Abstract
Objective:
To examine whether higher plasma urate concentrations are associated with a lower risk of developing Parkinson disease (PD) and whether there is a sex difference in the potential urate–PD relationship.
Methods:
We conducted a nested case-control study based on 90,214 participants of 3 ongoing US cohorts. We identified 388 new PD cases (202 men and 186 women) since blood collection, which were then matched to 1,267 controls. PD cases were confirmed by medical record review. Conditional logistic regression estimated relative risks (RRs) and 95% confidence intervals (95% CIs), after adjustment for age, smoking, caffeine intake, plasma concentrations of cholesterol and ferritin, and other covariates. We also conducted a meta-analysis to combine our study with 3 previously published prospective studies on urate and PD risk.
Results:
In the present nested case-control study, the multivariate-adjusted RRs of PD comparing extreme quartiles of urate were 0.63 (95% CI 0.35, 1.10; ptrend = 0.049) in men and 1.04 (95% CI 0.61, 1.78; ptrend = 0.44) in women (pheterogeneity = 0.001). In the meta-analysis, the pooled RRs comparing 2 extreme quartiles of urate were 0.63 (95% CI 0.42, 0.95) in men and 0.89 (95% CI 0.57, 1.40) in women.
Conclusion:
We observed that men, but not women, with higher urate concentrations had a lower future risk of developing PD, suggesting that urate could be protective against PD risk or could slow disease progression during the preclinical stage of disease.
Urate is a potent antioxidant and contributes to approximately 60% of the free radical scavenging activity in human serum.1,2 Serum urate concentrations in humans and apes are many-fold higher than those in other mammals as a consequence of evolutionary urate oxidase gene mutations.1 Results from in vitro and in vivo experimental studies suggest that urate could be a potential neuroprotective agent via its capacity to modify the cerebral damage induced by reactive nitrogen and oxidative species.2,3 In most,4–8 but not all,9 prospective observational studies in humans, higher concentrations of plasma urate were associated with a lower risk of developing Parkinson disease (PD). Further, patients with PD with higher urate levels have been found to have a slower disease progression relative to those with lower urate concentrations.10,11 However, significant associations of higher urate levels with lower PD risk and slower progression were observed only in men.4–9 Studies relating blood urate levels to PD risk were limited by their small sample sizes (PD case number < 160 in all studies) and underrepresentation of women. Therefore, it remains unclear whether this sex difference reflects a true difference in underlying biology or is due to lack of statistical power because women generally have lower urate concentration and lower PD incidence compared with men. Further, only one previous study7 controlled for plasma ferritin and cholesterol levels, which have been found to be associated with both urate and altered PD risk.12–16 To address these questions, we examined whether plasma urate was related to future risk of PD among participants in 3 ongoing US cohorts including over 90,000 men and women with blood samples available.
METHODS
The current nested case-control study was based on 3 ongoing US cohorts: the Health Professionals Follow-up Study (HPFS), the Nurses' Health Study (NHS), and the Cancer Prevention Study II Nutrition (CPS-IIN). As an extension of our previous reports based on the HPFS5 and NHS,7 we extended the follow-up of PD from 2002 to 2008 in the HPFS and from 2004 to 2010 in the NHS.
In 1986, we recruited 51,529 male health professionals, who were 40–75 years of age, in the HPFS. These participants returned a detailed mailed questionnaire regarding their diet, lifestyle practices, and medical history. The NHS comprises 121,700 female registered nurses, aged 30–55 years, who lived in one of 11 US states at the time of enrollment in 1976. The HPFS and NHS have been followed via mailed questionnaires every 2 years with overall response rate above 94%.17,18 The CPS-IIN began in 1992, comprising 77,048 men and 85,360 women, aged 50–79 years old, to study the relationship between lifestyles factors (e.g., dietary habits) and the risk of cancer.19 Questionnaires were sent in 1992, 1997, and every 2 years thereafter. Similarly, the follow-up rate was consistently over 90%.19
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Human Research Committees at the Harvard School of Public Health and the Brigham and Women's Hospital with receipt of each questionnaire accepted as participant's consent.
Case ascertainment and control selection.
Confirmation of PD cases among these 3 cohorts was described elsewhere.17,18,20 In brief, cases were confirmed based on a detailed questionnaire regarding PD diagnosis that was completed by treating neurologist/internists or review of medical records, conducted by our movement disorder specialist (M.A.S.). In the current analysis, we included only definite or probable PD with at least 2 cardinal signs of PD (resting tremor, rigidity, bradykinesia) without features suggesting other diagnoses in the current study. Overall, more than 90% of the cases were confirmed by the treating neurologist or movement disorder specialist. Participants were followed for incident PD from blood draw until they returned the 2010 (NHS/HPFS) or 2007 (CPS-IIN) questionnaire or died.
For each case, we randomly selected from 2 to 6 controls, who were alive and had not reported PD at the time of diagnosis, in the NHS and HPFS cohorts, and 1 control in the CPS-IIN cohort. All controls were matched to the cases based on the cohort, sex, birth year (±1 year), race (white vs other), fasting status (>8 hours vs less/unknown), and year, month, and time of blood draw (2-hour intervals).
Measurement of plasma urate and potential confounders.
Fasting blood samples were collected from 32,825 women in the NHS during 1989–1990, 18,018 men in the HPFS during 1993–1995, and 39,371 CPS-IIN members during 1998–2001, and were stored at approximately −130°C. Plasma urate concentrations were assessed via a colorimetric enzyme assay on the Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN). The day-to-day variabilities of the assay at concentrations of 4.86, 7.20, and 9.39 mg/dL are 1.3%, 1.7%, and 1.6%, respectively.
We used questionnaires to collect data on potential confounders, including age, smoking status, height, weight, presence of chronic diseases, and consumption of caffeinated coffee and alcohol. Body mass index (BMI) was computed as weight in kilograms divided by height in meters squared. Plasma low-density lipoprotein (LDL) and high-density lipoprotein cholesterol were determined enzymatically.21 Ferritin concentrations were assessed using a particle-enhanced immunoturbidimetric assay with the Hitachi 917 analyzer (Roche Diagnostics) and Kamiya Biomedical reagents (Seattle, WA).
Statistical analyses.
Analyses were carried out with SAS software (version 9; SAS Institute, Inc., Cary, NC). Because of correlation within each matched set, we compared average plasma urate levels across groups by using random-effects models. We used conditional logistic regression to calculate relative risks (RRs) and 95% confidence intervals (95% CIs). Study- and sex-specific quartiles were based on the distribution among controls. Trend test was conducted by treating urate as a continuous variable. We explored potential interaction between urate and potential modifiers, including age at blood collection (y), age at PD onset (y), BMI (kg/m2), alcohol consumption (g/d), and plasma concentrations of LDL and ferritin, by modeling the product of urate and the interested factors.
We conducted a meta-analysis to combine our study with previously published prospective studies on urate and PD risk in men and women separately. Relevant studies were identified through searches of PubMed using the keywords (urate or uric acid) and (Parkinson's disease or Parkinson) for all published studies in English by September 1, 2014. In addition, the reference lists from the relevant publications were used to identify additional studies. To be included in our meta-analysis, studies had to (1) be a prospective study, in which blood samples were collected before PD onset (or diagnosis), and (2) present RRs or odds ratios for men and women separately. We identified 6 prospective studies4–9 (table e-1 on the Neurology® Web site at Neurology.org). An article based on the Rotterdam study8 had to be excluded because sex-specific RRs were not provided. The results of 2 previous NHS/HPFS-based studies5,7 were pooled with those of the present investigation, so that each cohort is represented by only one RR estimate that is based on all the incident PD cases for that cohort. In this meta-analysis, we treated the CPS-IIN as a separate study and its sex-specific results were pooled with other relevant studies. The Q-statistic was used to assess heterogeneity across the studies. We used fixed-effects models to calculate the pooled hazard ratio as we did not observe significant heterogeneity across studies (p > 0.1). We used the Begg test to assess publication bias.
RESULTS
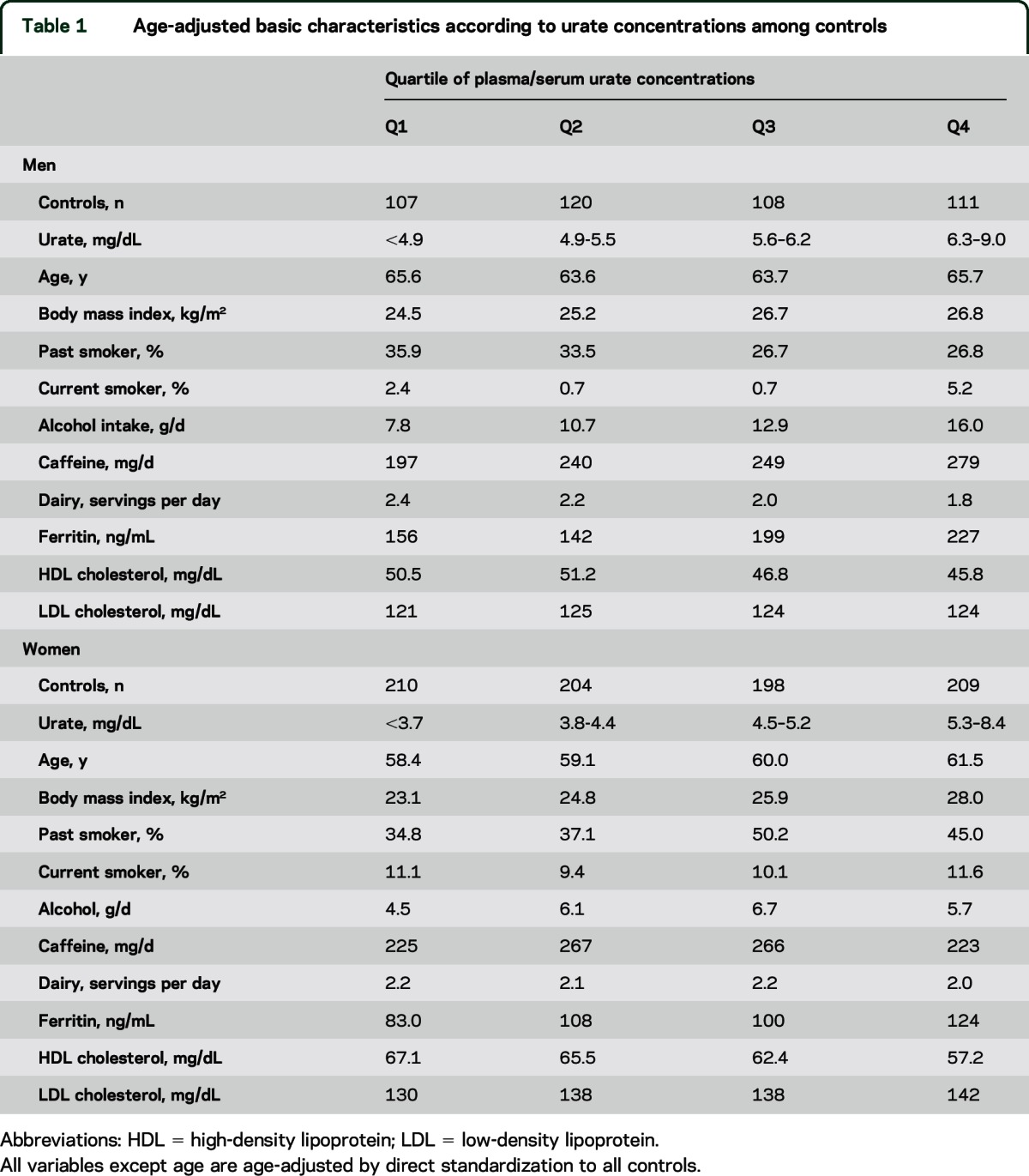
We identified 388 new incident PD cases (202 men and 186 women; 162 from the HPFS, 156 from the NHS, and 70 from the CPS-IIN) since blood collection and matched with 1,267 controls (446 men and 821 women). The number of incident PD cases has doubled since our previously published studies (n = 185) of urate and PD risk in HPFS and NHS.5,7 Urate concentration in controls was associated with higher BMI, ferritin, and LDL. Among men only, correlations were also found between urate and alcohol consumption (positive) and lower dairy consumption (negative) (table 1).
Table 1.
Age-adjusted basic characteristics according to urate concentrations among controls
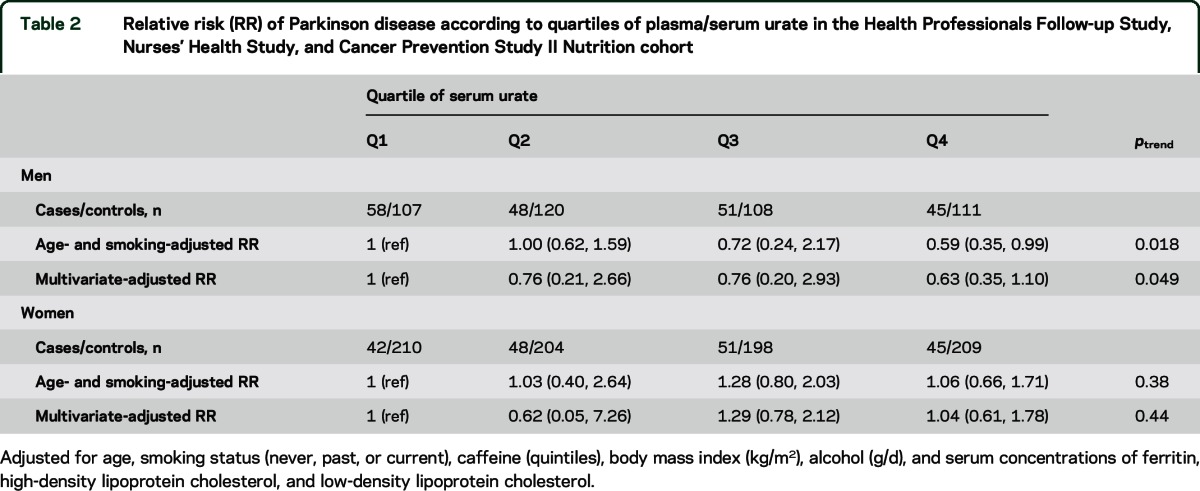
Higher baseline urate concentrations were associated with lower risk of PD in men but not in women (table 2). The multivariate-adjusted RRs of PD comparing 2 extreme quartiles of urate were 0.63 (95% CI 0.35, 1.10; ptrend = 0.049) in men and 1.04 (95% CI 0.61, 1.78; ptrend = 0.44) in women (pheterogeneity = 0.001) (table 2). Further adjustment for cardiovascular factors, including history of cardiovascular disease and diabetes, did not materially change the results (data not shown). Because men have higher levels of urate, relative to women, we also conducted a secondary analysis using the same cutoff points for both men and women to estimate the effects of similar levels of serum urate. We observed a similar sex difference for the urate–PD relationship (table e-2). There were no significant interactions between urate concentration and age, age at PD onset, smoking, BMI, caffeine intake, and plasma concentrations of ferritin and cholesterol (p > 0.05 for all).
Table 2.
Relative risk (RR) of Parkinson disease according to quartiles of plasma/serum urate in the Health Professionals Follow-up Study, Nurses' Health Study, and Cancer Prevention Study II Nutrition cohort
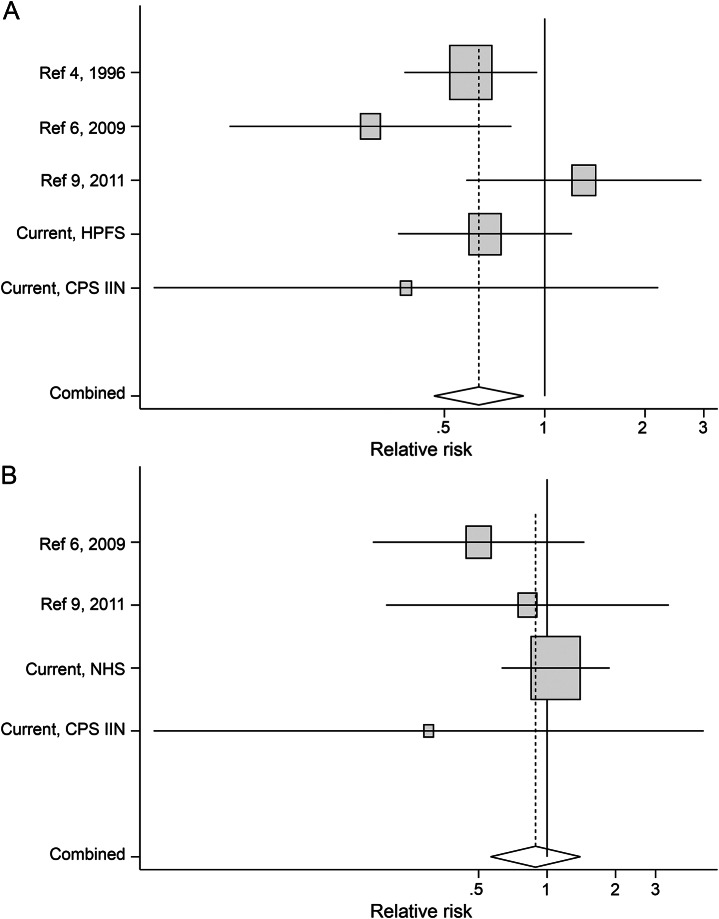
We further pooled the present study with 3 previously published studies4,6,9 with a total of 325 PD cases. The pooled RRs comparing 2 extreme categories of urate were 0.63 (95% CI 0.42, 0.95) in men and 0.89 (95% CI 0.57, 1.40) in women (figure). The Begg test did not support existence of publication bias.
Figure. Pooled relative risks (RRs) of Parkinson disease (PD) for the highest vs lowest quartiles of plasma/serum urate concentrations in men and women in the meta-analysis.
(A) Men. (B) Women. Pooled RR was 0.63 (95% confidence interval [CI] 0.42, 0.95; p = 0.03; pheterogeneity = 0.22) in men and 0.89 (95% CI 0.57, 1.40; p = 0.61; pheterogeneity = 0.52) in women. In the Health Professionals Follow-up Study (HPFS) cohort, 84 out of 162 PD cases were included in a previous case-control study (reference 5), and in the Nurses' Health Study (NHS) cohort, 101 out of 156 PD cases were included in a previous case-control study (reference 7). CPS IIN = Cancer Prevention Study II Nutrition.
DISCUSSION
In this large nested case-control study with 388 incident PD cases, we found that men with higher plasma urate concentration at baseline had a lower future risk of developing PD, independent of age, smoking, caffeine intake, serum ferritin, and other potential risk factors for PD. In contrast, we did not observe an association between urate and PD risk in women. Consistently, a meta-analysis summarizing the current study and previously published prospective data on this topic generated similar results.
The notion of urate as a neuroprotective agent has been supported by previous experimental studies. In a 6-hydroxydopamine (6-OHDA) mouse model of PD, knocking out urate oxidase resulted in increased brain urate concentrations and substantially attenuated toxic effects of 6-OHDA.22 On the other hand, transgenic urate oxidase, which lowers brain urate, exacerbated such deleterious effects.22 Administration of urate directly reduced motor and dopaminergic deficits in PD models of rodents. In vitro, urate attenuated the loss of dopaminergic neurons in neuron-enriched cultures induced by diverse neurotoxicants.23,24
Neuroprotective effects of urate were also supported by a series of human PD studies, as listed in table e-1. In a double-blind, placebo-controlled, randomized trial of urate precursor inosine in 75 patients with early PD, we observed nonfutility of inosine treatment for slowing disability.25 Further, in a PD case-control study, participants carrying more genetic alleles associated with lower serum urate concentrations were more likely to have PD.26 Individuals with gout, a chronic condition associated with hyperuricemia, have been also found to have lower risk of PD in 2 previous studies,27,28 but not in a recent one.29
Another possible interpretation for the observed urate–PD association is that urate may slow disease progression during the preclinical stage of PD. By the time of the PD diagnosis, substantial dopaminergic neurons in the substantia nigra pars compacta have been lost,30 suggesting that PD has a long preclinical period. Based on recent studies, it has been thought that the preclinical stage of PD could start at least 20 years before the motor manifestations.31 Using a Mendelian randomization approach, we recently showed that patients in the early stages of PD who carry the SLC2A9 genotypes associated with lower urate concentration had faster disease progression rate than those without the allele. This finding suggests possible causal protective effects of urate on PD.32 Interestingly, an inverse association between blood urate and PD was also observed in a small study among individuals with idiopathic REM sleep behavior disorder (RBD), a common symptom observed in the preclinical stage of PD. Individuals with more than 5 years of RBD without a diagnosis of PD had significantly higher concentrations of urate than patients with both RBD and PD.33
We observed a clear sex difference in the association of urate with PD risk in the current nested case-control study and in the meta-analysis. Similar sex difference pattern has been consistently observed in studies regarding urate and PD severity/progression among patients with PD.10,11,34,35 For example, in 2 prospective studies including approximately 800 US patients with PD, lower urate concentrations predicted prognosis of PD and the associations were stronger in men relative to women.10,11 In a case-control study including 161 individuals with PD living in southern Spain, serum urate concentration was inversely associated with disease severity, as assessed by the modified Hoehn & Yahr scale, in men but not in women.35 Similarly, in a cross-sectional study including 797 patients with early and untreated PD, a significant relation between higher urate concentrations and lower odds of having a scan without evidence of dopaminergic deficit was observed in men, but not in women.36 In a recent postmortem study, urate levels in cortical and striatal tissue was lower in PD compared to controls in men only.37
The biological mechanisms underlying such sex specificity remain unclear. Interestingly, several cardiovascular risk factors, such as hypercholesterolemia38 and hypertension,39 have been reported to be associated with increased risk of PD in women but not in men. This could play a part in the observed sex difference in the urate–PD relationship because higher urate concentrations are associated with these factors, which in turn might offset the potential neuroprotective effects of urate. Lack of a significant association between urate and PD risk in women could also be due to some potential factors other than urate, such as estrogen, predominating in determining the lower PD risk among women. In our study, we did not find significant interaction between use of hormone replacement therapy and urate in relation to PD risk; however, we were underpowered to see an interaction. Interestingly, in a second analysis based on the aforementioned inosine–PD trial,25 women benefited more regarding urate elevation and slowing disease progression after inosine administration, relative to men.40 More studies are warranted to understand sex differences in the urate–PD relationship.
Strengths of this study include its prospective design, large sample size, and availability of information on several important covariates that may confound the potential association between serum urate and PD risk. However, because of its observational nature, we cannot exclude the possibility of residual confounding. Another limitation is that the current case-control study and all previous studies included in the meta-analysis were based on a single measure of plasma urate and thus failed to account for within-person variability in urate levels. However, the effects of such within-person variability are likely to be random and the most likely effect would be a modest attenuation of any potential association between urate and PD risk. Generalizability is another concern for the current case-control study because the majority of participants were Caucasian with high educational attainment and socioeconomic status relative to the general US population. However, the associations between urate and PD risk were consistent across published prospective studies, as suggested by the insignificant heterogeneity test result in the meta-analysis. Similar observations were also reported in Chinese and Spanish populations.34,35
In this study we observed that men, but not women, with higher urate concentrations had a lower future risk developing PD, suggesting that urate could protect against PD risk or slow the progression of preclinical PD among individuals in a preclinical stage of PD. Our findings, together with previous observations that urate can elevated by administration of its precursor inosine, which is generally safe and tolerable, in early PD,25 provide strong evidence supporting the design of a randomized trial of urate elevation in patients with early PD or pre-Parkinson syndrome.
Supplementary Material
GLOSSARY
- 6-OHDA
6-hydroxydopamine
- BMI
body mass index
- CI
confidence interval
- CPS-IIN
Cancer Prevention Study II Nutrition
- HPFS
Health Professionals Follow-up Study
- LDL
low-density lipoprotein
- NHS
Nurses' Health Study
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
- RR
relative risk
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drafting/revising the manuscript (X.G., E.J.O., M.A,.S., and A.A.), study concept/design (X.G., M.A.S., and A.A.), analysis or interpretation of data (X.G., E.J.O., and A.A.), acquisition of data (X.G., M.A.S., and A.A.), study supervision (A.A.), and obtaining funding (A.A.).
STUDY FUNDING
Supported by grants (P01 CA055075, UM1 CA186107, UM1 CA167552, R01 NS061858, and R21 NS087235-01A1) from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURE
X. Gao received funding from the NIH/NINDS. E. O'Reilly received funding from the NHS/NINDS. M. Schwarzschild has received research support from the NIH/NINDS, the US Department of Defense, the Michael J. Fox Foundation, the Parkinson Disease Foundation, the RJG Parkinson's Disease Foundation, the American Parkinson Disease Association, and the American Federation for Aging Research. A. Ascherio serves on a scientific advisory board for the Michael J. Fox Foundation; serves on the editorial boards of Neurology®, Annals of Neurology, and the American Journal of Epidemiology; and receives research support from the NIH, the US Department of Defense, and the Michael J. Fox Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes: a new aspect of the antioxidant functions of uric acid. Biochem J 1986;235:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waring WS. Uric acid: an important antioxidant in acute ischaemic stroke. QJM 2002;95:691–693. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Wu G, Schwarzschild MA. Urate in Parkinson's disease: more than a biomarker? Curr Neurol Neurosci Rep 2012;12:367–375. [DOI] [PubMed] [Google Scholar]
- 4.Davis JS, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid and the risk of idiopathic Parkinson's disease. Am J Epidemiol 1996;144:480–484. [DOI] [PubMed] [Google Scholar]
- 5.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol 2007;166:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2009;169:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Reilly EJ, Gao X, Weisskopf MG, et al. Plasma urate and Parkinson's disease in women. Am J Epidemiol 2010;172:666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 2005;58:797–800. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Ton TG, Boudreau RM, et al. The risk of Parkinson disease associated with urate in a community-based cohort of older adults. Neuroepidemiology 2011;36:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ascherio A, LeWitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson's disease. Arch Neurol 2008;65:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghio AJ, Ford ES, Kennedy TP, Hoidal JR. The association between serum ferritin and uric acid in humans. Free Radic Res 2005;39:337–342. [DOI] [PubMed] [Google Scholar]
- 13.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson's disease. Am J Epidemiol 2006;164:998–1002. [DOI] [PubMed] [Google Scholar]
- 14.Dexter DT, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991;114:1953–1975. [DOI] [PubMed] [Google Scholar]
- 15.Logroscino G, Chen H, Wing A, Ascherio A. Blood donations, iron stores, and risk of Parkinson's disease. Mov Disord 2006;21:835–838. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch EC. Altered regulation of iron transport and storage in Parkinson's disease. J Neural Transm Suppl 2006:201–204. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol 2008;167:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology 2011;76:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer prevention Study II nutrition cohort: rationale, study design, and baseline characteristics. Cancer 2002;94:2490–2501. [DOI] [PubMed] [Google Scholar]
- 20.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol 2006;60:197–203. [DOI] [PubMed] [Google Scholar]
- 21.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–475. [PubMed] [Google Scholar]
- 22.Chen X, Burdett TC, Desjardins CA, et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci USA 2013;110:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J Neurochem 2002;80:101–110. [DOI] [PubMed] [Google Scholar]
- 24.Cipriani S, Desjardins CA, Burdett TC, Xu Y, Xu K, Schwarzschild MA. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson's disease. PLoS One 2012;7:e37331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson Study Group SURE-PD Investigators, Schwarzschild MA, Ascherio A, Beal MF, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 2014;71:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Aramburu I, Sanchez-Juan P, Jesus S, et al. Genetic variability related to serum uric acid concentration and risk of Parkinson's disease. Mov Disord 2013;28:1737–1740. [DOI] [PubMed] [Google Scholar]
- 27.Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology 2007;69:1696–1700. [DOI] [PubMed] [Google Scholar]
- 28.De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson's disease: a cohort study. Arthritis Rheum 2008;59:1549–1554. [DOI] [PubMed] [Google Scholar]
- 29.Schernhammer E, Qiu J, Wermuth L, Lassen CF, Friis S, Ritz B. Gout and the risk of Parkinson's disease in Denmark. Eur J Epidemiol 2013;28:359–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 1999;122:1437–1448. [DOI] [PubMed] [Google Scholar]
- 31.Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol 2010;67:798–801. [DOI] [PubMed] [Google Scholar]
- 32.Simon KC, Eberly S, Gao X, et al. Mendelian randomization of serum urate and Parkinson disease progression. Ann Neurol 2014;76:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin RU, Francke PV, Illanes FL, et al. Plasma urate in REM sleep behavior disorder. Mov Disord 2013;28:1150–1151. [DOI] [PubMed] [Google Scholar]
- 34.Zhang HN, Guo JF, He D, et al. Lower serum UA levels in Parkinson's disease patients in the Chinese population. Neurosci Letters 2012;514:152–155. [DOI] [PubMed] [Google Scholar]
- 35.Jesus S, Perez I, Caceres-Redondo MT, et al. Low serum uric acid concentration in Parkinson's disease in southern Spain. Eur J Neurol 2013;20:208–210. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzschild MA, Marek K, Eberly S, et al. Serum urate and probability of dopaminergic deficit in early “Parkinson's disease.” Mov Disord 2011;26:1864–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarland NR, Burdett T, Desjardins CA, Frosch MP, Schwarzschild MA. Postmortem brain levels of urate and precursors in Parkinson's disease and related disorders. Neurodegener Dis 2013;12:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007;69:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu C, Hu G, Kivipelto M, et al. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension 2011;57:1094–1100. [DOI] [PubMed] [Google Scholar]
- 40.Schwarzschild MA, Macklin EA, Ascherio A; Parkinson Study Group SURE-PD Investigators. Urate and neuroprotection trials. The Lancet Neurol 2014;13:758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.