Abstract
Background:
Evidences from ethnopharmacological practices have shown that combination of Abutilon indicum and Piper longum are traditionally used to treat symptoms of the liver disorder. The hypothesis is phytosomes of a combination of both crude drug extract will be more effective and safe as hepatoprotective agent.
Aim:
Present work is aimed at development and characterization of phytosomes containing ethanolic extract of both drugs to meet the need for better effectiveness and safety.
Materials and Methods:
Phytosomes were formulated by using Indena's patented process. Characterization involved following parameters: Particle size determination, percentage yield, entrapment efficiency, differential scanning calorimetry, scanning electron microscope, fourier transform infrared spectroscopy, and high performance thin liquid chromatography. Liver damage was induced in adult Charles foster rats (150 ± 10 g) with CCl4 in olive oil (1:1 v/v, i.p) 1 ml/kg once daily for 7 days. LIV 52 (1 ml/kg per oral [p.o]), ethanolic extract of A. indicum and P. longum combination (100, 200, and 400 mg/kg p.o) and phytosomes (100 mg/kg p.o.) was given 3 days prior to CCl4 administration. Estimation of liver marker enzymes and histopathological studies were done. Result was analyzed by using (analysis of variance) followed by Student-Newman–Keuls test.
Result:
Combined extract has shown hepatoprotective activity but phytosomal formulation has more potent hepatoprotective effect on CCl4 induced liver toxicity at very low dose comparative to a higher dose of combined extract.
Conclusion:
Novel approach for herbal drug delivery is more prominent than conventional which improves bioavailability of polar extract and also patient compliance.
SUMMARY
Standardised ethanolic extract of leaves of abutilon indicum and piper longum fruits by microwave assisted extraction was used for phytosomal complex formation and phytosomal complex was characterised by various parameters and finally the hepatoprotective activity of phytosomes and crude extract was evaluated by different biochemical markers and histopathological study.

Abbreviations Used: DSC: Differential scanning calorimetry, SEM: Scanning electron microscope, FTIR: Fourier transform infrared spectroscopy, HPTLC: High performance thin liquid chromatography, p.o: Per oral, A. indicum: Abutilon indicum, P. longum: Piper longum.
Keywords: Abutilon indicum, Characterization, Hepatoprotective activity, Phytosome, Piper longum
INTRODUCTION
According to the ethnobotanical literature of Odisha, paste of Abutilon indicum leaves and Piper longum fruits were used to treat symptoms of jaundice by tribes of Kalahandi district, Odisha, India.[1] The crude drug used were leaves of A. indicum (Malvaceae) and fruits of P. longum (Piperaceae) A. indicum (Malvaceae) commonly known as Indian Mallow, Atibala in Sanskrit and Kanghi in Hindi is an important medicinal plant of Indian traditional system of medicine and found to possess hypoglycemic activity,[2] anti-inflammatory activity,[3] antibacterial activity,[4] anticonvulsant activity,[5] lipid lowering activity,[6] antiulcer activity,[7] antidiarrheal activity,[8] immunomodulatory activity,[9] analgesic activity,[10] wound healing activity,[11] acetylcholinesterase inhibitory activity,[12] and hepatoprotective activity.[13] Chemically, the active constituents present in A. indicum are fatty acids, flavonoids, quercetin, glycosides, alkaloids, steroids, terpenoids, saponins, sesquiterpenes, lactones, gallic acid, β-setosterol, geraniol, caryophyllene, and phenolic compound.[14] Quercetin is the bioactive marker of A. indicum and ethanolic extract of leaves contains 72% more quercetin than flowers.[15] The mechanism mainly involved in CCl4 induced liver injury is lipid peroxidation by free radicals derivatives of CCl4.[16] P. longum (Piperaceae) also known as Indian long pepper, it has many medicinal and dietary uses and its fruits are taken in combination with other herbs in Ayurvedic medicine. The main active constituent piperine (1-piperoyl piperidine) has been demonstrated for its bioavailability enhancing property may be due to inhibition of drug metabolizing enzymes and renal clearance, enhancing blood supply for absorption or modulating active transporters.[17,18] Phytosomes are defined as “phyto” means plants and “some” means cell-like. The phytosome (technology was developed by Indena s.p.a of Italy), are used to enhance the bioavailability of phytomedicines by incorporating phospholipids into standardized plant extract.[19] It is novel drug delivery system in which hydrophilic choline moiety (head) binds to phytoconstituents (polar) and lipophilic phosphatidyl moiety surrounds choline bound phytoconstituents or form outer layer, hence water soluble phytoconstituents become lipid soluble.[20] Phytosomes contains naturally occurring phospholipid, phosphatidylcholine (PC) like soylecithin. It is also a cellular component which is biodegradable and has reported hepatoprotective activity. Phytosomes have improved pharmacokinetic and pharmacological parameter.[21,22,23]
MATERIALS AND METHODS
Plant material
The fresh green leaves of A. indicum were collected in the month of November from the botanical garden of Department of Dravyaguna, Banaras Hindu University, Varanasi. Dried fruits of P. longum were brought from Gola market of Varanasi, and identification of these drugs were done by Prof. N. K. Dubey, Department of Botany, Faculty of Science, Banaras Hindu University, Varanasi.
Drugs and chemicals
LIV 52 (Himalaya Drug Company) was purchased from the local medicinal shop of Varanasi. Carbon tetrachloride used to induce hepatotoxicity was obtained from the Merck Ltd. Mumbai. The biochemical estimation was performed by standard procedures in biochemical kit obtained from RAGINI DIAGNOSTIC, Varanasi. All other chemicals used were of analytical grade and were obtained from the Hi-Media Laboratories Pvt. Ltd, Mumbai.
Experimental animals
Adult Charles Foster albino rats (150 ± 10 g) of either sex, were procured from the Central Animal House (CPCSEA), Institute of Medical Science, Banaras Hindu University, Varanasi, India. The certified pathogen free animals were housed in polypropylene cages under standard condition (ambient temperature of 25°C ± 1°C and 45–55% RH, with a 12:12 h light/dark cycle). They were fed with commercially available rat feed (Amrut Rat and Mice Feed Pvt. Ltd., Sangli, India) and water ad libitum. Animals were acclimatized for at least 1 week before commencement of experiments and exposed only once to every experiments. All experimental protocols were performed after approval from the Central Animal Ethical Committee of Banaras Hindu University (No. Dean 10-11/60 dated 07/01/2011) and were conducted in accordance with accepted standard guidelines of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Extraction procedure
Dried leaves of A. indicum and fruits of P. longum were dried and coarsely powdered, accurately weighed homogenous powder 100 g in ratio of 3:1, respectively, were mixed with ethanol (1 g in 25 ml of ethanol) followed by preleaching for 5 min, then irradiated in microwave in an intermittent way, that is, irradiation: Cooling: Irradiation for 6 min. The microwave irradiation time was 1 min and cooling time of 1 min. After irradiation, the samples were centrifuged at 4000 rpm and the supernatant evaporated under reduced pressure. Dried residue was defatted with petroleum ether and then extracted in ethanol by maceration process for 7 days. The filtrate was dried in vacuum rotary evaporator to yield solid residue of 7.4 g (yield, 7.4%).[24]
Acute toxicity studies
Oral acute toxicity study of plant extract was performed according to the Organisation for Economic Cooperation and Development guidelines number 26/241, Nulliparous and nonpregnant healthy rats were used for this study. To the overnight fasted rats, combined extract of both drugs administered orally as per the guidelines and the rats were observed individually up to 48 h for any behavioral and neurological changes such as tremors, convulsions, salivation, diarrhoea, sleep, lacrimation, and feeding behavior in extract treated rats as a sign of acute toxicity. The observation was further extended up to 14 days to see any sign of mortality.
High performance thin liquid chromatography quantification of quercetin in extract
Instrument CAMAG Linomat 5 “Linomat5_170634”.
Application parameters
Inert gas used as spray gas, methanol as sample solvent, dosage speed was 150 nl/s, syringe size was 100 μl, predosage volume was 0.2 μl, number of tracks were 14, application position Y was 8.00 mm, and band length was 8.00 mm.
Development
Chamber type was Twin trough chamber 20 cm × 10 cm, Mobile phase used toluene:ethyl acetate:formic acid (5:3:0.5), solvent front position was 70.00 mm, volume as 10.00 ml, drying device used was CAMAG TLC plate heater III, temperature up to 60°C, Time was 5 min.
Formulation of phytosomes
Phytosomes were prepared by reaction of natural phospholipid (soy PC) with the extract at a molar ratio of 1:1. The reaction is carried out by refluxing in 100 ml round bottom flask with 20 ml aprotic solvent, methylene chloride, the mixture was refluxed at a temperature not exceeding 40°C for 2 h. The resultant clear solution was evaporated and 10 ml of n-hexane was added to it with continuous stirring, the precipitate was filtered and dried under vacuum to remove traces of solvents, resulting in a thin film formation. This thin film was separated and kept in an amber colored glass bottle, flushed with nitrogen, and stored at room temperature.[25,26]
Evaluation of phytosomes
Particle size determination
The particle size of the phytosomes was determined by Dynamic Light Scattering (NANO ZS Malvern instrument) and Zeta potential was estimated on the basis of electrophoretic mobility under an electric field. Particle size measurement was performed following 1/100 (v/v) dilution of phytosomal suspension in redistilled water at 25°C. Zeta potential was measured using the same instrument at 25°C following the same dilution in 1 mM NaCl solution.
Percentage yield
The prepared phytosomes were dried properly and weighed accurately. This weight was divided by total weight of drugs nonvolatile recipients.
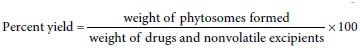
Drug entrapment efficiency
100 mg of prepared phytosomes were dried properly and dispersed in 50 ml distilled water. The content was stirred for 2 h and filtered through Whatman filter paper of pore size 45 μm. It was then suitably diluted and analyzed spectrophotometrically at λ max 266. The amount of drug entrapped was calculated from the standard calibration curve.

Differential scanning calorimetry
Thermal curves of phytosomes and physical mixture of PC extract were obtained using Differential Scanning Calorimeter (Mettler-Toledo TGA/differential scanning calorimetry [DSC] STAR SW9.20, USA). Each sample weighing was scanned at a rate of 10°C/min over the range of −25–200°C. The flow rate of nitrogen was maintained at 5 ml/min.
Scanning electron microscopy
Morphological characteristics were observed by environmental scanning electron microscope (SEM)-quanta 200. The phytosomes were spread on a circular aluminium stub precoated with silver glue (for enhancing conductivity of electrons) and placed observation area of the instrument. It was then observed under the SEM in varying magnifications and micrographs were recorded. Samples were analyzed in SEM at low vacuum. The pressure was maintained in the range of 5.99–6.02e−1 torr. The detector used was secondary electron detector.
Fourier transform infrared spectroscopy
FTIR spectra of pure drug Abutilon indicum, PC and formulated phytosomes were obtained by the conventional KBr pellet/disc method using FT-IR (SHIMADZU, Model 8400, Japan). The scanning range was 400–4000 cm−1.
Drug treatment
Animals after acclimatization (6–7 days) in the animal house, animals were fasted overnight and randomly divided into seven groups of six animals each and treatment is given
Group I - Served as a normal control and received olive oil (1 ml/kg, i.p) daily for 7 days
Group II - Served as toxicant control and received 1:1 (v/v) mixture of CCl4 and olive oil (1 ml/kg, i.p.) daily for 7 days
Group III - Treated with standard drug Liv-52 at a dose of 1 ml/kg, per oral (p.o.) for 10 days and a 1:1 (v/v) mixture of CCl4 and olive oil (1 ml/kg, i.p.) for 7 days from day 4 to day 10
Group IV - Treated with extract 100 mg/kg, p.o. daily for 10 days and a 1:1 (v/v) mixture of CCl4 and olive oil (1 ml/kg, i.p.) for 7 days from day 4 to day 10
Group V - Treated with extract 200 mg/kg, p.o daily for 10 days and a 1:1 (v/v) mixture of CCl4 and olive oil (1 ml/kg, i.p.) for 7 days from day 4 to day 10
Group VI - Treated with extract 400 mg/kg, p.o daily for 10 days and a 1:1 (v/v) mixture of CCl4 and olive oil (1 ml/kg, i.p.) for 7 days from day 4 to day 10
Group VII - Treated with phytosomes 100 mg/kg, p.o daily for 10 days and a 1:1 (v/v) mixture of CCl4 and olive oil (1 ml/kg, i.p.) for 7 days from day 4 to day 10.
Animals were killed 24 h after the last dosing. Blood was collected by a cardiac puncture and allowed to clot for 30 min at room temperature. The serum was separated by centrifugation and used for the assay of marker enzymes and histopathological studies.[27,28]
Estimation of liver marker enzymes
Lysosomal enzymes serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT),[29] total bilirubin (TB) and direct bilirubin (DB),[30] and alkaline phosphate (ALP)[31] were analyzed according to standard method.
Histopathological studies
Rats were killed on the day of withdrawal of blood and livers were dissected out immediately, washed with ice-cold saline, and fixed in 10% formalin solution. After dehydration, the liver pieces were embedded in paraffin wax, sections were cut into 4–6 mm, and stained using hematoxylin and eosin and observed under a microscope for histopathological changes in liver architecture.
Statistical analysis
All values were expressed as a mean ± SEM. Statistical significance differences between control and treatment groups were determined by one-way analysis of variance followed by Student-Newman–Keuls test unless otherwise stated. Statistical significance were determined at the level of confidence P < 0.05. GraphPad Prism (version 5.0) software, Inc. La Jolla, California, (USA) was used for all statistical analysis.
RESULTS AND DISCUSSION
Acute toxicity study
The combined ethanolic extract was found to be practically nontoxic when administered orally to rats and its LD50 value was found to be higher than 5000 mg/kg body weight.
High performance thin liquid chromatography quantification of quercetin in ethanolic extract of Abutilon indicum
After performing the high performance thin liquid chromatography (HPTLC) quantification of quercetin in ethanolic extract of A. indicum, the quantity of quercetin measured was found to be 3.65% w/w. Chromatogram of standard quercetin and extract shown in Figure 1a and b, respectively. Statistical analysis proved that the HPTLC method of quantification will useful for quality control and standardization of extract and also useful in differentiating the desired species from the adulterant and act as a biochemical marker for this important medicinal plant.
Figure 1.
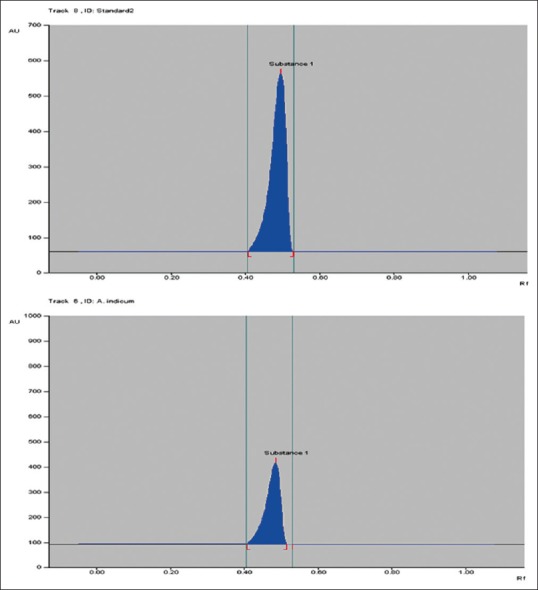
High performance thin liquid chromatography chromatogram of standard quercetin and ethanolic extract of Abutilon indicum
Preparation of phytosomes
Phytosomes were prepared by patented simple and reproducible method. Phytosomes were found to be as free flowing particles. Formulation was further characterized.
Evaluation of phytosomes
Particle size determination
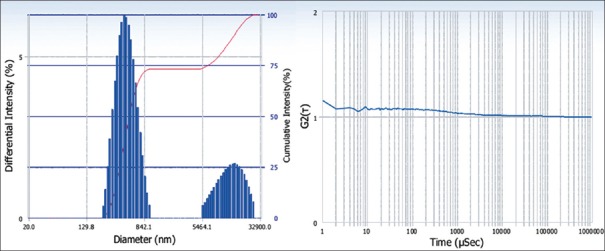
The sample was diluted with distilled water (1:10) before analysis. After performing the particle size determination, the average diameter of phytosomal formulation was found to be 1357.6 nm [Figure 2] and polydispersity index was 0.451 shown in Table 1. This nanoscale range is useful to circumvent the issues of poor bioavailability and solubility of the herbal conventional drug delivery.
Figure 2.
Particle size and Zeta potential of phytosomes
Table 1.
Particle size and polydispersity index

Percentage yield
Percentage yield varies with batches, so the average percentage yield was found to be 66.2%
Drug entrapment efficiency
Entrapment efficiency of different batches of phytosomal formulations are listed [Table 2], the maximum entrapment efficiency was 79.02 ± 0.48, and the minimum was 48.30 ± 0.78 shown in Graph 1. Phytosomes showed a good percent loading of the extract which might be an essential aspect for feasibility of the clinical or therapeutic delivery of the drug.
Table 2.
Entrapment efficiency of different batches of phytosomes
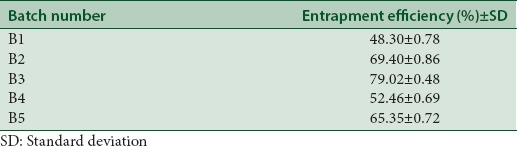
Graph 1.
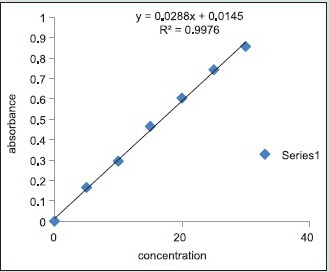
Standard curve of entrapment efficiency
Differential scanning calorimetry
DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. The result of a DSC experiment is a curve of heat flux versus temperature. In this experiment, DSC analysis of phytosomal complex and the physical mixture of PC and extract is reported. DSC profile phytosomes shown by black solid line in Figure 3 shows an endothermic peak with maximum at about 160°C corresponding to a 3% weight loss, and a less intense and broader endothermic signal with a maximum at about 148°C DSC profile of physical mixture green solid line Figure 3 show two broad endothermic peaks, the first one with a maximum at about 125°C and corresponding to a weight loss of 2%, and the second one at about 152°C with a loss of weight of 3% followed by the massive weight loss expected for degradation.
Figure 3.
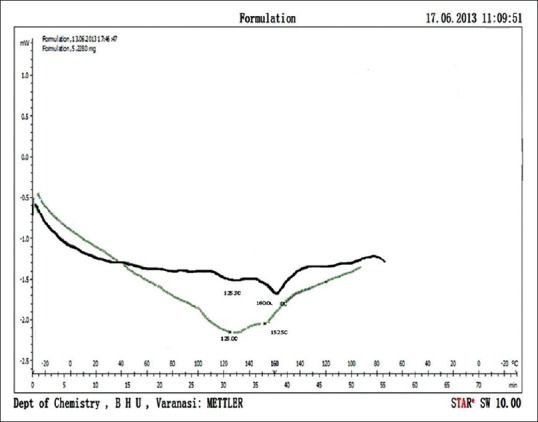
Differential scanning calorimetry curve of phytosomal formulation shown by black solid line and differential scanning calorimetry curve of physical mixture of phosphatidylcholine and extract is shown by green solid line
Scanning electron microscopy
The scanning electron microscopic view indicated the presence of spheroid or irregular shape with rough surface morphology of the phytosomes [Figure 4].
Figure 4.
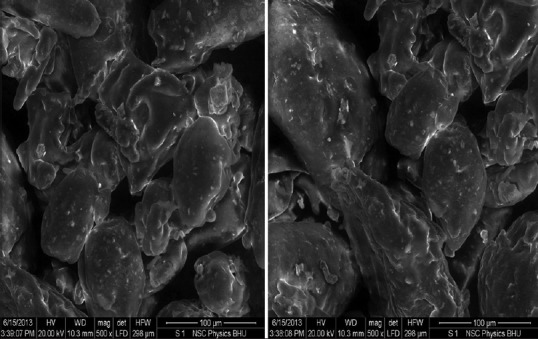
SEM images of phytosomes developed
Fourier transform infrared spectroscopy
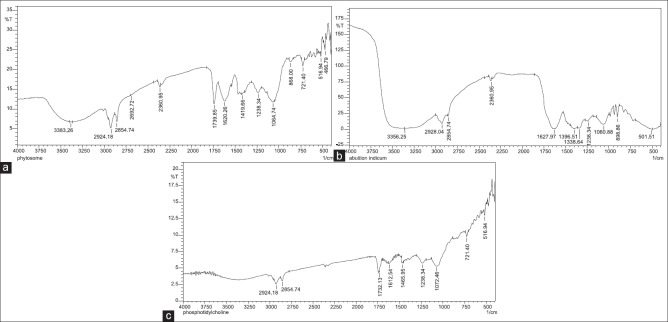
The formation of the complex can be confirmed by FTIR spectroscopy comparing the spectrum of the complex with the individual components and their complex. The spectrum of phytosomal formulation Figure 5a-c shows the additive effect of individual components, that is, the spectrum of ethanolic extract of A. indicum and spectrum of PC. The presence of ethanolic extract of A. indicum in the phytosomal complex can be revealed by absorption peaks at 2924.18 cm−1 and 2360.95 cm−1 describes the presence of N-H structure groups. Absorption peaks at 1060.74 cm−1 and 1060.88 cm−1 describes the presence of C-O (Primary alcohol). The PC can be revealed by absorption peaks at 2854.74 cm−1 and 2924.18 cm−1 describes the presence of N-H group (Ammonium ion), absorption peaks at 1238.34 cm−1 for C-O (Aromatic ether), absorption peak at 721.40 for C-X (chloromethane) and 516.94 cm−1 for C-X (bromomethane).
Figure 5.
(a) Fourier transform infrared spectroscopy spectrum of phytosomal formulation showing combined effect of ethanolic biherbal extract and phosphatidylcholine, (b) Fourier transform infrared spectroscopy spectrum of ethanolic extract Abutilon indicum (c) Fourier transform infrared spectroscopy spectrum of soy phosphatidylcholine
Estimation of liver marker enzymes
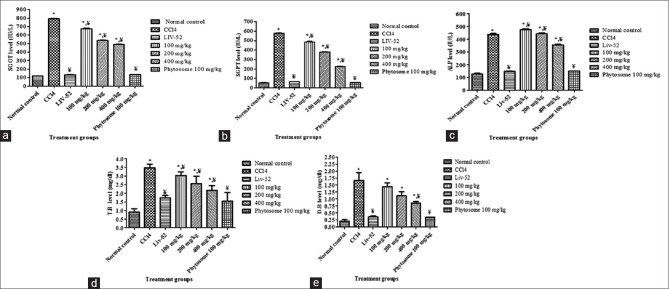
Administration of carbon tetrachloride to rats caused significant liver damage, and this damage results in altered liver marker enzymes that may result from the interference with cytochrome p450, which results in the hindrance of the formation of hepatotoxic free radicals which in turn alters the structure and function of liver cells. Treatment of rats with combined ethanolic extract exhibited remarkable protection against carbon tetrachloride induced hepatotoxicity in dose dependent manner, which is shown in Table 3. 400 mg/kg of combined ethanolic extract of A. indicum and P. longum showed significant hepatoprotective activity against injurious effect of carbon tetrachloride comparable with the standard LIV 52 and Phytosome at 100 mg/kg has shown better effect than 400 mg/kg of combined ethanolic extract. The higher dose of herbal extract is required due to poor absorption of their polar phytoconstituents. Phospholipid-based drug delivery system have been found a promising effect for better and effective delivery of drug and can enhance the rate and extent of drug absorption across the lipoidal biomembrane. SGOT, SGPT, ALP, TB, and DB levels of different groups are shown in Figure 6a-e.
Table 3.
Values are mean±SEM, (n=6)
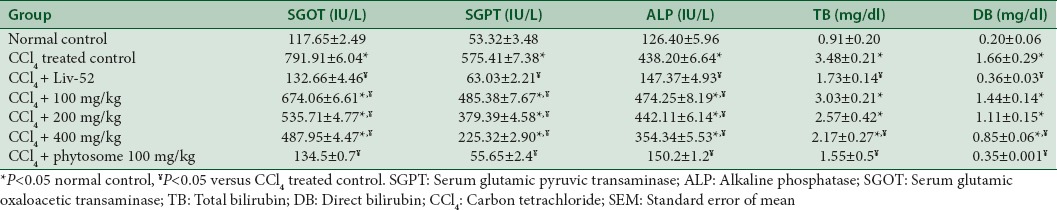
Figure 6.
(a) Comparative effect of combined ethanolic extract, phytosome, and Liv-52 treatment on serum glutamic oxaloacetic transaminase level in CCl4 induced liver injury in rats, (b) Comparative effect of combined ethanolic extract, phytosome, and Liv-52 treatment on serum glutamic pyruvic transaminase level in CCl4 induced liver injury in rats, (c) Comparative effect of combined ethanolic extract, phytosome, and Liv-52 treatment on serum alkaline phosphate level in CCl4 induced liver injury in rats, (d) Comparative effect of combined ethanolic extract, phytosome, and Liv-52 treatment on serum total bilirubin level in CCl4 induced liver injury in rats, (e) Comparative effect of combined ethanolic extract, phytosome, and Liv-52 treatment on serum direct bilirubin level in CCl4 induced liver injury in rats
Histopathological studies
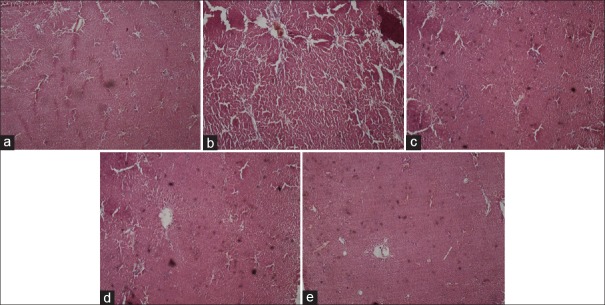
Histopathological images of the liver are shown in Figure 7a-e.
Figure 7.
(a) The liver tissue of rats belonging to control group showing normal histological architecture, with central vein from which chords of hepatocytes are radiating, (b) Carbon tetrachloride induced hepatic injury showing extensive areas of confluent necrosis and also showing fatty changes and hydropic degeneration (c) Pretreatment with 1 ml/kg Liv-52 showing partial protection of hepatocytes (d) Pretreatment with ethanolic biherbal extract 400 mg/kg showing hepatoprotection with some necrotic areas (e) Pretreatment with 100 mg/kg of phytosomal complex showing complete protection of hepatocytes and normalization of liver architecture
CONCLUSION
In this study, the combined ethanolic extract of A. indicum and P. longum in ratio of 3:1 found to exhibit significant hepatoprotective activity in carbon tetrachloride induced hepatotoxicity in rats by reducing carbon tetrachloride induced rise in liver injury markers such as SGPT, SGOT, ALP, and bilirubin. Extract-phospholipid complex or phytosomes were prepared and characterized for various physicochemical parameters. The characterization showed that extract formed complex with phospholipids with better lipid solubility. Hepatoprotective activity of developed phytosomes was found to be more potent than crude extract of both drugs. Phytosomes 1 ml/kg, p.o was more potent than 400 mg/kg p.o dose of combined ethanolic extract in reducing liver injury markers up to control level. Hence, phytosomal formulation of this herbal drug combination can be used for clinical application to enhance the therapeutic effect and patient compliance. Hence, further study is required to investigate in vitro drug release profile and pharmacokinetic activity.
Financial support and sponsorship
University Grant Commission for financial assistance.
Conflicts of interest
Authors declare that there are no conflicts of interest.
ABOUT AUTHORS

Sonam Sharma
Sonam sharma, Research scholar, Department of Pharmaceutics, Indian Institute of Technology, Banaras Hindu University, Varanasi – 221 005. E-mail: sonam.sharma.rs.phe13@itbhu.ac.in, Sonam.sharma.phe11@itbhu.ac.in

Alakh Niranjan Sahu
Alakh Niranjan Sahu, Assistant Professor, Department of Pharmaceutics, Indian Institute of Technology Banaras Hindu University, Varanasi – 221 005. E-mail: ansahu.phe@iitbhu.ac.in, alekh78@gmail.com
Acknowledgment
Financial assistance from the Ministry of Human Resource Development (MHRD) in the form of student support grant is highly acknowledged for the research work with thanks.
REFERENCES
- 1.Rout SD, Panda SK. Ethnomedicinal plant resources of Mayurbhanj district, Orissa. Indian J Tradit Knowledge. 2010;9:68–72. [Google Scholar]
- 2.Krisanapun C, Lee SH, Peungvicha P, Temsiririrkkul R, Baek SJ. Antidiabetic Activities of Abutilon indicum (L.) sweet are mediated by enhancement of adipocyte differentiation and activation of the GLUT1 promoter. Evid Based Complement Alternat Med 2011. 2011 doi: 10.1093/ecam/neq004. 167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajurkar R, Jain R, Matake N, Aswar P, Khadbadi SS. Antiinflammatory action of Abutilon indicum (L.) sweet leaves by HRBC membrane stablisation. Res J Pharm Techn. 2009;2:415–6. [Google Scholar]
- 4.Razia M, Rajalakshmi BS, lavanya K, Karthiga V, Bernala W, Deboral P. GC-MS, FTIR and in-vitro antibacterial activity of Abutilon indicum. Int J Biol Pharm Res. 2012;4:3959–65. [Google Scholar]
- 5.Golwala DK, Patel LD, Vaidya SK, Bothara SB, Mani M, Patel P. Anticonvulsant activity of Abutilon indicum leaves. Int J Pharm Pharm Sci. 2010;2:66–71. [Google Scholar]
- 6.Giri RK, Kanungo SK, Patro VJ, Das S, Sahoo DC. Lipid lowering activity of Abutilon indicum (L.) leaf extract in rats. J Pharm Res. 2009;2:1725–7. [Google Scholar]
- 7.Dashputre NL, Naikwade NS. Evaluation of anti-ulcer activity of methanolic extract of Abutilon indicum leaves in experimental rats. Int J Pharm Sci Drug Res. 2011;3:97–100. [Google Scholar]
- 8.Chandrashekhar VM, Nagappa AN, Channes TS, Habbu PV, Rao KP. Antidiarrhoeal activity of Abutilon indicum Linn. leaf extract. J Nat Remedies. 2000;4:12–6. [Google Scholar]
- 9.Dixit SP, Tiwari PV, Gupta RM. Experimental studies on the immunological aspects of Atibala (Abutilon indicum Linn.), Mahabala (Sida rhombifolia Linn.), Bala (Sida cardifolia Linn.) and Bhumibala (Sida vernonicaefolia Lam.) J Res Indian Med Yoga Homoeopathy. 1978;13:50–60. [Google Scholar]
- 10.Bagi MK, Kalyani GA, Dennis TJ, Kumar AK, Kakrani HK. Preliminary pharmacological screening of Abutilon indicum analgesic activity. Fitoterapia. 1985;6:169–71. [Google Scholar]
- 11.Roshan S, Ali S, Khan A, Tazneem B, Purohit MG. Wound healing activity of Abutilon indicum. Pharmacogn Mag. 2008;4:85–8. [Google Scholar]
- 12.Mukherjee PK, Kumar V, Mal M, Houghton JP. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Porchezhian E, Ansari SH. Hepatoprotective activity of Abution indicum on experimental liver damage in rats. Phytomedicine. 2005;12:62–4. doi: 10.1016/j.phymed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Ramasubramaniaraja R. Pharmacognostical phytochemical including GC-MS identification components of ethanolic leaf extract of Abutilon indicum Linn. IJPI's. J Pharmacogn Herb Formul. 2011;1:38–46. [Google Scholar]
- 15.Rajalakshmi PV, Kalaiselvi SK. Direct HPLC analysis of quercetin in exudates of Abutilon Indicum Linn. Malvaceae. J Pharm Sci Technol. 2009;1:80–3. [Google Scholar]
- 16.Sharma A, Mathur R, Shukla S. Hepatoprotective action of a propriety herbal preparation against carbon tetrachloride intoxication. Indian Drugs. 1994;32:120–8. [Google Scholar]
- 17.Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drugs bioavailability by piperine evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985;232:258–62. [PubMed] [Google Scholar]
- 18.Patil UK, Singh A, Chakraborty AK. Role of piperine as a bioavailability enhancer. Int J Recent Adv Pharm Res. 2011;4:16–23. [Google Scholar]
- 19.Bombardelli E, Spelta M. Phospholipid-polyphenol complex a new concept in skin care ingredients. Cosmet Toiletries. 1991;106:69–76. [Google Scholar]
- 20.Bombardelli E, Mustich G. Bilobalide Phospholipid Complex, their Uses and formulation Containing them. U.S. Patent EPO- 275005. 1991 [Google Scholar]
- 21.Jain PK, Khurana N, Pounikar Y, Gajbhiye A, Kharya MD. Enhancement of absorption and hepatoprotective potential through soya-phosphatidylcholine-andrographolide vesicular system. J Liposome Res. 2013;23:110–8. doi: 10.3109/08982104.2012.753456. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke H, Werner C, Wendel A. Disposition and hepatoprotection by phosphatidyl choline liposomes in mouse liver. Chem Biol Interact. 1987;64:127–37. doi: 10.1016/0009-2797(87)90066-4. [DOI] [PubMed] [Google Scholar]
- 23.Canty DJ, Zeisel SH. Lecithin and choline in human health and disease. Nutr Rev. 1994;52:327–9. doi: 10.1111/j.1753-4887.1994.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 24.Mandal V, Mohan Y, Hemalatha S. Microwave assisted extraction an innovative and promising extraction tool for medicinal plant research. Pharmacogn Rev. 2007;1:7–18. [Google Scholar]
- 25.Marena C, Lampertico M. Preliminary clinical development of silipide a new complex of silybin in toxic liver disorders. Planta Med. 1991;57(Suppl 2):A124–5. [Google Scholar]
- 26.Maiti K, Mukherjee K, Gantait A, Ahmed HN, Saha BP, Mukherjee PK. Enhanced therapeutic benefit of quercetin-phospholipid complex in carbon tetrachloride induced acute liver injury in rats: A comparative study. Iran J Pharmacol Ther. 2005;4:84–90. [Google Scholar]
- 27.Rao PG, Rao SG, Kumar V. Effects of Hepatogard against carbon tetrachloride induced liver damage in rats. Fitoterapia. 1993;64:108–13. [Google Scholar]
- 28.Shenoy AK, Somayaji SN, Bairy KL. Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharmacol. 2001;33:260–6. [Google Scholar]
- 29.Reitman S, Frankel S. In vitro determination of transaminase activity in Serum. Am J Clin Pathol. 1957;28:56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 30.Malloy HT, Evelyn KA. Estimation of serum bilirubin level. J Biol Chem. 1937;119:481. [Google Scholar]
- 31.Kind PR, King EJ. Determination of Serum alkaline phosphatase. J Clin Pathol. 1954;7:132–6. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]