Abstract
Background:
Various bone graft materials have been used in the treatment of periodontal defects. A synthetic bone substitute material composed of P-15 with anorganic bone mineral has been scantly studied. Hence, the present study was aimed to evaluate and compare the efficacy of anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) in human periodontal infrabony defects with that of open flap debridement (OFD) alone.
Materials and Methods:
A split-mouth, randomized controlled clinical study was designed to investigate the efficacy of ABM/P-15. In this clinical trial, 10 patients having bilateral periodontal infrabony defects were treated either with ABM/P-15 or OFD and followed for a period of 9 months. At baseline and at 9 months probing pocket depth (PPD), relative attachment level (RAL), depth of a defect, and radiographic bone level were measured; and compared between test and control sites.
Results:
A statistically significant reduction (P < 0.001) in PPD was observed in test sites compared to control sites. Both sites showed a gain in RAL without any significant difference. Similarly, the radiographic evaluation revealed significantly higher radiographic defect fill in test sites as compared to control sites (P < 0.001).
Conclusion:
ABM/P-15 bone graft material appears to be useful and beneficial in the treatment of human periodontal infrabony defects.
Keywords: Cell binding peptide P-15, grafts, hydroxyapatite, periodontal disease, periodontal regeneration
INTRODUCTION
Regeneration of supporting tissues of the teeth is the primary goal of periodontal therapy, and it includes modalities such as bone grafts, root conditioning, and guided tissue regeneration.[1] Various bone graft materials such as allografts, xenografts, alloplasts, bioactive glasses, and polymers have shown positive clinical results in treating infrabony defects.[2] However, these bone graft materials do not induce bone formation instead they act as a scaffold to enhance bone deposition. In spite, these materials demonstrates clinical effectiveness, functional periodontal repair, defect fill, and reduction of the pocket depth to manageable levels.
Intense research is currently underway in different laboratories to identify, characterize, purify, and synthesize a variety of biologic modulators that may enhance wound healing and regeneration of lost tissues by periodontal therapy. One such approach has been to identify the specific cell binding domain of Type I collagen. Based on this approach Qian and Bhatnagar developed a synthetic bone substitute material composed of P-15 with an organic bone mineral derived from bovines.[3] The peptide component (P-15) is a synthetic clone of 15 amino acid sequence (GTPGPQGIAGQRGVV) with steric similarities to the cell-binding site of Type I collagen. The ABM represents a natural hydroxyapatite skeleton that appears more physiological during wound healing. It has been shown that the P-15 helps in binding of cells particularly fibroblasts and osteoblasts.[4] The combination of above two approaches in the form of ABM/P-15 bone graft material (PepGen P-15™) has shown to enhance the attachment of cells and to promote attachment of periodontal fibroblasts to the ABM carrier in in vitro studies by regulating specific gene expressions of cells during early wound healing.[5]
Thus, PepGen P-15™ participates in the initial phase of bone regeneration as the P-15 helps in the fundamental requirement of wound healing by cell migration, differentiation, and cell attachment. This material could bypass many of the safety; supply and consistency issues associated with autografts and allografts; and provide a more beneficial material for periodontal regeneration. Until now, scant published literature exists from the controlled clinical studies evaluating the healing process of periodontal disease infrabony defects after treatment with ABM/P-15 graft material. Therefore, the present study was undertaken with objectives to evaluate and compare the treatment of infrabony periodontal defects with ABM/P-15 particulate bone replacement graft (test site) with that of open flap debridement (OFD) therapy alone (control site).
MATERIALS AND METHODS
Experimental design and study subjects
A longitudinal, split mouth, randomized controlled clinical study was designed to investigate the efficacy of ABM/P-15 particulate (PepGen P-15 particulate, Dentsply Friadent, Germany) in infrabony periodontal defects, as compared to conventional flap debridement therapy alone.
Ten generally healthy patients belonging to either gender (3 males and 7 females, aged 30–60 years) having bilateral, moderate to severe (≥6 mm) periodontal osseous defects were selected from the outpatient Department of Periodontics, Dental College; and followed for a period of 9 months.
Ethical clearance for the study was obtained from the Institutional Review Board of the Dental College. A written informed consent was obtained from the patients after explaining the surgical re-entry procedure involved in the study. The inclusion criteria comprised of: (1) The absence of any medical conditions that contraindicate periodontal surgical procedure, (2) not undergone any periodontal treatment in the past 6 months, (3) no history of use of antibiotics for the last 6 months, (4) no pregnancy or lactation, (5) agree to follow-up compliance.
Clinical parameters
A single calibrated examiner evaluated the clinical parameters at baseline before regenerative therapy and 9 months after surgery by using University of North Carolina-15 probe. Clinical parameters such as probing pocket depth (PPD), relative attachment level (RAL), and depth of defect (DOD) were recorded. PPD was measured from the free margin of the gingiva to the base of the pocket. An acrylic stent was used to measure RAL and DOD. RAL was recorded after occlusal adjustment.
Stent fabrication
Alginate impressions were taken, casts were poured, and a stent was fabricated by using clear acrylic. A groove was made in order to duplicate the placement of the probe both apico-coronal and mesiodistally at the end of 9 months to minimize the error in postoperative measurements. Standardized radiographs were also taken at baseline [Figures 1–8].
Figure 1.
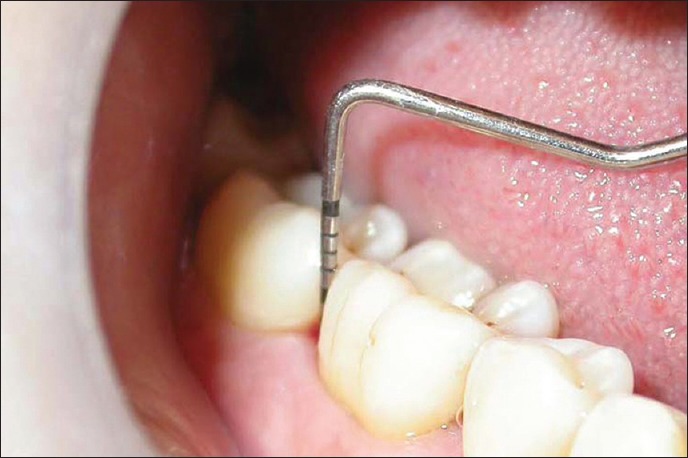
Probing pocket depth in test site at baseline
Figure 8.
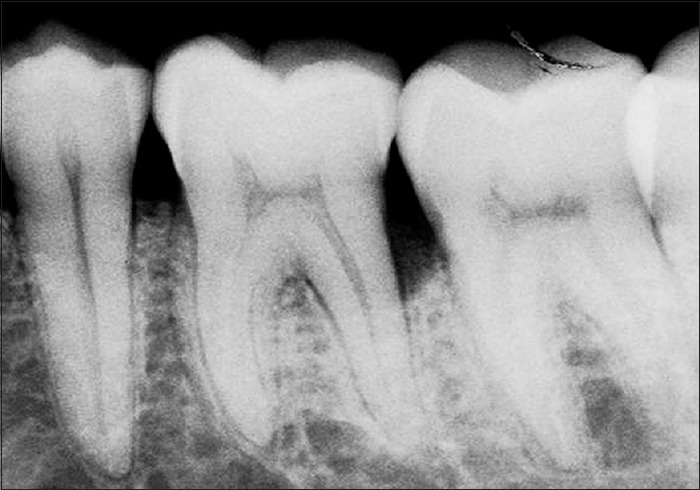
Radiograph in control site at baseline
Figure 2.
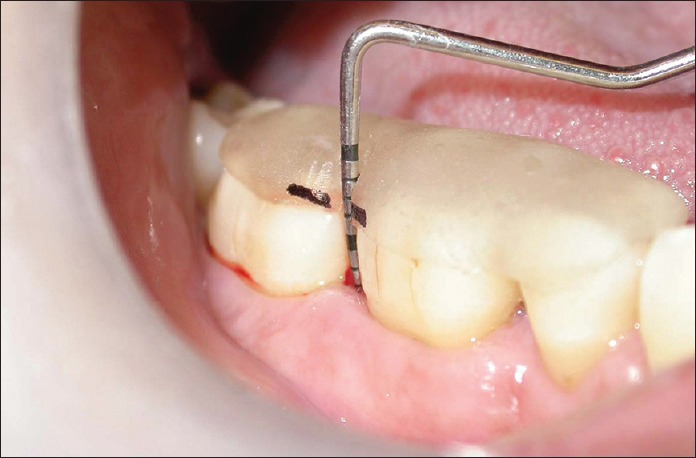
Relative attachment level in test site at baseline
Figure 4.
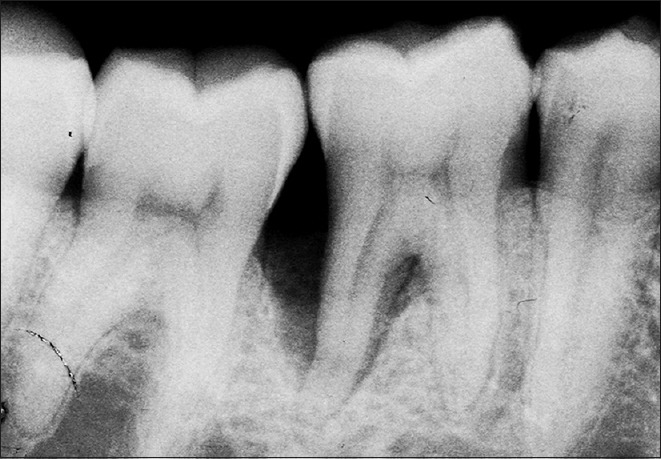
Radiograph in test site at baseline
Figure 5.
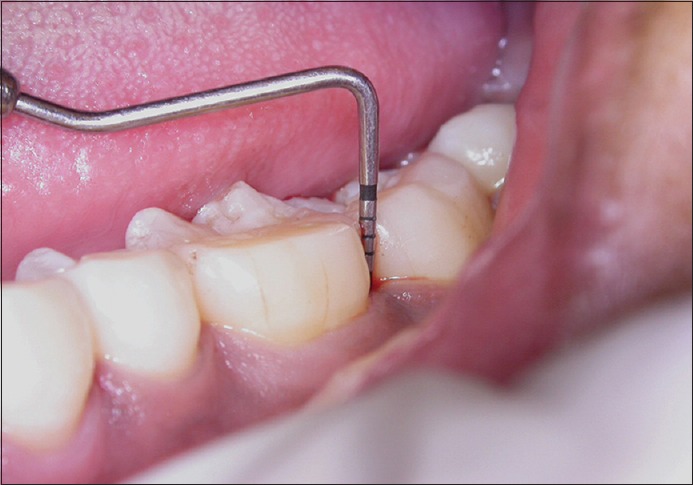
Probing pocket depth in control site at baseline
Figure 6.
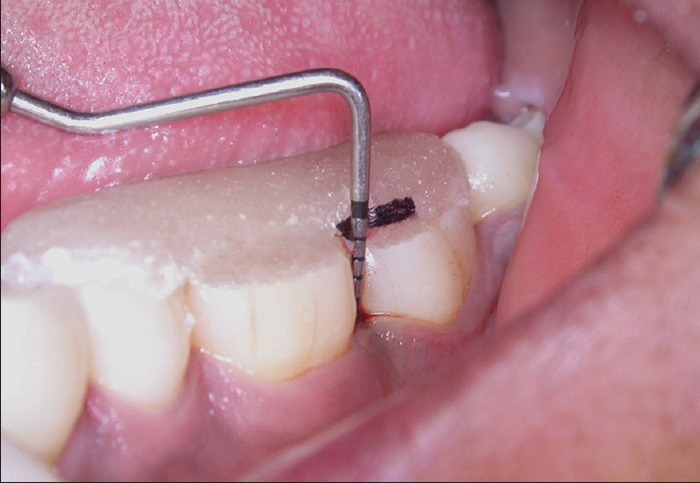
Relative attachment level in control site at baseline
Radiographs
Standardized periapical radiographs were taken at baseline and at 9 months for all defects by using long cone paralleling technique and Kodak Ekta speed films. Exposures were made at 70 kVp, 8 mA for 0.6 s (Planmeca, Finland). The radiographs were taken by a single radiologist throughout the study to minimize discrepancies. All the radiographs were scanned and digitized, and linear measurements were made on the digitized images by using Adobe Photoshop 7.0 (Adobe systems Incorporated) computer software program
Presurgical preparation
Prior to the surgical appointment, participants received oral hygiene instructions, scaling and root planning, and occlusal adjustments if needed. Surgical therapy was initiated only after patients demonstrated an acceptable efficiency in plaque control procedures and a satisfactory tissue response was observed.
Surgical procedure
The selected sites were randomly assigned as either control or experimental site. After adequate anesthesia of the surgical site, a full thickness mucoperiosteal flap was reflected bucally and lingually, and the osseous defect was exposed. A thorough surgical degranulation of the infected tissue was done, and exposed root surfaces were thoroughly scaled and root planed using hand instruments. The surgical site was thoroughly irrigated with saline. No attempt was made to perform osteoplasty or osteotomy at the surgical site. After a complete debridement of the surgical site, DOD was measured [Figures 3 and 7]. Then, subjects were treated with either ABM/P-15 or OFD. The ABM/P-15 test graft material was hydrated with saline as per the manufacturer's instructions in a sterile dappen dish and delivered in small increments to the defect by lightly condensing to adapt the particles to the configuration of the defect in the test site [Figure 9]. In the control site, only OFD was performed.
Figure 3.
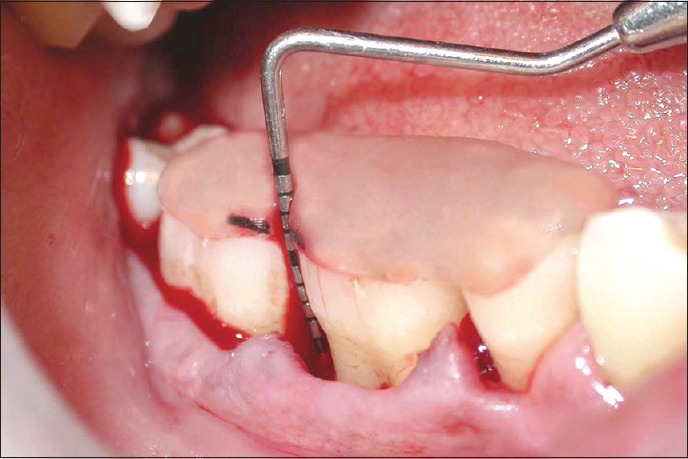
Depth of the defect in test site at baseline
Figure 7.
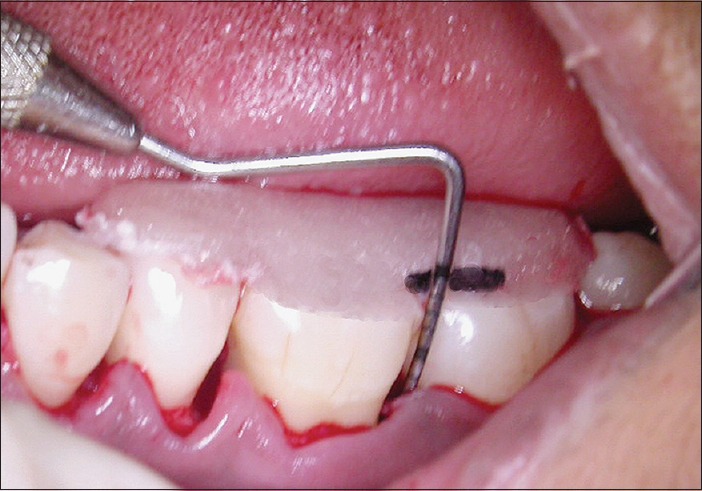
Depth of the defect in control site at baseline
Figure 9.
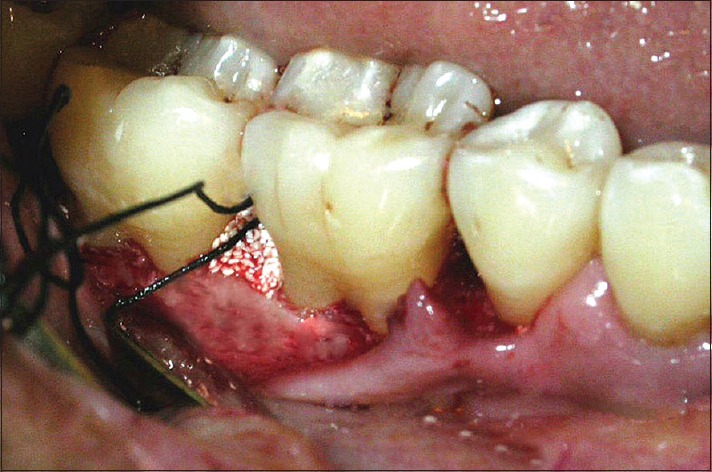
Placement of graft material in test site
The mucoperiosteal flaps were repositioned and secured in place using black braided (3–0) silk suture. The surgical site was protected with a noneugenol periodontal dressing to avoid tissue irritation.
Postoperative infection control
The patients were prescribed with chlorhexidine digluconate 0.2% mouth rinse twice daily for 2 weeks. Postoperative antibiotic therapy included Amoxicillin 250 mg 3 times daily for 7 days. Pain was controlled with ibuprofen 400 mg 3 times daily for 3 days. The dressing and sutures were then removed after 1-week. All study subjects were placed on a regular maintenance schedule of every 1, 3, 6, and 9 months. At each recall visit, oral hygiene was assessed, and oral hygiene instructions were reinforced. Neither probing nor deep scaling was attempted until 9 months.
At 9 months postsurgically, standardized radiographs were taken, and clinical parameters were recorded, and re-entry flap procedure was performed to expose the defects under investigation for documentation [Figures 10–17]. The radiographic bone level (RBL) was measured using software program (Adobe Photoshop 7.0).
Figure 10.
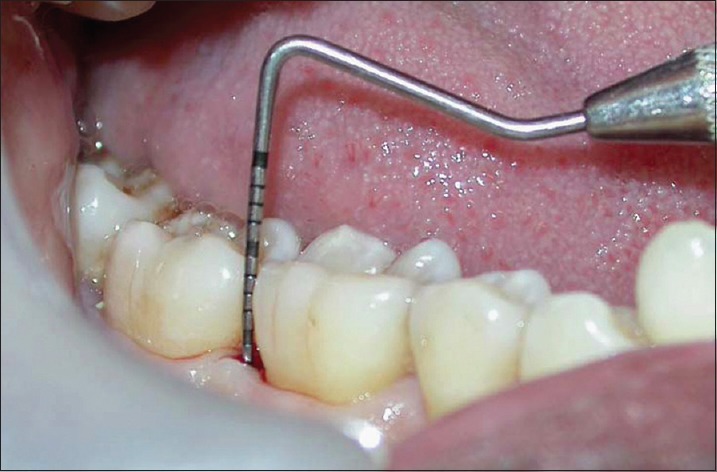
Probing pocket depth in test site at 9 months
Figure 17.
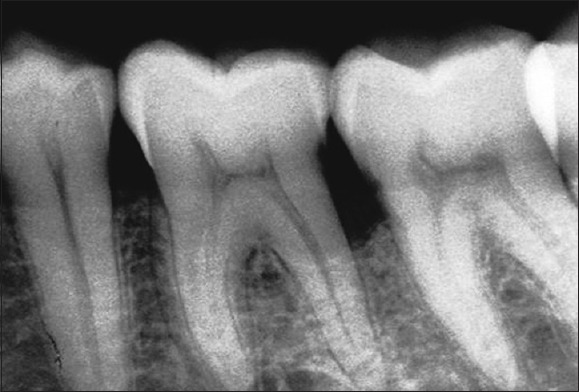
Radiograph in control site at 9 months
Figure 11.
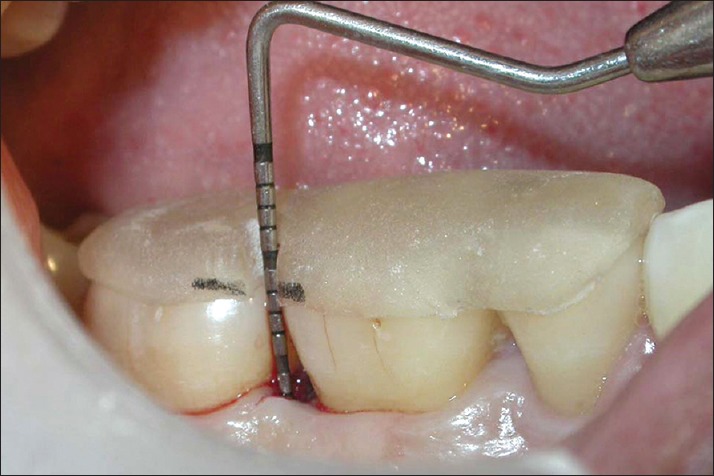
Relative attachment level in test site at 9 months
Figure 12.
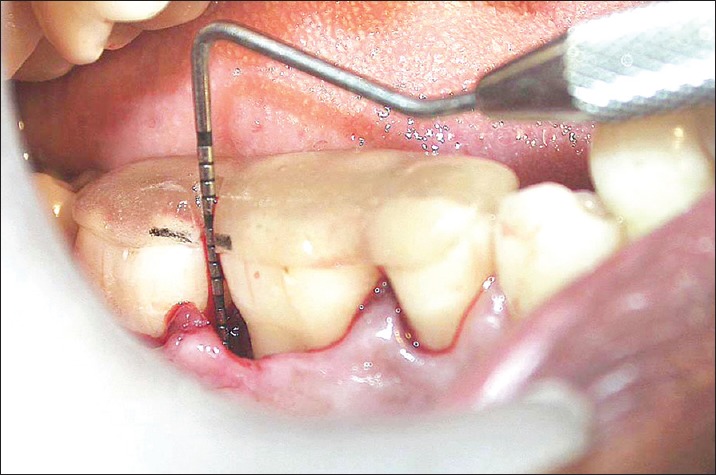
Depth of the defect in test site at 9 months
Figure 13.
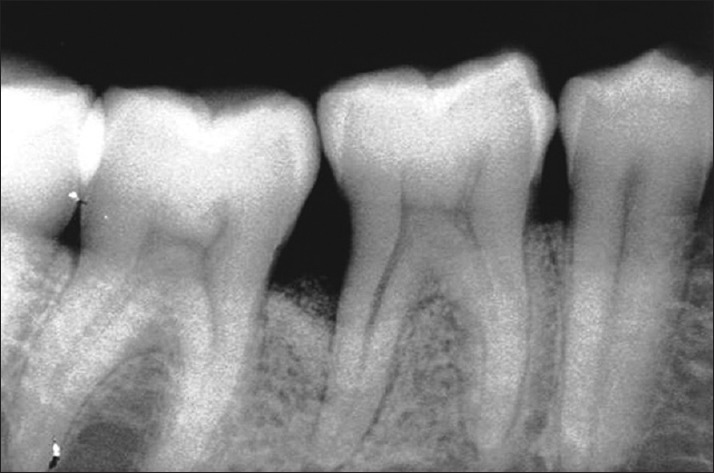
Radiograph in test site at 9 months
Figure 14.
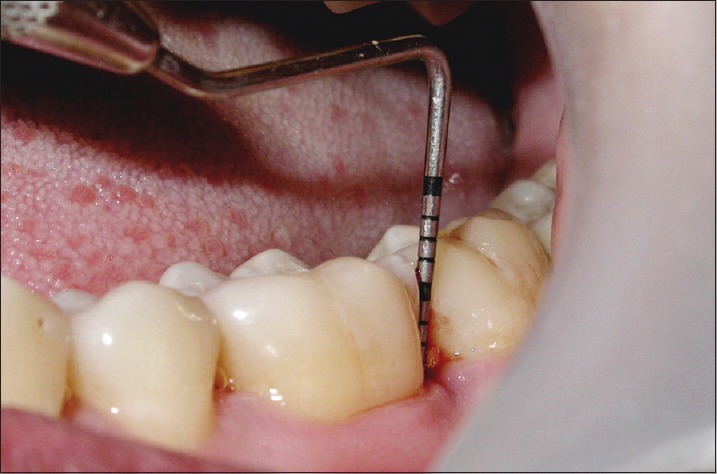
Probing pocket depth in control site at 9 months
Figure 15.
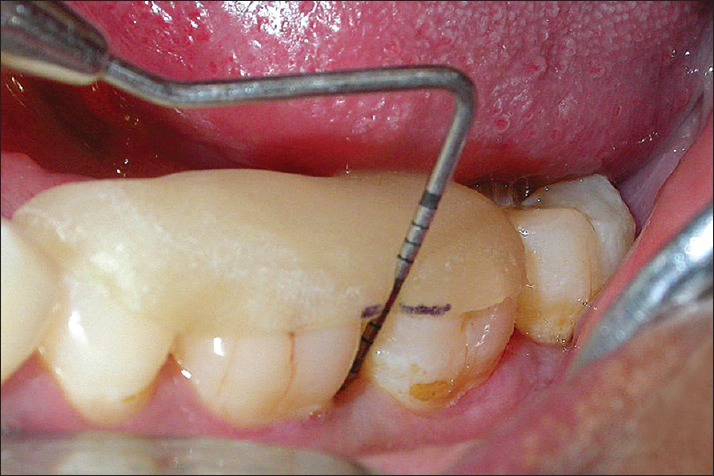
Relative attachment level in control site at 9 months
Figure 16.
Depth of the defect in control site at 9 months
Statistical analysis
Statistical Package for the Social Sciences (SPSS, 14.0 for Windows, Chicago, IL, USA) was used to perform all statistical analysis. Mean and standard deviation were calculated for each clinical parameter. The differences between the test and control site were analyzed by using the Student's paired t-test and unpaired t-test from baseline to 9 months. The level of significance was set at P = 0.05.
RESULTS
A total of 10 patients having bilateral, moderate to severe (≥6 mm) periodontal osseous defects (test site, n = 10, the control site, n = 10) completed the 9 months follow-up period. In all cases, postoperative healing was without any complications and infections.
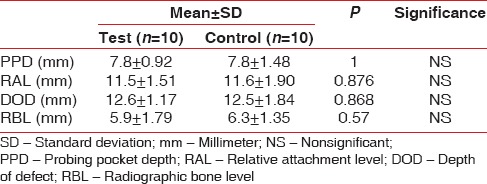
At baseline, the defect characteristics of the test and control sites showed that the mean and standard deviation of PPD (mm) in test site were 7.8 ± 0.92, and in control site was 7.8 ± 1.48. RAL was found to be 11.5 ± 1.51 in test site and 11.6 ± 1.90 in control site. Mean and standard deviations of DOD (mm) in test site were 12.6 ± 1.17 and control site 12.5 ± 1.84. Similarly, RBL (mm) was 5.9 ± 1.79 at test site and 6.3 ± 1.35 at control site, respectively, at baseline.
Defect characteristics did not yield any statistical significant difference at baseline as shown in Table 1.
Table 1.
Baseline variables of the test and control sites
The clinical results (PPD, RAL, DOD, and RBL) at baseline and 9 months after surgery are summarized in Table 2. When compared to the baseline data both test and control sites showed statistically significant reductions of PPD, RAL, DOD, and RBL (P < 0.001).
Table 2.
Clinical and radiological parameters at baseline and 9 months for the test and control sites
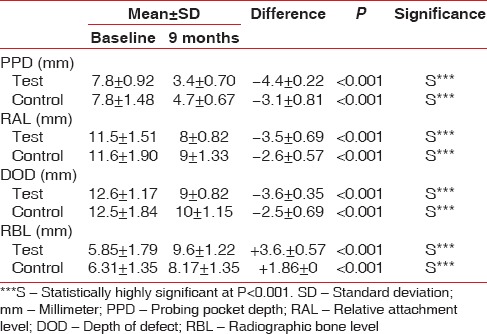
At 9 months after therapy test site showed a reduction in the mean PPD from 7.8 ± 0.92 mm to 3.4 ± 0.70 mm, and a change in mean RAL from 11.5 ± 1.51 mm to 8 ± 0.82 mm (P < 0.001). Mean DOD change of 12.6 ± 1.17 mm to 9 ± 0.82 mm (P < 0.001) was seen. Similarly, mean RBL in test site increased from 5.85 ± 1.79 mm to 9.6 ± 1.22 mm (P < 0.001). Likewise, mean values PPD, RAL, DOD, and RBL showed statistically significant difference in control sites when compared to the baseline values (P < 0.001). In control site a mean PPD reduction of 7.8 ± 1.48 mm to 4.7 ± 0.67 mm (P < 0.001) and a mean change of RAL 11.6 ± 1.90 mm to 9 ± 1.33 mm (P < 0.001) was observed. A mean DOD change of 12.5 ± 1.84 mm to 10 ± 1.15 mm (P < 0.001) was noticed in the control site. However, a mean RBL in control site increased from 6.31 ± 1.35 mm to 8.17 ± 1.35 mm (P < 0.001).
The mean PPD was very significantly reduced in the test sites as compared to the control sites (P < 0.001). Similarly, mean DOD was also significantly reduced in the test sites as compared to the control sites (P < 0.05). However, mean RAL did not show any significant difference [Table 3]. A further radiographic evaluation revealed that the mean radiographic defect fill (RDF) was significantly higher in the test sites as compared to control sites (P < 0.001) as shown in Figure 18. The frequency distribution of clinical attachment gains in the test and control sites is depicted in Figure 19. For the test, sites mean clinical attachment level gains ranged from 4.4 mm to 3.5 mm. However, the control sites showed mean clinical attachment level gains of 1.86 mm to 3.1 mm.
Table 3.
Comparison of clinical parameters between test and control sites at 9 months
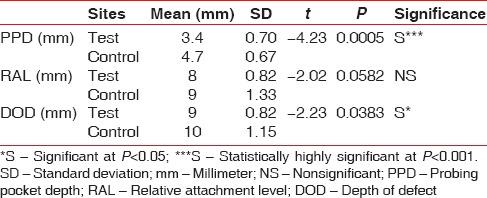
Figure 18.
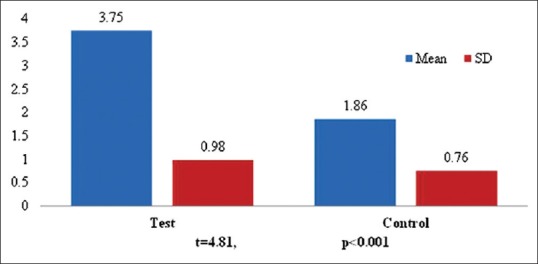
Comparison of mean Radiographic Defect Fill between test and control sites
Figure 19.
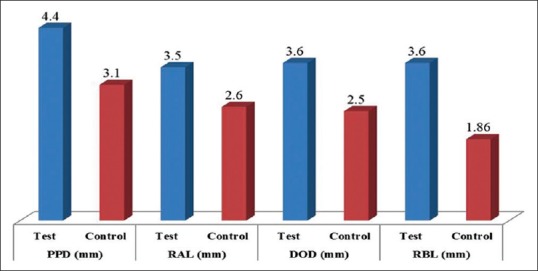
Changes in Clinical and Radiologic parameters in test and control sites at 9 months
DISCUSSION
The ideal goal of periodontal therapy is to provide a dentition that functions in health and comfort for the life of the patient.[6] In order to achieve this goal, regenerative periodontal therapy is carried out. Bone grafting is the most common form of regenerative therapy today and is essential for restoring the periodontal supporting tissues.[1] A variety of biological and synthetic materials are available for regeneration of alveolar bone. The ideal material should be nonantigenic and induces bone formation but, synthetic hydroxyapatite does not meet these criteria since, they act as inert fillers and have relatively few osteogenic properties.[7,8] Several studies have shown that they heal by fibrous encapsulation.[8,9,10] Subsequently, a nonantigenic ABM material was introduced which retains more physiological characteristics of bone mineral than synthetic hydroxyapatite and has been shown to be efficacious as an osteoconductive biomaterial in several experimental animal and clinical studies.[3] With improved technology, this material could serve as a delivery vehicle for growth or differentiation factors helping in regeneration. Jiang et al. showed that the platelet-derived growth factor with anorganic bovine bone stimulates bone regeneration.[7] Based on these data, a synthetic peptide (P-15) adsorbed on anorganic bovine bone was developed and made commercially available as PepGen P-15™.[3]
The aim of the present controlled clinical trial was to evaluate and compare the effectiveness of filling the infrabony defect with ABM/P-15 (test) in its particulate form that of OFD alone (control). The study was conducted over a period of 9 months as the duration of three or six was not enough to assess the bone formation. Patients showing signs of chronic periodontitis were enrolled into the study. PPD, DOD, RAL, and RBL were recorded at baseline and at 9 months between test and control sites. Both the treatments resulted in clinically significant improvement between baseline and 9 months.
PPD was assessed to determine the response of the soft tissue. RAL was measured using a customized acrylic stent, to serve as a fixed reference point.[11] DOD was measured to assess the defect fill. There was no statistically significant difference observed in the mean baseline values between the test and control sites.
At re-entry, the test sites showed a good amount of defect fill compared to the control site. At 9 months, the results for the ABM/P-15 particle sites demonstrate RAL gain of 3.5 ± 0.69 mm and an average probing pocket reduction of 4.4 ± 0.22 mm. The size of the RAL gain (3.5 mm) observed in the test (ABM/P-15 particle) sites is well within the best results ranging from 1.3 mm to 4 mm obtained from previous clinical trials using bovine-derived grafts alone or combined with barrier membranes or enamel matrix proteins or ABM/P-15 flow material in the treatment of infrabony defects.[2],8,12,[13,14,15] The reduction in PPD in the test site was in accordance with studies reported by Yukna et al.,[2,16,17,18] and Radhakrishnan and Anusuya.[19] Other studies conducted by different authors also showed a similar reduction in PPD with the use of anorganic bovine bone.[20,21] The control sites also showed a reduction in PPD similar to the study reported by Froum et al. in which significant improvement in PPD at 6–7 months following OFD was observed.[9] However, the test site showed more reduction in PPD as compared to the control site.
The gain in RAL in the test and control sites was found to be statistically insignificant indicating that both the treatment modalities showed increased gain in RAL. The gain in the DOD at the test site was more than in control sites. These findings were similar to those reported by previous studies.[2,16,17,18,19] Available published clinical data suggests that in human periodontal osseous defect any bone replacement graft material will produce 60–70% of the bone fill independent of the material used by the clinician. Therefore, defect fill of 25–35% following surgical debridement could be a common observation.[16] Although clinical studies have proved that clinical attachment gain following regenerative periodontal surgery is mainly dependent on the defect depth and configuration.[22,23,24,25] It has been reported that the greater the initial defect depth, the better the amount of clinical attachment gain, Deeper defects have a higher potential for bone healing due to their enhanced source of osteogenic cells from periodontal ligament and adjacent alveolar bone.[26] The use of ABM/P-15 alone in the treatment of grade II furcation was found to be beneficial and cost-effective as compared to the ABM/P-15 with the biodegradable membrane.[27] However, in the present study no attempt was made to treat any Grade II furcation defects.
In the test sites, residual graft particles were attached to the soft tissue, as well as embedded in the defect fill, showing an appearance of partially calcified matrix [Figure 20]. This condition was in agreement with the reported series of studies by Yukna et al., in which they found graft particles clinically, as well as in histologic sections.[2,16,17,18] This shows that anorganic bovine bone mineral matrix/cell binding peptide P-15 (PepGen P-15™) takes a longer time to undergo complete resorption and replacement by new bone. It has been shown that ABM material resorbs very slowly, and histologic evidence suggests new bone formation on, around and among the ABM/P-15 particles, incorporating them into the new bone.[2] In the present study, clinically the bone fill did not show the complete bone formation or hard tissue formation even at 9 months. The grafted areas, as well as the control sites, resisted penetration by the probe or removal with a curette, which is similar to the study reported by Rabalais et al.,[28] and Meffert et al.[29] However, it should be borne in mind that the placement of graft material itself into the defect could have modified gingival tissue consistency limiting the penetration of probe without inducing the actual gain in clinical attachments.[30]
Figure 20.
Defect fill with graft particles in test site at 9 months
Radiographic assessment of the hard tissue was done using the Adobe Photoshop 7.0 software. Radiographs at its best represent a two-dimensional image of a three-dimensional defect and probably this could be the cause for the discrepancies in the clinical and RDF. Moreover, it is difficult to achieve absolutely reproducible radiographs in all cases. Thus, in order to minimize the variations and to provide a degree of standardization the present study utilized radiographs with a paralleling sensor holder technique. Krejci et al. suggested that radiographs should not be used as the sole method to assess the osseous defect fill in infrabony defects due to the difficulties in estimation of the amount of defect fill.[31]
CONCLUSION
From the results of the present study, it can be concluded that the natural hydroxyapatite, anorganic bone matrix combined with a synthetic cell binding peptide (ABM/P-15) appears to be a useful and beneficial material in the treatment of human periodontal infrabony defects. The use of this material may effectively circumvent many of the safety; supply and consistency issues associated with autografts and allografts; and provide a more beneficial material for regeneration. However, further studies including human histologic evidences are needed to elucidate true periodontal regeneration by using ABM/P-15 in its particulate form in the treatment of infrabony periodontal defects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank the staff members and postgraduate students of Department of Periodontics and Department of Oral Medicine SDM Dental College and Hospital Dharwad, and the patients who participated in the study.
REFERENCES
- 1.Rosenberg E, Rose LF. Biologic and clinical considerations for autografts and allografts in periodontal regeneration therapy. Dent Clin North Am. 1998;42:467–90. [PubMed] [Google Scholar]
- 2.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects 6-month results. J Periodontol. 2000;71:1671–9. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- 3.Barboza EP, de Souza RO, Caúla AL, Neto LG, Caúla Fde O, Duarte ME. Bone regeneration of localized chronic alveolar defects utilizing cell binding peptide associated with anorganic bovine-derived bone mineral: A clinical and histological study. J Periodontol. 2002;73:1153–9. doi: 10.1902/jop.2002.73.10.1153. [DOI] [PubMed] [Google Scholar]
- 4.Lallier TE, Yukna R, St Marie S, Moses R. The putative collagen binding peptide hastens periodontal ligament cell attachment to bone replacement graft materials. J Periodontol. 2001;72:990–7. doi: 10.1902/jop.2001.72.8.990. [DOI] [PubMed] [Google Scholar]
- 5.Emecen P, Akman AC, Hakki SS, Hakki EE, Demiralp B, Tözüm TF, et al. ABM/P-15 modulates proliferation and mRNA synthesis of growth factors of periodontal ligament cells. Acta Odontol Scand. 2009;67:65–73. doi: 10.1080/00016350802555525. [DOI] [PubMed] [Google Scholar]
- 6.Zander HA, Polson AM, Heijl LC. Goals of periodontal therapy. J Periodontol. 1976;47:261–6. doi: 10.1902/jop.1976.47.5.261. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Dziak R, Lynch SE, Stephan EB. Modification of an osteoconductive anorganic bovine bone mineral matrix with growth factors. J Periodontol. 1999;70:834–9. doi: 10.1902/jop.1999.70.8.834. [DOI] [PubMed] [Google Scholar]
- 8.Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss: A bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999;26:421–8. doi: 10.1034/j.1600-051x.1999.260702.x. [DOI] [PubMed] [Google Scholar]
- 9.Froum SJ, Coran M, Thaller B, Kushner L, Scopp IW, Stahl SS. Periodontal healing following open debridement flap procedures. I. Clinical assessment of soft tissue and osseous repair. J Periodontol. 1982;53:8–14. doi: 10.1902/jop.1982.53.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Stahl SS, Froum SJ, Kushner L. Periodontal healing following open debridement flap procedures. II. Histologic observations. J Periodontol. 1982;53:15–21. doi: 10.1902/jop.1982.53.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Pihlstrom BL. Measurement of attachment level in clinical trials: Probing methods. J Periodontol. 1992;63:1072–7. doi: 10.1902/jop.1992.63.12s.1072. [DOI] [PubMed] [Google Scholar]
- 12.Barros RR, Novaes AB, Jr, Roriz VM, Oliveira RR, Grisi MF, Souza SL, et al. Anorganic bovine matrix/p-15 “flow” in the treatment of periodontal defects: Case series with 12 months of follow-up. J Periodontol. 2006;77:1280–7. doi: 10.1902/jop.2006.050161. [DOI] [PubMed] [Google Scholar]
- 13.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Djordjevic M, Kenney EB. The use of bovine porous bone mineral in combination with enamel matrix proteins or with an autologous fibrinogen/fibronectin system in the treatment of intrabony periodontal defects in humans. J Periodontol. 2001;72:1157–63. doi: 10.1902/jop.2000.72.9.1157. [DOI] [PubMed] [Google Scholar]
- 14.Sculean A, Berakdar M, Chiantella GC, Donos N, Arweiler NB, Brecx M. Healing of intrabony defects following treatment with a bovine-derived xenograft and collagen membrane. A controlled clinical study. J Clin Periodontol. 2003;30:73–80. doi: 10.1034/j.1600-051x.2003.10192.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasaj A, Röhrig B, Reichert C, Willershausen B. Clinical evaluation of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide (P-15) in the treatment of human infrabony defects. Clin Oral Investig. 2008;12:241–7. doi: 10.1007/s00784-008-0191-y. [DOI] [PubMed] [Google Scholar]
- 16.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, et al. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects 6-month results. J Periodontol. 1998;69:655–63. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]
- 17.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Thirty-six month follow-up of 25 patients treated with combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell-binding peptide (P-15) bone replacement grafts in human infrabony defects. I. Clinical findings. J Periodontol. 2002;73:123–8. doi: 10.1902/jop.2002.73.1.123. [DOI] [PubMed] [Google Scholar]
- 18.Yukna R, Salinas TJ, Carr RF. Periodontal regeneration following use of ABM/P-1 5: A case report. Int J Periodontics Restorative Dent. 2002;22:146–55. [PubMed] [Google Scholar]
- 19.Radhakrishnan S, Anusuya CN. Comparative clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and open flap debridement (DEBR) in human periodontal osseous defects: A 6 month pilot study. J Int Acad Periodontol. 2004;6:101–7. [PubMed] [Google Scholar]
- 20.Sogal A, Tofe AJ. Risk assessment of bovine spongiform encephalopathy transmission through bone graft material derived from bovine bone used for dental applications. J Periodontol. 1999;70:1053–63. doi: 10.1902/jop.1999.70.9.1053. [DOI] [PubMed] [Google Scholar]
- 21.Paolantonio M. Combined periodontal regenerative technique in human intrabony defects by collagen membranes and anorganic bovine bone. A controlled clinical study. J Periodontol. 2002;73:158–66. doi: 10.1902/jop.2002.73.2.158. [DOI] [PubMed] [Google Scholar]
- 22.Klein F, Kim TS, Hassfeld S, Staehle HJ, Reitmeir P, Holle R, et al. Radiographic defect depth and width for prognosis and description of periodontal healing of infrabony defects. J Periodontol. 2001;72:1639–46. doi: 10.1902/jop.2001.72.12.1639. [DOI] [PubMed] [Google Scholar]
- 23.Liñares A, Cortellini P, Lang NP, Suvan J, Tonetti MS. European Research Group on Periodontology (ErgoPerio). Guided tissue regeneration/deproteinized bovine bone mineral or papilla preservation flaps alone for treatment of intrabony defects. II: Radiographic predictors and outcomes. J Clin Periodontol. 2006;33:351–8. doi: 10.1111/j.1600-051X.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 24.Tonetti MS, Cortellini P, Lang NP, Suvan JE, Adriaens P, Dubravec D, et al. Clinical outcomes following treatment of human intrabony defects with GTR/bone replacement material or access flap alone. A multicenter randomized controlled clinical trial. J Clin Periodontol. 2004;31:770–6. doi: 10.1111/j.1600-051X.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsitoura E, Tucker R, Suvan J, Laurell L, Cortellini P, Tonetti M. Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J Clin Periodontol. 2004;31:643–7. doi: 10.1111/j.1600-051X.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 26.Gottlow J, Nyman S, Lindhe J, Karring T, Wennström J. New attachment formation in the human periodontium by guided tissue regeneration. Case reports. J Clin Periodontol. 1986;13:604–16. doi: 10.1111/j.1600-051x.1986.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 27.Khashu H, Vandana KL. Clinical and radiographic evaluation of human periodontal osseous defect (mandibular grade II furcation) treated with PepGen P-15 and a bioresorbable membrane (Atrisorb) J Indian Soc Periodontol. 2012;16:569–76. doi: 10.4103/0972-124X.106917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabalais ML, Yukna RA, Mayer ET. Evaluation of durapatite ceramic as an alloplastic implant in periodontal osseous defects. 1. Initial 6 months results. J Clin Periodontol. 2000;71:752–9. [Google Scholar]
- 29.Meffert RM, Thomas JR, Hamilton KM, Brownstein CN. Hydroxylapatite as an alloplastic graft in the treatment of human periodontal osseous defects. J Periodontol. 1985;56:63–73. doi: 10.1902/jop.1985.56.2.63. [DOI] [PubMed] [Google Scholar]
- 30.Camargo PM, Lekovic V, Weinlaender M, Nedic M, Vasilic N, Wolinsky LE, et al. A controlled re-entry study on the effectiveness of bovine porous bone mineral used in combination with a collagen membrane of porcine origin in the treatment of intrabony defects in humans. J Clin Periodontol. 2000;27:889–96. doi: 10.1034/j.1600-051x.2000.027012889.x. [DOI] [PubMed] [Google Scholar]
- 31.Krejci CB, Bissada NF, Farah C, Greenwell H. Clinical evaluation of porous and nonporous hydroxyapatite in the treatment of human periodontal bony defects. J Periodontol. 1987;58:521–8. doi: 10.1902/jop.1987.58.8.521. [DOI] [PubMed] [Google Scholar]


