Abstract
Background:
Stress plays a significant role in the pathogenesis of neuropsychiatric disorders such as anxiety and depression. Beta vulgaris is commonly known as “beet root” possessing antioxidant, anticancer, hepatoprotective, nephroprotective, wound healing, and anti-inflammatory properties.
Objective:
To study the protective effect of Beta vulgaris Linn. ethanolic extract (BVEE) of leaves against acute restraint stress (ARS)-induced anxiety- and depressive-like behavior and oxidative stress in mice.
Materials and Methods:
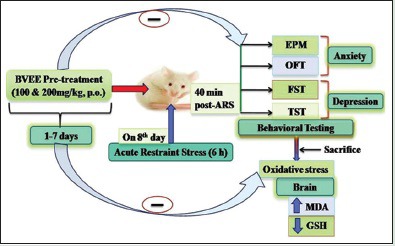
Mice (n = 6) were pretreated with BVEE (100 and 200 mg/kg, p. o.) for 7 days and subjected to ARS for 6 h to induce behavioral and biochemical changes. Anxiety- and depressive-like behavior were measured by using different behavioral paradigms such as open field test (OFT), elevated plus maze (EPM), forced swim test (FST), and tail suspension test (TST) 40 min postARS. Brain homogenate was used to analyze oxidative stress parameters, that is, malondialdehyde (MDA) and reduced glutathione (GSH) level.
Results:
BVEE pretreatment significantly (P < 0.05) reversed the ARS-induced reduction in EPM parameters, that is, percentage entries and time spent in open arms and in OFT parameters, that is, line crossings, and rearings in mice. ARS-induced increase in the immobility time in FST and TST was attenuated significantly (P < 0.05) by BVEE pretreatment at both the dosage. An increase in MDA and depletion of GSH level postARS was prevented significantly (P < 0.05) with BVEE pretreatment at both the dosage (100 and 200 mg/kg).
Conclusion:
BVEE exhibits anxiolytic and antidepressant activity in stressed mice along with good antioxidant property suggesting its therapeutic potential in the treatment of stress-related psychiatric disorders.
SUMMARY
Stress plays major role in the pathogenesis of anxiety and depression
ARS-induced anxiety- and depressive-like behavior through oxidative damage in mice
BVEE pretreatment reversed ARS-induced behavioral changes, that is, anxiety and depression
ARS-induced oxidative stress was prevented by BVEE pretreatment in mice.
Abbreviations Used: ANOVA: Analysis of variance, ARS: Acute restraint stress, BVEE: Beta vulgaris ethanolic extract, BV: Beta vulgaris, CMC: Carboxymethylcellulose, CNS: Central nervous system, CPCSEA: Committee for the purpose of control and supervision of experiments on animals, cms: Centimeter, DNA: Deoxyribose nucleic acid, EPM: Elevated plus maze, FST: Forced swim test, GSH: Reduced glutathione, g: Gram, h: Hour, IAEC: Institutional Animal Ethics Committee, mg: Milligram, μM: Microgram, MDA: Malondialdehyde, SEM: Standard error of mean, TST: Tail suspension test, UV: Ultraviolet, w/v: Weight by volume.
Keywords: Acute restraint stress, antioxidant, anxiety, Beta vulgaris, depression, oxidative stress
INTRODUCTION
World Health Organization report says that approximately 450 million people are suffering from psychiatric disorders contributing 12.3% of the global burden of disease, and this figure will rise to 15% by 2020.[1] Worldwide, there are 10–20 million suicide attempts every year which are associated with mental disorders.[1] Unfortunately, these disorders are under-diagnosed and undertreated.[2] Moreover, most of the clinically available anxiolytics and antidepressants are not effective for all patients and inundated by adverse effects, slow onset of actions, and poor patient compliance.[3,4] The knowledge of basic neuroscience helps us in improving our understanding about disease pathophysiology, identifying novel mechanisms which can be targeted for effective treatments, and screening of drugs from herbal sources. These considerations implicate the search for novel anxiolytic and antidepressant agents which have a fast onset of action with maximum efficacy and minimum toxicity.[1,5,6] Several plant species have shown their pharmacological effectiveness in the variety of animal models of psychiatric disorders,[7] and are being used as complementary and alternative medicines for the management of these disorders.
There are ample evidences which demonstrates that stress play a significant role in the pathogenesis of neuropsychiatric problems including anxiety and depression.[7,8,9,10] Stress may be defined as a state of body to reestablish homeostasis by producing adaptive physiological and neurobehavioral changes, and depends on severity, type, and duration of stressful events.[11] A consequence of stressful events is the altered physiological and psychological responses that affect different organs and systems, including the central nervous system (CNS),[11] and increased susceptibility to different psychiatric diseases, including depression.[7]
Moreover, stress induces immunological and neurobehavioral responses such as anxiety, depression, cognitive impairment, insomnia, anorexia, and activate the hypothalamic-pituitary-adrenal axis resulting in elevated corticosterone levels in animals and humans.[12,13] Stress exerts detrimental effects on cellular functions through oxidative damage due to release of free radicals. Acute restraint stress (ARS) stimulates several cellular events resulting in enhanced reactive oxygen species (ROS) production. These free radicals damage the body parts, especially CNS because of brain's high oxygen consumption, abundant lipid content, and relative paucity of antioxidant enzymes.[8] Similarly, restraint stress in rodents precipitates many neurochemical, hormonal, and behavioral abnormalities that are often associated with an imbalance in the brain's intracellular redox state. Numerous studies have reported that restraint stress enhances lipid peroxidation and decreases antioxidant enzyme activities in rodents.[8,14,15]
Various diseases are now being treated with the help of indigenous drugs, and rebirth of herbal treatment options are happening all over the globe.[16,17] At present, the herbal products are considered to be safe while synthetics are regarded as unsafe to human and the environment due to their side effects. Nowadays, peoples are showing faith on products of natural origin due to safety and security and decreasing their dependence on products of synthetic origin.[17] Epidemiological studies have suggested positive association between the consumption of phenolic-rich foods or beverages and the prevention of diseases. These effects have been attributed to antioxidant components such as plant phenolics, flavonoids, and phenyl propanoids among others.
Beta vulgaris (BV) Linn. (BV, family: Chenopodiaceae), popularly known as “chukandar” or “beet root,” is a native to Mediterranean region and extensively cultivated in America, Europe, and throughout India.[18] The parts of this plant are harbored with numerous medicinal values and used in traditional Indian medicine. Leaves possess diuretic, purgative, and anti-inflammatory activity and useful in alleviating paralysis, spleen, and liver diseases. Seeds are expectorant and having carminative effect. Roots possess expectorant, diuretic, sedative, emmenagogue effects, and employed in the treatment of mental problems and liver diseases.[18,19] It is also used as a natural food colorant for dairy and meat products.[20] In addition, leaves contain various phytoconstituents such as betalains, that is, betacyanins (red-violet pigments) and betaxanthines (yellow pigments), flavonoids, polyphenols, vitamins, and minerals.[18] BV possesses antioxidant, anticancer, hepatoprotective, nephroprotective, wound healing, and anti-inflammatory activities.[20,21,22,23,24] Leaves are a good source of natural antioxidant and widely consumed as vegetables due to its high nutritional value. On the basis of literature review, it can be concluded that little work has been done to explore its biological activities. Moreover, no scientific report is available regarding its anti-anxiety and antidepressant activity, to the best of our knowledge. Therefore, to validate the traditional use, the present study was undertaken to evaluate the effect of BV leaves extract against restraint stress-induced neurobehavioral and biochemical alterations in mice.
MATERIALS AND METHODS
Chemicals
Fluoxetine, thiobarbituric acid, 5,5′- dithiobis (2-nitrobenzoic acid), and L-reduced glutathione (GSH) were purchased from Sigma-Aldrich, St. Louis, MO, USA. All other chemicals were of analytical grade purchased from Sigma-Aldrich chemicals unless otherwise mentioned.
Animals
The protocol of this study was approved by the Institutional Animal Ethics Committee of Sapience Bioanalytical Research Lab., Bhopal, India (Approval no. 1413/PO/a/11/Committee for the Purpose of Control and Supervision of Experiments on Animals [CPCSEA]) in accordance with the CPCSEA guidelines for the safe use and care of the experimental animals. The adult male Swiss albino mice (22–30 g) were procured from Sapience Bioanalytical Research Lab., Bhopal, (Madhya Pradesh). The animals were kept under the experimental housing and normal feeding conditions for 7 days prior to the test. All the experiments were carried out between 9:00 and 17:00 h. The animal room was maintained at 22°C ± 2°C temperature, 60% ±5% relative humidity, with 12:12 h light–dark cycle. Food and water were available ad libitum. Considering the animal ethical issues, all animals were kept under best hygienic conditions and animals were inspected daily for any signs of pain, discomfort, or distress.
Plant material and extraction
The fresh leaves of BV were procured from the market of Bhopal, India, and authenticated by Dr. Zia Ul Hasan, Professor, Department of Botany, Saifia College, Bhopal (Madhya Pradesh), India (Specimen voucher no. 483/Bot/Saifia/14). The fresh leaves were washed with tap water, dried under shade, and powdered. The powdered leaf material (160 g) was successively extracted with petroleum ether (50–60°C) and 95% ethanol (60–70°C) using Soxhlet extractor. The resultant ethanol extract was air dried for 5 days to remove solvents and kept in a refrigerator until use and used in this study without any further purification. The suspension of plant extract in 0.5% carboxymethylcellulose (CMC) was prepared, and the dose level 100 and 200 mg/kg body weight was selected based on the previous studies.[20]
Experimental design
Thirty-six mice were randomly divided into six experimental groups, each consisting of six mice. Group I (normal control) mice received 0.5% CMC (1 mL/kg, p.o.) daily for 7 days. Group II (stress control) mice received 0.5% CMC (1 mL/kg, p.o.) daily for 7 days and subjected to restraint stress on 8th day [Figure 1]. Group III-IV mice were treated with Beta vulgaris Linn. ethanolic extract (BVEE) (100 and 200 mg/kg, p.o.) daily for 7 days and subjected to ARS on 8th day. Groups V-VI mice were treated with BVEE (100 and 200 mg/kg, p.o.) daily for 7 days and no stress was given on 8th day. Anxiety- and depressive-like behavior were measured by submitting the mice to different behavioral paradigms such as open field test (OFT), elevated plus maze (EPM) test, forced swim test (FST), and tail suspension test (TST) 40 min poststress procedure. Oxidative stress parameters such as lipid peroxidation and reduced GSH level were also analyzed following behavioral tests.
Figure 1.
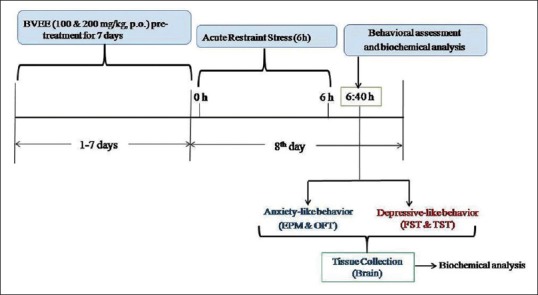
Illustration of experimental design. BVEE: Beta vulgaris ethanolic extract; EPM: Elevated plus maze; FST: Forced swim test; OFT: Open field test; TST: Tail suspension test
Acute restraint stress procedure
Restraint stress protocol was adapted from a previously described procedure.[8,17,25] The animals were divided into six groups as mentioned above. Stressed groups were administered with vehicle and BVEE, and 1 h after the treatment; they were submitted to the stress protocol. The immobilization stress was accomplished by placing them in an individual rodent restraint device made of Plexiglas fenestrate for a period of 6 h. This restrained all physical movements without submitting the animal to pain. The animals were deprived of food and water during the entire period of exposure to stress. After 6 h, the animals were released from their enclosure and after 40 min postrelease, the animals were subjected to the behavioral tests or biochemical estimations. In unstressed group, the mice were kept in the animal cage with soft bedding in the experimental room.
Behavioral tests
Elevated plus maze test
EPM test has been proposed for selective screening of anxiolytic and anxiogenic drugs. It was made of two open arms (35 cm × 5 cm) perpendicular to two closed arms (35 cm × 5 cm × 20 cm) size with a small central square (5 × 5) between arms. The maze was elevated 50 cm from the floor in a dim room. Each mouse was placed at the center of EPM with head facing toward the open arm. During 5 min test period, the parameters measured were: Number of open arm entries and closed arm entries. Subsequently, the percentages of open arm entries and time spent on open arms were calculated from open arm entries and time spent on open arms was divided by the total number of entries in both open and closed arms and time spent on open arm exploration was divided by total time spent in both open and closed arms, respectively. The procedure was conducted in sound attenuated room.[13,26]
Open field test
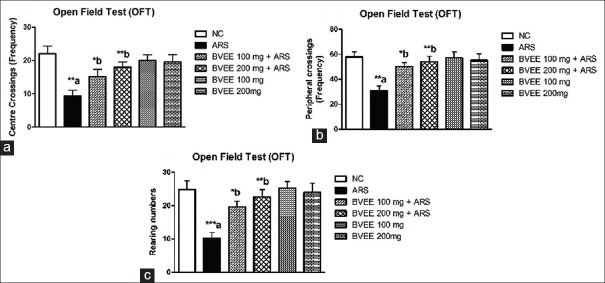
OFT is a useful tool to assess the effect of restraint stress on motor and behavioral changes in the mice. Wooden box (60 cm × 60 cm × 30 cm) with its floor divided into 16 equal sized squares (15 cm × 15 cm) was used. Four squares were considered as the center and the 12 squares along the walls were considered the periphery. Each mouse was placed in the very center of the open field and (a) number of central crossing, (b) number of peripheral crossing, and (c) rearing were observed during a 5 min exposure period for both the control and treated animals.[13,26]
Forced swim test
The FST, the most commonly employed behavioral model for screening antidepressant agents in rodents, was performed as per the method described by Porsolt et al.[27] with some modifications. Briefly, mice were forced to swim in a cylinder (diameter 15 cm, height 25 cm) containing 15 cm of fresh water maintained at 25°C ± 1°C. At this height of water, mice were not able to support themselves by touching the bottom or the side walls of the cylinder with their paws or tail. Water in the cylinder was changed after each animal to prevent the behavioral alteration among animals due to used water. Each animal showed vigorous movement during initial 2 min period of the test. The duration of immobility was manually recorded during the next 4 min of the total 6 min testing period by the observers blind to the treatment conditions. Mice were considered to be immobile when they floated in an upright position, making only small movements to keep their head above the water. Following swimming session, mice were dried using room heater or towel and returned to their home cages.[1] A decrease in the duration of immobility is indicative of an antidepressant-like effect,[27] whereas an increase of immobility time, when compared with the control group, is associated with depressive-like effect.[25]
Tail suspension test
TST is a frequently used behavioral model to study the antidepressant‐like activity in mice. The TST was performed as previously described by Steru et al.[28] with some modifications. Briefly, each mouse was individually suspended to the edge of a table, 50 cm above the floor, by adhesive tape, placed approximately 1 cm from the tip of the tail in dim lighted room that was acoustically and visually isolated. The immobility time of each mouse was manually recorded for 6 min but immobility time during the last 4 min was analyzed and presented. Animal was considered to be immobile when it did not show any body movement, hung passively, and completely motionless. Recording of the immobility of animals was done by the observers blind to the treatments given to the animals under study.[1,4]
Biochemical analysis
Preparation of brain homogenate
All the animals were sacrificed by decapitation on the same day immediately after behavioral assessments. The brains were quickly removed, washed in ice cold sterile isotonic saline, and weighed. A 10% (w/v) tissue homogenates were prepared with 0.1 M phosphate buffer (pH 7.4). The supernatant was obtained by centrifugation of the homogenate at 12000 ×g for 20 min at 4°C and used for further biochemical analysis.
Glutathione levels
Reduced GSH levels in the brain homogenates were determined according to the Ellman's method.[29] Briefly, homogenates were mixed with 50% trichloroacetic acid solution. After centrifugation (3000 rpm/15 min), the supernatant of the homogenate was collected and mixed with 0.4 M tris HCl buffer (pH 8.9) and 0.01 M 5,5-dithiobis (2-nitrobenzoic acid). The resultant yellow color was immediately read at 412 nm using a ultraviolet (UV)-visible spectrophotometer. Results were calculated based on a standard GSH curve and expressed as μM of GSH/mg of protein.
Lipid peroxidation assay
The quantitative measurement of lipid peroxidation in the whole brain was assessed according to the method of Jangra et al.[30] The amount of malondialdehyde (MDA) formed was measured by the reaction with thiobarbituric acid at 532 nm using Shimazdu UV-visible spectrophotometer. The results were expressed as μM of MDA per mg of protein using the molar extinction coefficient of chromophore (1.56 × 105/M/cm).
Protein estimation
The protein content was measured according to the method described by Lowry et al.[31] using bovine serum albumin as standard.
Statistical analysis
All data are presented as mean ± standard error of mean and compared by one-way ANOVA followed by Tukey's test as post-hoc test. The results were considered significant at P ≤ 0.05. The statistical program used was GraphPad Prism 5.0 Version for Windows, (GraphPad Software, Inc., San Diego, California, USA).
RESULTS
Effect of Beta vulgaris Linn. ethanolic extract pretreatment on restraint stress-induced anxiety - like behavior tested in elevated plus maze
Analysis of EPM data revealed that ARS (6 h) induced an anxiety - like behavior as observed by significant reduction in the percentage open arm entries (P < 0.01) and percentage time spent in open arms (P < 0.01) as compared to control group. BVEE pretreatment, at both the dosage (100 and 200 mg/kg p.o. for 7 days), reversed the restraint stress-induced changes in EPM parameters. Chronic pretreatment of BVEE increased the percentage of open arm entries [Figure 2a] and percentage time spent in open arms [Figure 2b] significantly at both the dosage 100 and 200 mg/kg, respectively (P < 0.05 and P < 0.01), as compared to the stress group.
Figure 2.
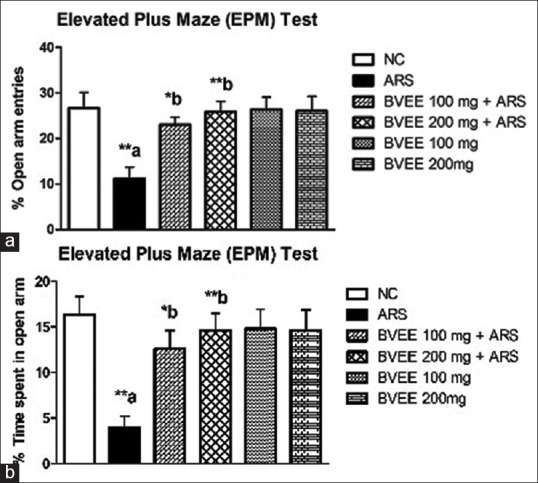
Effect of Beta vulgaris ethanolic extract pretreatment on ARS-induced anxiety-like behavior tested in elevated plus maze. (a) Percentage of open arm entries (b) Percentage time spent in open arm. NC: Normal control; ARS: Acute restraint stress; BVEE: Beta vulgaris ethanolic extract. Values are expressed as mean ± standard error of mean (N = 6 mice). **P < 0.01, *P < 0.05. a versus NC group and b versus ARS group
Effect of Beta vulgaris Linn. ethanolic extract pretreatment on restraint stress-induced anxiety - like behavior tested in open field test
Restraint stress-induced a significant decrease in the number of central crossing (P < 0.01), peripheral crossing (P < 0.01), and rearing (P < 0.001) as compared to the control group. Pretreatment of BVEE attenuated the restraint stress-induced changes in the open field parameters. BVEE pretreatment at low dose (100 mg/kg) produced significant increase in the frequency of central (P < 0.05) and peripheral crossings (P < 0.05) and rearings (P < 0.05) whereas higher dose of BVEE (200 mg/kg) showed more significant effect on the number of central (P < 0.01) and peripheral crossings (P < 0.01) and rearings (P < 0.01) [Figure 3a-c].
Figure 3.
Effect of Beta vulgaris ethanolic extract pretreatment on ARS-induced anxiety-like behavior tested in open field. (a) Number of Central and (b) Peripheral crossings, and (c) Rearings. NC: Normal control; ARS: Acute restraint stress; BVEE: Beta vulgaris ethanolic extract. Values are expressed as mean ± standard error of mean (N = 6 mice). ***P < 0.001, **P < 0.01, *P < 0.05. a versus NC group and b versus ARS group
Effect of Beta vulgaris Linn. ethanolic extract pretreatment on restraint stress-induced changes in immobility time in forced swim test
To evaluate the influence of treatment of mice with BVEE on the depressive-like behavior elicited by ARS procedure, the immobility time of mice in the FST was measured [Figure 4a]. ARS caused a significant increase in the immobility time, which is in agreement with its ability to induce depressive-like behavior. In the FST, our results showed that the immobility time was increased significantly (P < 0.001) in the mice subjected to ARS as compared to control group. Pretreatment with BVEE attenuated restraint stress-induced increase in the immobility time at both the dosage 100 mg/kg (P < 0.05) and 200 mg/kg (P < 0.01) significantly.
Figure 4.
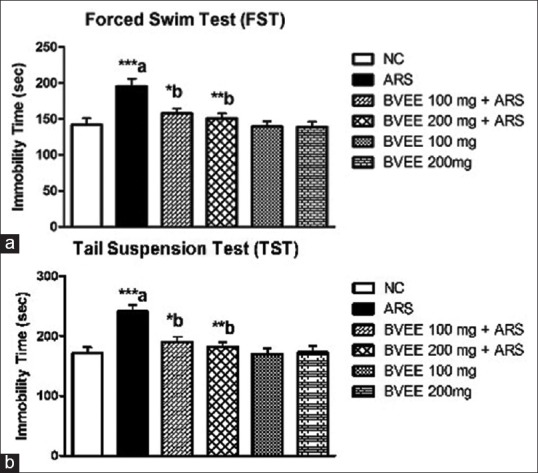
Effect of Beta vulgaris ethanolic extract pretreatment in ARS-induced changes in immobility time in (a) forced swim test (FST) and (b) tail suspension test. NC: Normal control; ARS: Acute restraint stress; BVEE: Beta vulgaris ethanolic extract. Values are expressed as mean ± standard error of mean (N = 6). ***P < 0.001,**P < 0.01,*P < 0.05. a versus NC group and b versus ARS group
Effect of Beta vulgaris Linn. ethanolic extract pretreatment on restraint stress-induced changes in immobility time in tail suspension test
Mice subjected to ARS exhibited a significant increase in the immobility time (P < 0.001) as compared with that of control group, whereas pretreatment with BVEE significantly reversed ARS-induced increase in the immobility time in TST at both the dosage 100 mg/kg (P < 0.05) and 200 mg/kg (P < 0.01) [Figure 4b]. BVEE pretreatment exhibited significant protective effect against ARS-induced increase in the immobility time in TST.
Effect of Beta vulgaris Linn. ethanolic extract pretreatment on restraint stress-induced oxidative stress parameters
ARS (6 h) significantly decreased the reduced GSH level in brain tissues (P < 0.001) of mice as compared to that of vehicle treated group. Pretreatment of BVEE resulted in significant improvement in GSH level at both the dosages, that is, 100 mg/kg (P < 0.05) and 200 mg/kg (P < 0.01) when compared to the restraint stress group [Figure 5a].
Figure 5.
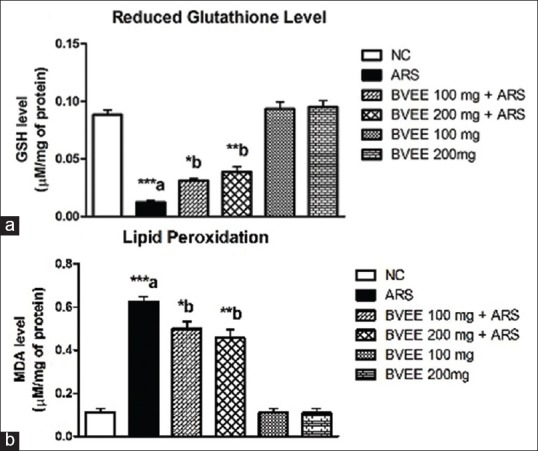
Effect of BVEE pre-treatment on ARS-induced changes in oxidative stress parameters (a) GSH (b) MDA. MDA: Malondialdehyde; GSH: Glutathione; NC: Normal control; ARS: Acute restraint stress; BVEE: Beta vulgaris ethanolic extract. Values are expressed as Mean±S.E.M (N = 6). ***P < 0.001, **P < 0.01, *P < 0.05. a Vs NC group and b Vs ARS group
The exposure of 6-h ARS significantly increased MDA level (P < 0.001) in mice brain as compared to control (unstressed) mice. Chronic (7 days) pretreatment with BVEE significantly attenuated the rise in MDA level at both the dosage, that is, 100 (P < 0.05) and 200 mg/kg (P < 0.01) as compared to the restraint stress group [Figure 5b].
DISCUSSION
Stressful events are known to influence the physiological homeostasis of the organism and complex mechanisms, lead to the changes in immunological and neurobehavioral profile in the course of adaptational processes. Stress plays the significant role in the pathophysiology of psychiatric disorders including anxiety and depression.[32] It alters various neurological functions at both central and peripheral level through activation of hypothalamus-pituitary-adrenal axis. Any type of stress influences brain functions by causing long-term changes in the multiple neural systems.[8] ARS has been reported to precipitate anxiety- and depression-like behaviors in the animals.[8,13]
Stress-induced behavioral alterations can be monitored in the rodents effectively. EPM and OFT are the most commonly employed test to study the effect of anxiolytics on behavioral parameters of animals.[13] In the present study, ARS (6 h) caused anxiety-like behavior as evidenced by significant reduction in the EPM parameters such as percentage number of entries and percentage time spent in open arms. Similarly, restraint stress caused significant decrease in central and peripheral ambulation and rearing in the OFT, indicating fear or anxiety. The results are in an agreement with previous studies.[33,34] Increase in the percentage number of entries and percentage time spent in open arms in EPM was reversed by the chronic pretreatment of BVEE at both the dosage level, might be due to the chemical constituents such as betaine and betalains present therein. Similarly, pretreatment of BVEE increased number of central and peripheral crossings and rearings. The observed results indicate decreased fear or anxiety. Thus, BVEE pretreatment provided anxiolytic effects against ARS.
There are ample of evidences which showed association of depression with stressful events. Therefore, stress-induced depression models in rodents are used to evaluate antidepressant drugs. FST and TST are the two most commonly employed tests in rodents for screening of antidepressants, are quite sensitive and relatively specific to all the major classes of antidepressants.[13] In the current study, ARS significantly increased the immobility time in the FST and TST indicating depressive-like behavior. These results are in accordance with earlier studies which demonstrated that restraint stress-induced depressive-like behavior as evidenced by increased immobility time in FST and TST.[11] Pretreatment of BVEE provided significant protection against ARS-induced increased in the immobility time in FST and TST. This protective activity of BVEE might be due the presence of betalains and betaine, an active constituent of BV Linn., which are useful in the treatment of depression.[35,36,37]
Oxidative stress generates ROS that exerts detrimental effects on the cellular components such as lipids, proteins, and DNA resulting in cellular damage and neurodegeneration.[8,26,38] Restraint stress is a well-known method to produce oxidative damage by causing derangement in the antioxidant defense mechanism.[17] In the present study, 6-h restraint stress caused significantly oxidative damage as indicated by increased lipid peroxidation and depleted reduced GSH level. The results are in line with the earlier findings that exposure of restraint stress for 6 h cause imbalance in antioxidant defense mechanism resulting in oxidative stress.[8,17] Study, reported an increased oxidative damage and weak antioxidant defense mechanisms, are implicated in anxiety and depression.[8] In the present study, BVEE pretreatment significantly attenuated lipid peroxidation and restored GSH activity suggesting its antioxidant-like effect. The protection offered by BVEE pretreatment might be attributed due to the presence of antioxidant constituents such as polyphenols (e.g., betalains and betaine), flavonoids, and Vitamin C in the leaves of BV Linn.[18,39] Our results also indicate that BVEE could be a beneficial dietary supplement to combat various neurodegenerative diseases.
CONCLUSION
The results of the current study demonstrate the anxiolytic and antidepressant-like activities of BVEE against ARS-induced anxiety- and depressive-like behavior and oxidative damage in mice. These observed effects may be mediated by the central serotonergic neuro-transmission (5-HT) and antioxidant profile of BVEE. Further research is needed to be performed to characterize the specific phytoconstituents involved as well as to ascertain their individual contributions and exact mechanism of action pertaining to CNS activity. However, this study provides indication that BVEE can be used in the treatment and management of stress-induced disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Kunjbihari Sulakhiya
Dr. Kunjbihari Sulakhiya, has worked as Assistant Professor at the Department of Pharmacology, Ravishankar College of Pharmacy, Bhopal. He has published 9 research papers and 1 review article in reputed peer reviewed international and national journals, and also contributed 1 book chapter. He has presented various research papers at national and international conferences. He served as editorial member and reviewer for various reputed journals. His research area of interest is animal model development and pre-clinical research in the field of neuropharmacology

Vikas Kumar Patel
Mr. Vikas Kumar Patel, is currently working as Pharmacist under NACO India in ART Center Dhar. His main job in ART center is to diagnose the clinical toxicity produced by the anti-retroviral drugs used for the treatment of HIV in AIDS patients. He has obtained his master degree (M. Pham) in Pharmacology from RGPV University, Bhopal. His main research area of interest is to find out the toxicity associated with drugs and to serve as community pharmacist.

Rahul Saxena
Mr. Rahul Saxena, presently working as Assistant Professor at the Department of Pharmacology, Ravishankar College of Pharmacy, Bhopal. He has 4 years of teaching and research experience. He has 13 publications in his credit including both research and review international and national journals, and presented various research papers at national and international conferences. He has skilled in pre-clinical model development, clinical studies, expertise in interpretation of drug use and biochemical analysis.

Jagrati Dashore
Ms. Jagrati Dashore, presently working as Assistant Professor at Rajeev Gandhi College of Pharmacy, Bhopal. She has 1 year of teaching experience as lecturer. She has submitted her M. Pharm (Pharmacology) thesis in RGPV University, Bhopal. Her area of research interest is pre-clinical and clinical research, and would like to make her career in R&D field.

Amit Kumar Srivastava
Mr. Amit Kumar Srivastava, Presently working as Research co-ordinator in the Department of Pharmacology, Sapience Bioanalytical Research Lab, Bhopal. He has published 12 papers (both original and review articles) in peer reviewed international and national journals and presented various research papers at national and international conferences. His area of expertise and interest includes preclinical pharmacological screening model development aimed at mechanistic studies, safety pharmacology and regulatory toxicological studies, and Bio-analytical method development of new chemical entities in the preclinical discovery phase.

Manoj Rathore
Mr. Manoj Rathore, presently working as Lecturer at School of Pharmacy, Devi Ahilya Vishwavidhyalaya (DAVV), Indore. He is having 3 years teaching experience. He has published 1 research paper and 3 review articles in reputed peer reviewed journals. He has obtained diploma certificates in Clinical trials and Pharmacovigilance programme, and associated with both pre-clinical and clinical research work.
Acknowledgments
The authors are thankful to the Director, Sapience Bioanalytical Research Laboratory, Bhopal, India, and for providing necessary assistance during the research work.
REFERENCES
- 1.Santosh P, Venugopl R, Nilakash AS, Kunjbihari S, Mangala L. Antidepressant activity of methanolic extract of Passiflora foetida leaves in mice. Int J Pharm Pharm Sci. 2011;3:112–5. [Google Scholar]
- 2.Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M. Canadian Anxiety Guidelines Initiative Group on behalf of the Anxiety Disorders Association of Canada/Association Canadienne des troubles anxieux and McGill University. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1. doi: 10.1186/1471-244X-14-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesfin M, Asres K, Shibeshi W. Evaluation of anxiolytic activity of the essential oil of the aerial part of Foeniculum vulgare Miller in mice. BMC Complement Altern Med. 2014;14:310. doi: 10.1186/1472-6882-14-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulakhiya K, Kumar P, Jangra A, Dwivedi S, Hazarika NK, Baruah CC, et al. Honokiol abrogates lipopolysaccharide-induced depressive like behavior by impeding neuroinflammation and oxido-nitrosative stress in mice. Eur J Pharmacol. 2014;744:124–31. doi: 10.1016/j.ejphar.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Bradley BF, Bridges NJ, Starkey NJ, Brown SL, Lea RW. Anxiolytic and anxiogenic drug effects on male and female gerbils in the black-white box. Behav Brain Res. 2011;216:285–92. doi: 10.1016/j.bbr.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Kumar D, Bhat ZA. Anti-anxiety activity of methanolic extracts of different parts of Angelica archangelica Linn. J Tradit Complement Med. 2012;2:235–41. doi: 10.1016/s2225-4110(16)30105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese F, Molteni R, Riva MA. Antistress properties of antidepressant drugs and their clinical implications. Pharmacol Ther. 2011;132:39–56. doi: 10.1016/j.pharmthera.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Garg R, Prakash AK. Effect of St. John's Wort (Hypericum perforatum) treatment on restraint stress-induced behavioral and biochemical alteration in mice. BMC Complement Altern Med. 2010;10:18. doi: 10.1186/1472-6882-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masood A, Banerji B, Vijayan VK, Ray A. Pharmacological and biochemical studies on the possible role of nitric oxide in stress adaptation in rats. Eur J Pharmacol. 2004;493:111–5. doi: 10.1016/j.ejphar.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Yadav AK. Protection of stress induced behavioural and physiological alteration by Marsilea quadrifolia in rodents. J Chem Pharm Res. 2014;6:2207–17. [Google Scholar]
- 11.Budni J, Zomkowski AD, Engel D, Santos DB, dos Santos AA, Moretti M, et al. Folic acid prevents depressive-like behavior and hippocampal antioxidant imbalance induced by restraint stress in mice. Exp Neurol. 2013;240:112–21. doi: 10.1016/j.expneurol.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Subakanmani S, Umadevi P. Evaluation of Anxiolytic potential of ethanolic extract of Hypericum hookerianum in stress induced Swiss albino mice. Int Res J Pharm. 2013;3:1–5. [Google Scholar]
- 13.Tabassum I, Siddiqui ZN, Rizvi SJ. Effects of Ocimum sanctum and Camellia sinensis on stress-induced anxiety and depression in male albino Rattus norvegicus. Indian J Pharmacol. 2010;42:283–8. doi: 10.4103/0253-7613.70108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balk Rde S, Bridi JC, Portella Rde L, Carvalho NR, Dobrachinski F, da Silva MH, et al. Clomipramine treatment and repeated restraint stress alter parameters of oxidative stress in brain regions of male rats. Neurochem Res. 2010;35:1761–70. doi: 10.1007/s11064-010-0240-1. [DOI] [PubMed] [Google Scholar]
- 15.García-Fernández M, Castilla-Ortega E, Pedraza C, Blanco E, Hurtado-Guerrero I, Barbancho MA, et al. Chronic immobilization in the malpar1 knockout mice increases oxidative stress in the hippocampus. Int J Neurosci. 2012;122:583–9. doi: 10.3109/00207454.2012.693998. [DOI] [PubMed] [Google Scholar]
- 16.Khan MS, Tabrez S, Priyadarshini M, Priyamvada S, Khan MM. Targeting Parkinson's-tyrosine hydroxylase and oxidative stress as points of interventions. CNS Neurol Disord Drug Targets. 2012;11:369–80. doi: 10.2174/187152712800792848. [DOI] [PubMed] [Google Scholar]
- 17.Zaidi SK, Hoda MN, Tabrez S, Ansari SA, Jafri MA, Shahnawaz Khan M, et al. Protective effect of Solanum nigrum leaves extract on immobilization stress induced changes in rat's brain. Evid Based Complement Alternat Med. 2014;2014:912450. doi: 10.1155/2014/912450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain NK, Singhai AK. Protective role of Beta vulgaris L. leaves extract and fractions on ethanol-mediated hepatic toxicity. Acta Pol Pharm. 2012;69:945–50. [PubMed] [Google Scholar]
- 19.Khare CP. New York: Springer Science and Business Media LLC; 2007. Indian Medicinal Plants: An Illustrated Dictionary. [Google Scholar]
- 20.Babu VL, Gowri R. Evaluation of Antioxidant activity of Beta vulgaris root extract in rats. Asian J Chem. 2010;22:3385–9. [Google Scholar]
- 21.El Gamal AA, AlSaid MS, Raish M, Al-Sohaibani M, Al-Massarani SM, Ahmad A, et al. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflamm. 2014;2014:983952. doi: 10.1155/2014/983952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain S, Garg VK, Sharma PK. Anti-inflammatory activity of aqueous extract of Beta vulgaris L. J Basic Clin Pharm. 2011;2:83–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Kapadia GJ, Rao GS, Ramachandran C, Iida A, Suzuki N, Tokuda H. Synergistic cytotoxicity of red beetroot (Beta vulgaris L.) extract with doxorubicin in human pancreatic, breast and prostate cancer cell lines. J Complement Integr Med. 2013;10 doi: 10.1515/jcim-2013-0007. pii:/j/jcim. 2013.10.issue-1/jcim-2013-0007/jcim-2013-0007.xml. [DOI] [PubMed] [Google Scholar]
- 24.Kapadia GJ, Azuine MA, Rao GS, Arai T, Iida A, Tokuda H. Cytotoxic effect of the red beetroot (Beta vulgaris L.) extract compared to doxorubicin (Adriamycin) in the human prostate (PC-3) and breast (MCF-7) cancer cell lines. Anticancer Agents Med Chem. 2011;11:280–4. doi: 10.2174/187152011795347504. [DOI] [PubMed] [Google Scholar]
- 25.Moretti M, Budni J, Dos Santos DB, Antunes A, Daufenbach JF, Manosso LM, et al. Protective effects of ascorbic acid on behavior and oxidative status of restraint-stressed mice. J Mol Neurosci. 2013;49:68–79. doi: 10.1007/s12031-012-9892-4. [DOI] [PubMed] [Google Scholar]
- 26.Sulakhiya K, Kumar P, Gurjar SS, Barua CC, Hazarika NK. Beneficial effect of honokiol on lipopolysaccharide induced anxiety-like behavior and liver damage in mice. Pharmacol Biochem Behav. 2015;132:79–87. doi: 10.1016/j.pbb.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 28.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 29.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 30.Jangra A, Lukhi MM, Sulakhiya K, Baruah CC, Lahkar M. Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur J Pharmacol. 2014;740:337–45. doi: 10.1016/j.ejphar.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 32.Kulkarni PM, Archana RJ. Effect of roots of Tylophora indica (Burm. f.) on stress and anxiety in animal models. Int J Pharm. 2009;8:1–5. [Google Scholar]
- 33.Chakraborti A, Gulati K, Banerjee BD, Ray A. Possible involvement of free radicals in the differential neurobehavioral responses to stress in male and female rats. Behav Brain Res. 2007;179:321–5. doi: 10.1016/j.bbr.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 34.Masood A, Banerjee B, Vijayan VK, Ray A. Modulation of stress-induced neurobehavioral changes by nitric oxide in rats. Eur J Pharmacol. 2003;458:135–9. doi: 10.1016/s0014-2999(02)02688-2. [DOI] [PubMed] [Google Scholar]
- 35.Kagan BL, Sultzer DL, Rosenlicht N, Gerner RH. Oral S-adenosylmethionine in depression: A randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 1990;147:591–5. doi: 10.1176/ajp.147.5.591. [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, Lee L, Kim JH, Lee TH, Shim I. Antidepressant-like effects of lycii radicis cortex and betaine in the forced swimming test in rats. Biomol Ther (Seoul) 2013;21:79–83. doi: 10.4062/biomolther.2012.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenbaum JF, Fava M, Falk WE, Pollack MH, Cohen LS, Cohen BM, et al. The antidepressant potential of oral S-adenosyl-l-methionine. Acta Psychiatr Scand. 1990;81:432–6. doi: 10.1111/j.1600-0447.1990.tb05476.x. [DOI] [PubMed] [Google Scholar]
- 38.Marzatico F, Bertorelli L, Pansarasa O, Guallini P, Torri C, Biagini G. Brain oxidative damage following acute immobilization and mild emotional stress. Int J Stress Manag. 1998;5:223–36. [Google Scholar]
- 39.Kanner J, Harel S, Granit R. Betalains – A new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49:5178–85. doi: 10.1021/jf010456f. [DOI] [PubMed] [Google Scholar]