concluded in their study that combination of GTR and bone graft was more effective that bone graft alone in the management of Grade II furcation Various researchers[12,13,14] used demineralized freeze-dried bone allograft (DFDBA) in conjunction with GTR membrane for the treatment of Grade II furcation defects. Rummelhart et al.[15] conducted a study comparing FDBA and demineralized freeze-dried bone allografts in human periodontal osseous defects. They found no significant difference in parameters recorded for both the groups recorded 6 months postsurgery. Apart from this, there are no studies in literature discussing the results obtained using FDBA in conjunction with GTR membrane in the treatment of Grade II furcation defects.
The results of the present study demonstrated a significant difference in the plaque score within the same arm at 3 months and 6 months from baseline for both control and test arm. The maintenance and significant improvement in the plaque scores and gingival index scores in the current study from baseline to 3 and 6 months, respectively, in the control arm and test arm which could be attributed to the fact that there was maintenance of strict plaque control and oral hygiene throughout the study period. The healing was uneventful in all the cases in both the arms. On a comparison between the test and control arms, there was no statistically significant difference between gingival index of both the arms. In the control arm, the probing depth, horizontal probing depth, and RAL showed a significant decrease from baseline to 6 month which was in accordance with the study of Tsao et al.[16]
In their study, Tsao et al.[16] evaluated clinical attachment level from baseline to 6 months by taking cemento-enamel junction as a fixed reference point. In the present study, a prefabricated stent with a guiding groove for reproducibility of probe insertion and angulations and a metal wire over the groove a fixed reference point were used to assess attachment level difference at 6 months. The results of the present study are in accordance to that of previous study of Tsao et al.[16]
The above-mentioned parameters (probing depth, horizontal furcation depth) attribute to the assessment of periodontal regeneration in control sites by the use of FDBA. The results demonstrated the positive effect of FDBA with a significant decrease in the parameters for regeneration. These results were similar with results of various studies demonstrating periodontal regeneration, mainly bone regeneration with the use of FDBA.[17,18,19,20]
In test arm, there was a significant decrease in probing depth evaluated from baseline to 6 months. This was in accordance with the results of various authors.[13,14,16] On comparison of test and control arms, there was no significant difference in probing depth at 3 months and 6 months from baseline. These results were contrary to that of Tsao et al.,[16] who found a significant difference in vertical bone fill in the control group and test group at 6 months with a mean difference of 1.9 ± 1.4 mm and 0.7 ± 0.9 mm at 6 months.
The present study also had a significant decrease in horizontal furcation depth from baseline at 3 months and 6 months with a mean difference of 1.0 ± 0.816 mm and 1.6 ± 1.26 mm, respectively in test arm. In previous studies,[13] similar results were demonstrated with a mean difference of 1.7 ± 1.22 mm.
The RAL assessed for test arm in present study showed significant changes at time interval from 3 months to 6 months and baseline to 6 months with a mean difference of 1.0 ± 1.15mm and 1.9 ± 1.52 mm, respectively. These were similar to those of Leonardis et al.,[14] as they found a significant change in clinical attachment level at 6 months with a mean difference of 2.3 ± 0.1 mm. There was no significant change in RAL on comparison of both the groups at any interval.
The parameters evaluated for regeneration (probing depth, horizontal furcation depth, and RAL) showed positive results attributing to successful periodontal regeneration in Grade II furcation defects treated in test arm. This could be explained by the fact apart from the effect of osteoconductive potential of FDBA which facilitated the formation of new host bone, additional placement of collagen membrane over FDBA could promote periodontal ligament fibroblast and osteoblast cell adhesion and enhance periodontal regeneration via collagen membrane hemostatic and chemotactic function.[21,22]
Piattelli et al. compared FDBA and DFDBA histologically and histochemically. The results showed that in the DFDBA sites, only those particles which were near the host bone were involved in mineralization whereas on the other hand in the FDBA sites, ethose particles which were even far from the host bone were involved in mineralization and were lined by osteoblasts. These results suggest more of an osteoconductive potential in FDBA than DFDBA.[23] Moreover, FDBA is economically more viable than DFDBA. Keeping these points in mind, in the present study, FDBA was used with and without bioabsorbable membrane in Grade II furcation defects.
The limitation of present study were:
Radiographic assessment was not done, as in cases of Grade II furcation radiographic assessment cannot be done only on the basis of Intraoral periapical, it requires advanced radiographic techniques which were not available
Ten defects each were treated in both the arms in the present study and the results demonstrated a lack of statistical difference between both the arms. A larger sample size could have given a statistically significant difference between both the arms.
CONCLUSION
From this study it could be concluded that:
Grade II furcation defects treated in both control and test arm demonstrated a significant improvement in the probing depth, horizontal furcation depth, and RAL at 6 months postsurgery
There was no statistically significant difference in the results obtained when compared between both the treatment modalities. Hence, further long-term studies with a larger sample size, radiographic assessment, and quality of bone assessment are required to substantiate the efficacy of FDBA with and without bioabsorbable GTR membrane in the treatment of Grade II furcation defects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank Dr. Sukhvinder Singh Oberoi for his contribution in statistical analysis of the present study.
REFERENCES
- 1.Ammons WF, Harrington GW. Furcation: Involvement and treatment. In: Newman MG, Takei HH, Klokkevol PR, Carranza FA, editors. Clinical Periodontology. 10th ed. St. Louis: Saunders; 2004. pp. 991–1004. [Google Scholar]
- 2.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol. 1986;13:604–16. doi: 10.1111/j.1600-051x.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 3.Houser BE, Mellonig JT, Brunsvold MA, Cochran DL, Meffert RM, Alder ME. Clinical evaluation of anorganic bovine bone xenograft with a bioabsorbable collagen barrier in the treatment of molar furcation defects. Int J Periodontics Restorative Dent. 2001;21:161–9. [PubMed] [Google Scholar]
- 4.Chen TH, Tu YK, Yen CC, Lu HK. A systemic review and meta analysis of guided tissue regeneration/osseous grafting for treatment of class II furcation defects. J Dent Sci. 2013;8:209–24. [Google Scholar]
- 5.Grover V, Kapoor A, Malhotra R, Sachdeva S. Bone allografts: A review of safety and efficacy. Indian J Dent Res. 2011;22:496. doi: 10.4103/0970-9290.87084. [DOI] [PubMed] [Google Scholar]
- 6.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 7.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 8.Mellonig JT. Freeze-dried bone allografts in periodontal reconstructive surgery. Dent Clin North Am. 1991;35:505–20. [PubMed] [Google Scholar]
- 9.Bunyaratavej P, Wang HL. Collagen membranes: A review. J Periodontol. 2001;72:215–29. doi: 10.1902/jop.2001.72.2.215. [DOI] [PubMed] [Google Scholar]
- 10.Khanna D, Malhotra S, Naidu DV. Treatment of Grade II furcation involvement using resorbable guided tissue regeneration membrane: A six-month study. J Indian Soc Periodontol. 2012;16:404–10. doi: 10.4103/0972-124X.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prathap S, Hegde S, Kashyap R, Prathap MS, Arunkumar MS. Clinical evaluation of porous hydroxyapatite bone graft (Periobone G) with and without collagen membrane (Periocol) in the treatment of bilateral Grade II furcation defects in mandibular first permanent molars. J Indian Soc Periodontol. 2013;17:228–34. doi: 10.4103/0972-124X.113083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderegg CR, Martin SJ, Gray JL, Mellonig JT, Gher ME. Clinical evaluation of the use of decalcified freeze-dried bone allograft with guided tissue regeneration in the treatment of molar furcation invasions. J Periodontol. 1991;62:264–8. doi: 10.1902/jop.1991.62.4.264. [DOI] [PubMed] [Google Scholar]
- 13.Luepke PG, Mellonig JT, Brunsvold MA. A clinical evaluation of a bioresorbable barrier with and without decalcified freeze-dried bone allograft in the treatment of molar furcations. J Clin Periodontol. 1997;24:440–6. doi: 10.1111/j.1600-051x.1997.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 14.De Leonardis D, Garg AK, Pedrazzoli V, Pecora GE. Clinical evaluation of the treatment of class II furcation involvements with bioabsorbable barriers alone or associated with demineralized freeze-dried bone allografts. J Periodontol. 1999;70:8–12. doi: 10.1902/jop.1999.70.1.8. [DOI] [PubMed] [Google Scholar]
- 15.Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989;60:655–63. doi: 10.1902/jop.1989.60.12.655. [DOI] [PubMed] [Google Scholar]
- 16.Tsao YP, Neiva R, Al-Shammari K, Oh TJ, Wang HL. Effects of a mineralized human cancellous bone allograft in regeneration of mandibular Class II furcation defects. J Periodontol. 2006;77:416–25. doi: 10.1902/jop.2006.050109. [DOI] [PubMed] [Google Scholar]
- 17.Mellonig JT, Bowers GM, Bright RW, Lawrence JJ. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. J Periodontol. 1976;47:125–31. doi: 10.1902/jop.1976.47.3.125. [DOI] [PubMed] [Google Scholar]
- 18.Sepe WW, Bowers GM, Lawrence JJ, Friedlaender GE, Koch RW. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects – Part II. J Periodontol. 1978;49:9–14. doi: 10.1902/jop.1978.49.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Altiere ET, Reeve CM, Sheridan PJ. Lyophilized bone allografts in periodontal intraosseous defects. J Periodontol. 1979;50:510–9. doi: 10.1902/jop.1979.50.10.510. [DOI] [PubMed] [Google Scholar]
- 20.Schrad SC, Tussing GJ. Human allografts of iliac bone and marrow in periodontal osseous defects. J Periodontol. 1986;57:205–10. doi: 10.1902/jop.1986.57.4.205. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel AK, Möhler H, Busch F, Mehl A. Preclinical and clinical studies of a collagen membrane (Bio-Gide®) Biomaterials. 1997;18:535–8. doi: 10.1016/s0142-9612(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 22.Locci P, Calvitti M, Belcastro S, Pugliese M, Guerra M, Marinucci L, et al. Phenotype expression of gingival fibroblasts cultured on membranes used in guided tissue regeneration. J Periodontol. 1997;68:857–63. doi: 10.1902/jop.1997.68.9.857. [DOI] [PubMed] [Google Scholar]
- 23.Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: A histological and histochemical study in man. Biomaterials. 1996;17:1127–31. doi: 10.1016/0142-9612(96)85915-1. [DOI] [PubMed] [Google Scholar]


