Abstract
Objective:
In Indian Ayurvedic system, Benincasa hispida (BH) and Carissa congesta (CC) are well-known plants used for major and minor ailments. BH has been regarded as Kushmanda, whereas CC has been used in immune-related disorders of the human system. Quercetin and rutin identified from the vast plethora of plant extracts have proved to possess ethnopharmacological relevance.
Materials and Methods:
In present studies, we have determined quercetin and rutin in terms of percentage in BH seeds and CC roots by high-performance thin layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC). After extraction and phytochemical screening, the extracts were subjected to quantification for the presence of quercetin and rutin by HPTLC and HPLC.
Results:
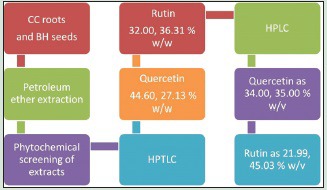
HPTLC showed quercetin as 44.60, 27.13% and rutin as 32.00, 36.31% w/w, whereas HPLC revealed quercetin as 34.00, 35.00% and rutin as 21.99, 45.03% w/v in BH and CC extracts, respectively.
Conclusion:
The BH and CC extracts have elucidated peaks that were corresponding with standard peaks on undertaking chromatographic studies.
SUMMARY
Quercetin and rutin are isolated from BH seeds and CC roots by High Performance. Thin Layer Chromatography and High Performance Liquid Chromatography. HPTLC revealed presence of quercetin as 44.60, 27.13 % and rutin as 32.00, 36.31 % w/w. HPLC revealed presence of quercetin as 34.00, 35.00 % and rutin as 21.99, 45.03 % w/v.
Abbreviation Used: HPTLC: High Performance Thin Layer Chromatography; HPLC: High Pressure Liquid Chromatography, UV: Ultraviolet, CC: Carissa congesta, BH: Benincasa hispida
Keywords: Benincasa hispida, Carissa congesta, high-performance liquid chromatography, high-performance thin layer chromatography, quercetin, rutin
INTRODUCTION
Much of the research currently is dedicated to the phytochemical investigation of higher plants, which have ethnopharmacological importance.[1] Phytochemical techniques are immensely important for the evaluation of phytoconstituents in identification of phytotoxins and phytolaxeins.[2] Plant systematics is one of the emerging fields in photochemistry. It is related to the chemical structural interpretation of different plant groups with a major focus on secondary constituents. Due to this recent upcoming stream, it has become easy for laboratory researchers to identify flavonoids classes of compounds.[3]
Flavonoids are water-soluble compounds present in vascular plants and found as bounded sugar glycosides with attachment of any one flavonoid as aglycone occurring as glycosidic combinations. Determination of flavonoids in the plant tissue is based on solubility profiles and color reactions followed by a one-dimensional examination of a hydrolyzed plant extract and two-dimensional chromatography of a direct alcoholic extract. However, they can be separated by different chromatographic techniques and spectral analysis using known biomarkers.[4,5,6,7]
The flavonols are well identified as copigments with anthocyanins in plant extracts of petals and leaves such as quercetin and rutin. Quercetin inhibits enzymes of the cytochrome P450 CYP1A, as well as CYP3A4, and is found to be beneficial in metabolizing a significant number of carcinogens and medications. Rutin is found effective in the treatment of inflammation during the chronic phase.[8,9,10]
Benincasa hispida (BH) and Carissa congesta (CC) are ethnobotanical and pharmacological important local plants in India and used for a number of natural remedies. We have reported the significance of these plants with lupeol presence in the extracts in previous studies.[11,12]
However, numbers of analytical methods are available and reported in literature for isolation and characterization of plant constituents by high-performance thin layer chromatography (HPTLC) and high-liquid chromatography (HPLC), there is no systematic approach designed toward chromatographic quantification studies for selected plant extracts. Therefore, in current research studies, we have attempted to identify phytoconstituents such as quercetin and rutin from BH and CC petroleum plant extracts.
MATERIALS AND METHODS
Collection, authentication and extraction
Our previous studies reported the presence of lupeol and β-sitosterol from these extracts.[11,12,13]
Reagents and biomarkers
All solvents and standard biomarkers used for identification purpose in chromatographic studies were procured from Merck Pvt. Ltd and Yucca Enterprises, Mumbai, India.
High-performance thin layer chromatography instrumentation
- Instrument: CAMAG Linomat 5
- Spray gas: Inert gas
- Sample solvent type: Methanol
- Dosage speed: 150 nL/s
- Predosage volume: 0.2 μL.
-
Sequence
- Syringe size: 100 μL
- Number of tracks: 10
- Application position Y: 8.0 mm
- Band length: 6.0 mm
- Solvent front position Y: 80.0 mm
- Distance between the tracks: 13.0 mm.
-
Calibration parameters
- Calibration mode: Multiple level
- Statistics mode: Coefficient of variation
- Evaluation mode: Area and peak height.
High-liquid chromatography instrumentation
Instrument Type: Shimadzu LC-10 ATVP
Software: Chromtech N 2000
Flow rate: 1.5 mL/min
Detector: Ultraviolet (Type 335) and wavelength: 360 nm
Run time: 30 min
Injection volume: 20 μL
Column dimensions: RP C-18, 250 mm × 4.6 mm, 5 μ.
EXPERIMENTATION
Thin layer chromatography
Quercetin
Mobile phase: Toluene: Ethyl acetate: Formic acid (5:4:0.2)
Dilution: The standard and the sample were dissolved in ethanol and was filtered using Whatman Filter paper no. 41 before spotting on thin layer chromatography (TLC) plate.
Saturation: Chamber was saturated for 30 min.
Rutin
Mobile phase: Methanol: Glacial acetic acid: Formic acid: Water (3: 0.9: 0.9: 0.5)
Dilution: The standard and the sample were dissolved in ethanol and was filtered using Whatman Filter paper no. 41 before spotting on TLC plate.
Saturation: Chamber was saturated for 30 min.
High-performance thin layer chromatography
The HPTLC studies were performed at Anchrom Test Labs Private Limited, Mumbai, India. The HPTLC plates (20 cm × 10 cm) were coated with silica gel 60 F254 and scanning of the developed plates was performed at 254 nm (before derivatization) and 540 nm (after derivatization). The standard and the sample were dissolved in the ratio of (1:2) of methanol each.
Quercetin: (Tracks 10)
Mobile Phase: Toluene: Ethyl Acetate: Formic Acid (5: 4:0.2)
Spots of 4 μg/L, 5 μg/L, 6 μg/L, 7 μg/L, 8 μg/L and 9 μg/L (std), BH -9 μg/L (2times) and CC- 6 μg/L (2times) were applied on plates.
Rutin: (Tracks 12)
Mobile Phase: Methanol: Glacial Acetic Acid: Formic Acid: Water (3: 0.9: 0.9: 0.5)
Spots of 1 μg/L, 2 μg/L, 3 μg/L, 4 μg/L, 5 μg/L, 6 μg/L, 7 μg/L, 8 μg/L (std), BH -7μg/L (2times) and CC- 5 μg/L (2times) were applied on plates.
Formula: Percentage of quercetin/rutin = sample area × standard dilution × purity × 100/standard area × sample dilution × 100 (purity of STD is 90%).
High-pressure liquid chromatography analysis
The HPLC studies were performed at Vivekanand Education Society's College of Pharmacy, Mumbai, India. The mobile phase used was acetonitrile and water (95:5) and Methanol: Water (0.1 % orthophosphoric acid) pH: 3.5 (60:40) (Only for CC extract in determination of quercetin). Two and five milligrams of standard and sample were weighed and dissolved in 2 mL and 5 mL of solvent respectively, from which 100 μL was taken and made up to 1 mL with solvent, from this stock solution 20 μL was injected.
Formula: Percentage of quercetin/rutin = sample area × standard dilution × purity × 100/standard area × sample dilution × 100 (purity of STD is 90%).
RESULTS
Extraction and preliminary phytochemical analysis
The results of extraction yield and preliminary analysis of the BH and CC extracts have been previously reported by us.[11,12]
Thin layer chromatography
Quercetin
In TLC, chromatographic analysis of extracts revealed the presence of quercetin.
Rf for standard quercetin = 3.1/5.6 = 0.55
Rf for BH petroleum ether extract (BH) =3.3/5.6 = 0.58
Rf for CC petroleum ether extract (CC) =3.1/5.4 = 0.57.
Rutin
In TLC, chromatographic analysis of extracts revealed the presence of rutin.
Rf for standard rutin = 4.4/5.1 = 0.86
Rf for BH petroleum ether extract (BH) = 4.2/5.1 = 0.82
Rf for CC petroleum ether extract = 3.9/4.8 = 0.81.
High-performance thin layer chromatography
HPTLC reports of BH and CC showed well-resolved spots:
Quercetin
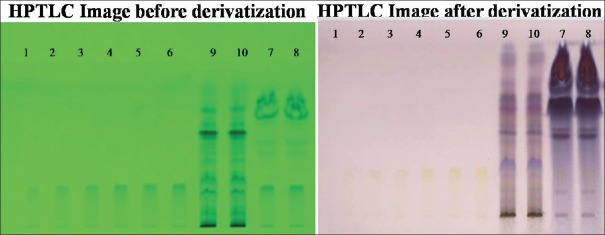
At tracks 9, 10 (BH), and 7, 8 (CC) in comparison to standard tracks at 1–6, Rf value was found to be identical to standard at 0.25 [Figure 1]. According to the formula, the amount of quercetin present was found to be 44.60% w/w (BH) and 27.13% w/w (CC).
Figure 1.
High-performance thin layer chromatography chromatograms of Benincasa hispida seeds, Carissa congesta roots and standard for quercetin
Rutin
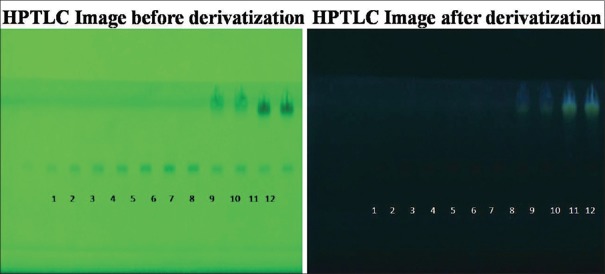
At tracks 9, 10 (BH), and 11, 12(CC) in comparison to standard at tracks 1–8, Rf value was found to be identical to standard at 0.47 [Figure 2]. According to the formula, the amount of rutin present was found to be 32.00% w/w (BH) and 36.31% w/w (CC).
Figure 2.
High-performance thin layer chromatography chromatograms of Benincasa hispida seeds, Carissa congesta roots and standard for rutin
High-performance liquid chromatography
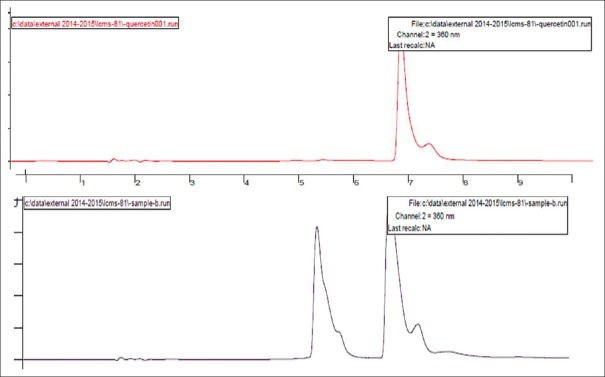
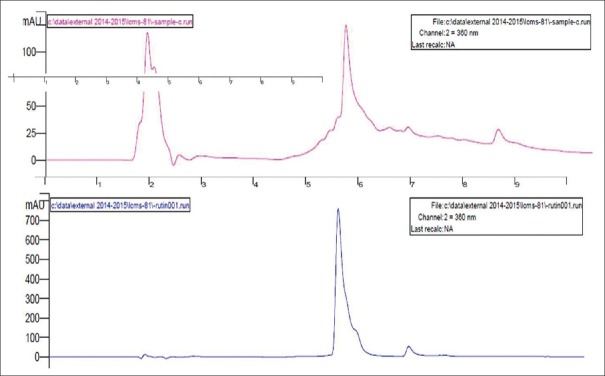
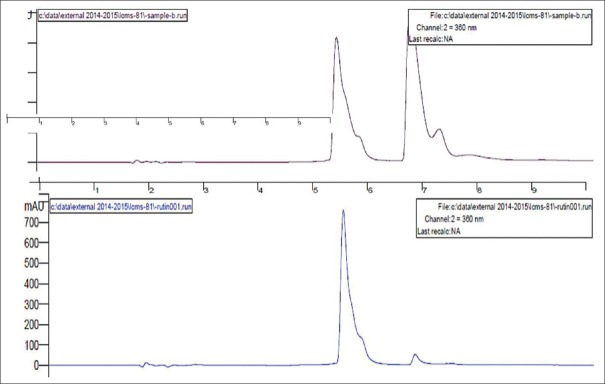
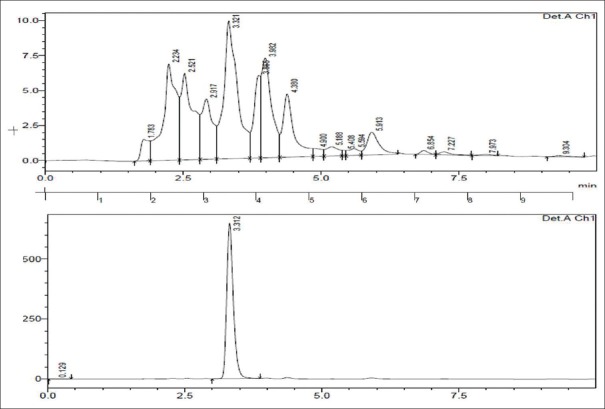
HPLC reports of BH (quercetin- retention time is 6.7 min, rutin- retention time is 5.380 min) at 360 nm and CC extracts (quercetin- retention time is 3.321 min, rutin- retention time is 5.488 min) have shown well-resolved peaks when compared with standard as depicted in Tables 1, 2 and Figures 3-6. According to the formula mentioned in methodology, the amount of quercetin was found to be 34.00 and 35.00%, whereas rutin as 21.99 and 45.03% w/v in BH and CC extracts respectively.
Table 1.
HPLC analysis of BH seeds, CC roots and standard quercetin
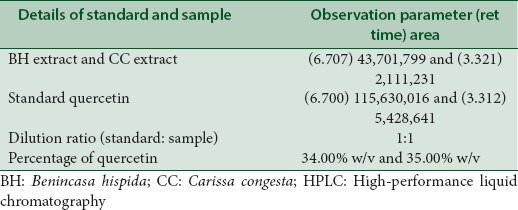
Table 2.
HPLC analysis of BH seeds, CC roots and standard rutin
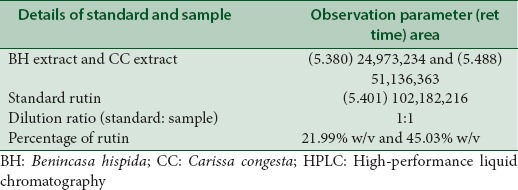
Figure 3.
High-performance liquid chromatography peaks of Benincasa hispida seeds and standard quercetin
Figure 6.
High-performance liquid chromatography peaks of Carissa congesta roots and standard rutin
Figure 4.
High-performance liquid chromatography peaks of Benincasa hispida seeds and standard rutin
Figure 5.
High-performance liquid chromatography peaks of Carissa congesta roots and standard quercetin
DISCUSSION
Isolation and characterization of the plant constituents are difficult due to the presence of ample quantities of components in the extracts. Although, 200–300 flavonols aglycone are known but there is wide existence and reporting of only three commonly known flavonols such as kaempferol, quercetin, and myricetin in significant amount in plant extracts.[14] In addition, more than 200 quercetin glycosides have been identified and among them one well-known analog is rutin chemically known as quercetin-3-rutinoside.[15] Quercetin and rutin are well cited many scientists in phytochemical studies and correlated to a number of pharmacological activities such as anti-inflammatory, anticancer, antiarthritic, etc., The current research studies paves the pathway for research thrust scientists to consider the other probable identified constituents in these extracts and correlate their utilities in terms of on pharmacological effects with in vivo and in vitro consideration.
CONCLUSCIONS
Our research studies directs light in the field of the pharmacognosy and its budding professionals toward these identified constituents in BH and CC extracts which were confirmed by simple methods such as TLC and HPTLC.
Financial support and sponsorship
The study was funded by Intra-mural fund of Haffkine Institute for Training, Research and Testing, Mumbai, India.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Gaurav Doshi
Mr. Gaurav Doshi, Presently working as Assistant Professor, Department of Pharmacology, Vivekanand Education Society's College of Pharmacy, Chembur (East), Mumbai- 400074. He has 31 journal papers; 72 citations to his credit. He is Ph.D Research Scholar of Department of Pharmaceutical Sciences, Pacific Academy of Higher Education and Research University, Udaipur, Rajasthan, India.

Hemant Devidas Une
Hemant Devidas Une, Working as Associate Professor, Vice- Principal, and Head of Department of Pharmacology, Y. B. Chavan College of Pharmacy, Rouzabagh, Aurangabad, Maharashtra, India. He has 22 journal paper; 98 citations to his credit. He has many research grants to his credit from government agencies.
Acknowledgments
We would like to acknowledge the college management who provided us all the facilities to do this work as well as Radiant Research Services Pvt. Ltd., for their help in analysis.
REFERENCES
- 1.Hostettmann K. Vol. 6. London: Academic Press; 1991. Methods in Plant Biochemistry. Assays for Bioactivity. [Google Scholar]
- 2.Grayer R, Harborne JB. A survey of antifungal compounds from higher plants. Phytochemistry. 1994;37:19. doi: 10.1016/s0031-9422(00)00450-7. [DOI] [PubMed] [Google Scholar]
- 3.Solitis PS, Solitis DE, Doyle JJ. New York: Chapman and Hall; 1992. Molecular Systematics of Plants. [Google Scholar]
- 4.Harborne JB. London: Academic Press; 1967a. Comparative Biochemistry of the Flavonoids. [Google Scholar]
- 5.Mabry TJ, Markham KR, Thomas MB. Berlin: Spring-Verlag; 1970. The Systematic Identification of Flavo. noids. [Google Scholar]
- 6.Markham KR. London: Academic Press; 1982. Techniques of Flavonoid Identification. [Google Scholar]
- 7.Harborne JB, Mabry TJ, Mabry H. London: Chapman and Hall; 1975. The Flavonoids. [Google Scholar]
- 8.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991;2:325–57. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Kohno H, Tsukio Y, Honjo S, Tanino M, Miyake M, et al. Citrus limonoids obacunone and limonin inhibit azoxymethane-induced colon carcinogenesis in rats. Biofactors. 2000;13:213–8. doi: 10.1002/biof.5520130133. [DOI] [PubMed] [Google Scholar]
- 10.Deepak M, Handa SS. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother Res. 2000;14:463–5. doi: 10.1002/1099-1573(200009)14:6<463::aid-ptr611>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Doshi GM, Une HD. Chromatographic studies on Benincasa hispida (thunb.) cogn seed extract scrutinized by HPLC and HPTLC. Pharmacogn J. 2014;6:42–8. [Google Scholar]
- 12.Doshi GM, Chaskar PR, Zine SP, Une HD. Cold extraction of Carissa congesta wight monitored by a comparative revision of HPLC and HPTLC. Pharmacogn Commun. 2014;4:29–33. [Google Scholar]
- 13.Doshi GM, Chaskar PR, Une HD. Elucidation of β-sitosterol from Benincasa hispida seeds, Carissa congesta roots and Polyalthia longifolia leaves by high performance thin layer chromatography. Pharmacogn J. 2015;7:221–7. [Google Scholar]
- 14.Harborne JB. London: Chapman and Hall; 1988. The Flavanoids: Advances in Research Since 1980. [Google Scholar]
- 15.Harborne JB. London: Chapman and Hall; 1994. The Flavanoids: Advances in Research Since 1986. [Google Scholar]