Abstract
Introduction:
With an ever increasing cause of cancer, it has been recommended to treat with conventional drugs, however because of the side effects caused by the conventional drugs, the research on medicinal plants has been intensified due to their less adverse and toxic effects.
Objectives:
The primary objective of the present study was to evaluate the protective effect of the medicinal plant Curcuma caesia Roxb. against free radicals ABTS+ and O2-. Also it was aimed to evaluate the protective effect of C.caesia Roxb. against the chemotherapeutic drug Cyclophosphamide and its side effects in liver and kidney.
Methods:
The rhizomes of the plant was extracted with methanol through soxhlet and its antioxidant activity was tested against ABTS+ and O2-. For antigenotoxic studies, animals were divided into eight groups and micronucleus assay was employed and for biochemical analysis serum sample was collected from the blood and SGOT, SGPT analysis was performed. Also the biochemical analysis was performed from both the liver and kidney.
Results:
The methanolic extract of Curcuma caesia Roxb. was found to scavenge the free radicals ABTS+ and O2-. the micronuclei formation was found to be increased in the positive control group as compared to the negative control group significantly (P<0.002) however increase in the number of micronuclei was found to be decrease with the pretreatment of the extract at different concentrations significantly as compared to the negative control groups (P<0.01, P<0.005, P<0.001). The increased level of serum SGPT and SGOT as well as peroxidation level in both liver and kidney due to treatment of cyclophosphamide was also found to be decreased with the pretreatment of the extract significantly as compared to the positive control groups. There was decreased in the level of endogenous antioxidant such as GSH and GR in the positive control group however decreased level of GSH and GR was found to be increased with the pretreatment of the methanolic extract of C. caesia Roxb.
Conclusion:
The present study suggested that the methanolic extract of C. caesia Roxb has not shown any genotoxicity and reduces the genotoxicity caused by cyclophosphamide. It was also to have the protective effects against the liver and kidney. So it could be provided as one of the herbal supplementation in chemoprevention of CP to ameliorate the side effects of it.
SUMMARY
Cancer is characterized by uncontrolled growth of cells and much research has been done for the past several years from various disciplines for the treatment of cancer but till now no therapy has been discovered. Treatment of cancer with chemotherapeutic drugs has been suggested to prevent cancer cells however they are often limited with their toxicity to normal cells. Therefore it has been suggested that the supplementation of medicinal plants which are rich source of antioxidants can decrease the toxic effect caused by chemotherapeutic drugs. Curcuma caesia Roxb is a medicinal plant which has high antioxidant activity, as per present study, methanolic extract of Curcuma caesia Roxb prevents the toxicity caused by cyclophosphosphamide (chemotherapeutic drug) in bone marrow cells by reducing the micronuclei formation; it also prevents the hepatotoxicity and nephrotoxicity caused by cyclophosphamide, so it can be used as a supplement in cancer treatment with cyclophosphamide.
Keywords: 2, 2’ azino bis (ethylbenzthiazolene-6-sulfonic acid) radical cation assay, Superoxide dismutase assay, Cyclophosphamide, micronucleus assay, Curcuma caesia Roxb., Oxidative stress
INTRODUCTION
With an ever increasing cause of cancer due to diet, environment, and carcinogenic virus[1,2] conventional drugs have been recommended for cancer patients. Such treatments lengthened the life or may permanently cure, but most treatments, experiences side effects such as miserable pain, blood clots fatigue, and infection.[3] However, due to less toxic and adverse effects, the research on medicinal plants or herbs has been intensified,[4] since they have profound active ingredients yielding important breakthrough in cancer prevention and treatment and they have been using as a first line of cancer-fighting agent in developing countries.[5]
Cyclophosphamide (CP) is a nitrogen mustard alkylating agent[6] used in various types of cancer chemotherapy, but the International Agency for Research Centre has identified it as a carcinogen for both animals and humans.[7] CP therapy causes injuries to normal tissue,[8] and peroxidative damage to kidney and other vital organs in which such side effects are supported by the reactive oxygen species (ROS)[9] such as acrolein (ROS) and phosphoramide mustard (ROS)[10] produced by the metabolic activation of CP in the liver by cytochrome P450 mix functional oxidase system.[11,12] Curcuma caesia Roxb. (black turmeric) is a perennial herb with bluish black rhizomes and one of the endangered species amongst the medicinal plants found in Manipur.[13] Many of the researches worked on it like anti-fungal activity,[14] smooth muscle relaxant and anti-asthmatic activity,[15] bronchodilating activity,[16] antioxidant activity,[17] anxiolytic and central nervous system depressant activity, locomotor depressant, anti-convulsant,[18] anthelmintic activity,[19] anti-bacterial activity,[20] and anti-ulcer activity.[21] Methanolic extract of the rhizome of Curcuma caesia Roxb. (MECC) has high phenol content,[22] it is also a good source of antioxidant and antimutagenic activity.[23] The rhizomes of the plant are also a rich source of many phytoconstituents such as essential oils with camphor, ar-turmerone, (Z) ocemene, ar-curcumene, 1,8-cineole, elemene, borneol, bornyl acetate, and curcumene etc.[24] Till now, no data are available on the antigenotoxic activity of the rhizome of MECC against CP, so the present study was undertaken to investigate the prevention of toxicity by the rhizome of MECC caused by CP in bone marrow cells and oxidative stress produced in the liver and kidney.
MATERIALS AND METHODS
Plant material collection and extraction
Rhizomes of C. caesia Roxb. were collected from the region of Nambol, Bishnupur District, Manipur, India and were cut into pieces and sun-dried. The dried rhizomes were coarsely powdered and extracted with methanol through soxhlet at a temperature of 50–60°C for a period of 12–24 h. The crude extract was dried in a water bath and kept for further uses.
Preliminary phytochemical screening
Qualitative preliminary phytochemical screening was performed following.[25]
Drugs and chemicals
All the drugs and chemicals used in this experiment were procured from Segma sales, Silchar, Assam, India, which were kindly provided by Himedia, India.
2,2’ azino bis (ethylbenzthiazolene-6-sulfonic acid) radical cation decolourisation assay
The assay was performed following[26] with slight modifications. ABTS radical cation was generated by the addition of 7 mM ABTS and 2.45 mM potassium persulphate. The reaction mixtures were allowed to stand for 12–16 h at 30°C in the dark. After 16 h, the reaction mixture was diluted with ethanol or phosphate buffer saline (pH = 7.4). Following that 0.3 mL of ABTS+ and 0.5 mL of extract solution (5–100 μg/mL) were mixed and absorbance was read at 734 nm without any incubation period against the sample blank prepared by mixing 0.3 mL of methanol and 0.5 mL of Dimethyl sulfoxide (DMSO). Similarly, control was also read in the same wavelength by adding together of 0.3 mL of 2,2’ azino bis (ethylbenzthiazolene-6-sulfonic acid) radical cation, 0.5 mL of DMSO and 1 mL of methanol. With the same procedure measurement of the gallic acid standard was also recorded. Percentage of inhibition was calculated using the formula given below:
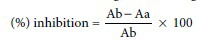
Where Ab is the absorption of the control and Aa is the absorption of the extract sample.
Superoxide dismutase assay
It was performed following[27] with slight modifications. The reaction mixture contain 1 mL of different concentrations of extract (20–200 μg), 1 mL of 156 μM nicotinamide adenine dinucleotide hydrogen (NADH), 1 mL of 60 μM nitroblue tetrazolium, and 1 mL of 468 tetrazolium(NBT), and 1 mL of 468 μM phenazine methosulphate(PMS) in phosphate buffer (pH = 8.3). The reaction mixture was incubated at 25°C for 10 min and absorbance was taken against blank at 560 nm. The standard taken was gallic acid. The inhibition mixture was calculated using the formula:
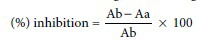
Where, Ab is the absorption of the control and Aa is the absorption of the extract sample.
Experimental design
The animals were kindly procured from the Pasteur Institute, Shillong, India and were acclimatized for 15 days. The study was conducted on 25–30 g body weight male Swiss albino mice. They were maintained under controlled conditions of temperature and light (12 h light: 12 h dark). They were provided standard mice feed. The study protocol was approved by the Institutional Ethical Committee (IEC/AUS/2-013-33, dt. 20/3/13 Assam University, Silchar, India).
The experimental animals were divided into eight groups each containing five mice designated as follows:
Group 1: Negative control (NC): Each animal received distilled water
Group 2: Positive control (PC): CP was administered intraperitoneally at a dose of 50 mg/kg. b.wt
Group 3: Animals received 100 mg/kg. b.wt of MECC only intraperitoneally
Group 4: Animals received 250 mg/kg. b.wt of MECC only intraperitoneally
Group 5: Animals received 500 mg/kg. b.wt of MECC only intraperitoneally
Group 6: Pretreatment: MECC was administered at a dose of 100 mg/kg. b.wt (i.p) followed by CP (i.p) treatment 2 h later
Group 7: Pretreatment: MECC was administered at a dose of 250 mg/kg b. wt (i.p) followed by CP (i.p) treatment 2 h later
Group 8: Pretreatment: MECC was administered at a dose of 500 mg/kg b. wt (i.p) followed by CP (i.p) treatment 2 h later.
After 7 days of the experimental period, the animals were sacrificed, and parameters described below were studied.
Serum sample collection
The blood sample was collected from the heart and kept it undisturbed for 2 h. Serum was then removed by centrifugation at 10,000 g for 10 min and isolated serum sample was kept in −80°C for further analysis of serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT). Besides, the removed kidney and liver were washed with phosphate buffer saline and blotted with filter paper and kept in the deep freezer for further analysis.
Micronucleus assay
Mice bone marrow micronucleus test was carried out according to.[28] Bone marrow cells from both the femurs of each animal were flushed out with fetal bovine serum albumin (FBS) in a centrifuge tube. The cell suspensions were centrifuged at 10,000 rpm for 10 min and supernatant was removed. The pellet was resuspended in FBS before being used for preparing slides. The air-dried slides were stained with May Grunwald stain and Geimsa stain. Thousand polychromatic erythrocytes (PCEs) were scored for each group of animals to determine the frequency of micronucleated polychromatic erythrocytes. All the slides were coded and scored by the same observer. The percentage reduction in the frequency of micronuclei was calculated using the formula given by[29]:
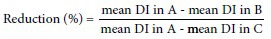
A = Group treated with CP
B = Group treated with CP plus methanol extract of the rhizome
C = Negative groups
DI = Damage index
Biochemical assays
Determination of serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase
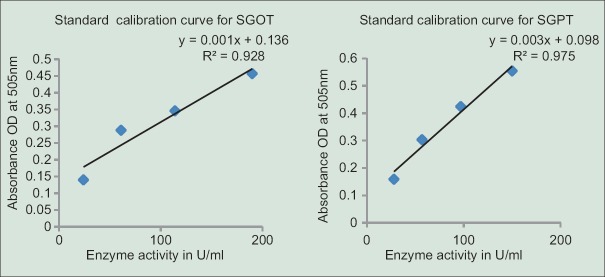
It was performed according to the protocol provided by[30] in a commercial kit (SGOT [ASAT] and SGPT [ALT] kits). Each enzyme activity (U/mL) was calculated from the standard curves generated [Figure 1].
Figure 1.
Standard calibration curve for serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase
Quantitative assay for lipid peroxidation
The assay was performed following[31] with slight modifications. 0.2 g of the sample was homogenized in 2 mL of 0.2 M KCl followed by centrifugation at 10,000 rpm for 10 min in cooling centrifuge (Heraeus Biofuge Startos centrifuge). 0.5 mL of the homogenate was mixed with 100 μL of 10 mM FeCl3 and incubated at 37°C for 30 min. After incubation 400 μL of TCA, 50 μL of BHT, 0.5 mL of TBA, and 50 μL of 0.25N HCl were added and heated at 100°C for 60 min. The reaction mixtures were cooled and then centrifuged it. Absorbance was recorded against blank. The percentage inhibition was calculated using the formula given below:

Estimation of glutathione reduced level
Glutathione reduced (GSH) was estimated following[32] with slight modifications. 0.1 g of each sample was homogenized in 2.5 mL of 10% TCA followed by the centrifugation at 10,000 rpm for 10 min. 0.1 mL of the supernatant was mixed with 0.9 mL of 0.2 M phosphate buffer and 0.2 mL of 0.6 mM (5,5′-dithiobis-[2-nitrobenzoic acid]) (DTNB) and the absorbance was read at 412 nm against blank. The level of GSH was expressed as nmole of GSH/g tissue.
Estimation of cytosolic glutathione reductase
Glutathione reductase (GR) was assayed in the liver and kidney following.[33] 0.1 g of tissue was homogenized in 1 mL of phosphate buffer followed by the centrifugation at 10,000 rpm. To 0.1 mL of the enzyme source (supernatant), 1 mL of (0.12 M, pH = 7.2) phosphate buffer, 0.1 mL of 15 mM ethylenediaminetetraacetic acid, 0.1 mL of 10 mM sodium azide, 0.1 mL of GSSG, 0.6 mL of dH2O were added, and the volume was made up to 1 mL with buffer. The reaction mixture was incubated for 3 min, followed by the addition of 0.3 mL of NADPH (9.6 Mm). The absorbance was read at 340 nm in spectrophotometer for every 15 s at an interval of 2–3 min. The enzyme activity will be expressed as μmoles of NADPH oxidized/minute/g tissue.
Protein estimation
Protein concentration was estimated following.[34] 0.025 g of each tissue was homogenized in 1 mL of phosphate buffer saline. 0.5 mL of each homogenate was diluted with 6 mL of phosphate-buffered saline and from this 0.5 mL of the diluted sample was used for the analysis. The reaction mixtures contain 0.5 mL of the homogenate, 0.7 mL of Lowry's solution. It was mixed through the vortex and incubated for 20 min. 0.1 mL Folin-Ciocalteau reagent was added and mixed in the vortex. After 30 min of incubation absorbance was read at 750 nm against the reagent blank. The amount of protein was estimated from the standard calibration curve obtained using bovine serum albumin [Figure 2].
Figure 2.
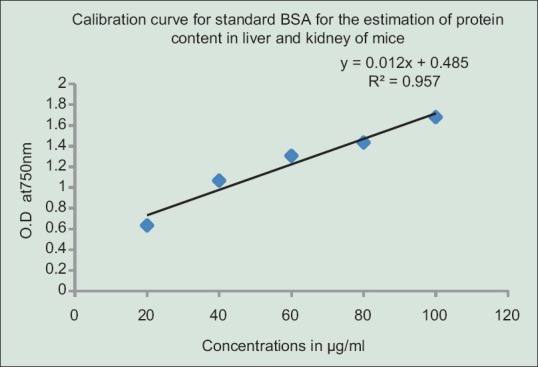
Standard calibration curve for bovine serum albumin to find out protein content
Statistical analysis
The results presented are expressed as mean ± standard deviation. The difference between treatment and control was analyzed by one-way ANOVA.
RESULTS
Preliminary phytochemical screening reveals the presence of alkaloids, carbohydrates, reducing sugars, flavonoids, terpenes, steroids, and tannins in the methanolic extract of C. caesia Roxb. as indicated in Table 1.
Table 1.
Preliminary phytochemical screening of methanolic extract of Curcuma caesia Roxb.

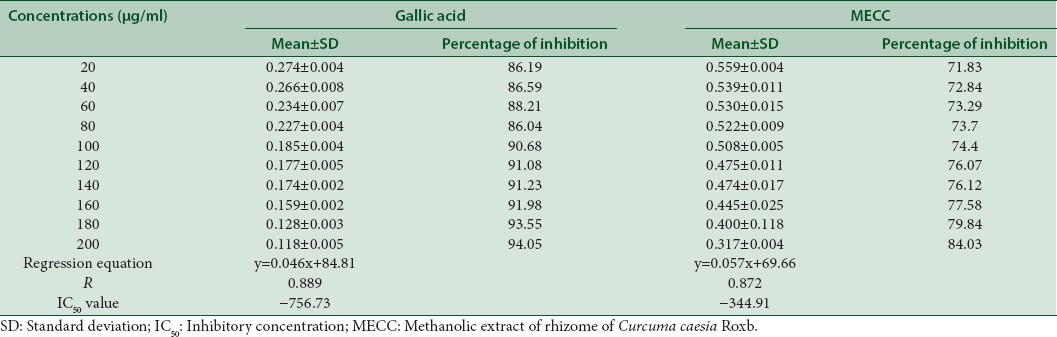
The inhibitory effect of MECC against ABTS+ and superoxide dismutase (SOD) was compared with standard compound gallic acid [Tables 2 and 3]. The highest inhibitory effect of MECC against ABTS+ was found to be 64.78% at its highest concentration (100 μg/mL) which lies in between 63.5% and 72.7% at the concentrations of 40 μg/mL and 60 μg/mL of gallic acid. The IC50 value of MECC is 59.99 μg/mL as compared to the IC50 value of gallic acid with −4.37 μg/ml. On the other hand, the highest inhibition (84.03%) shown at 200 μg/mL of MECC against SOD was comparable to the value of gallic acid standard at 20 μg/mL (86.19%) [Tables 2 and 3].
Table 2.
ABTS + radical scavenging activity of a standard and methanolic extract of Curcuma caesia Roxb.
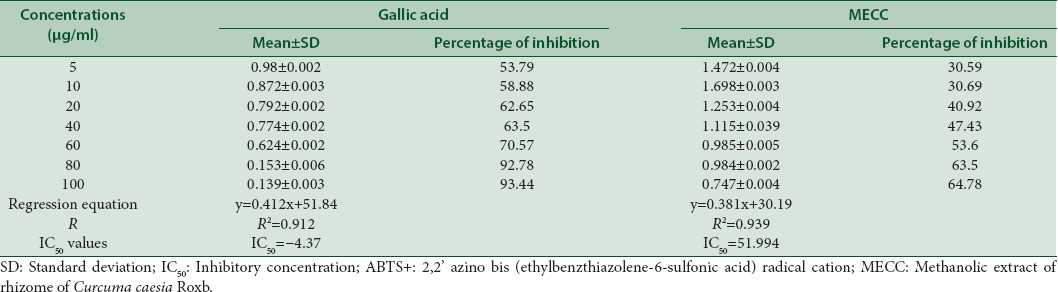
Table 3.
Superoxide anion scavenging ability of standard and methanolic extract of Curcuma caesia Roxb.
An increase in the number of micronuclei was observed when treated with CP only as shown in Table 4. But, the pretreatment of different concentrations of MECC followed by CP reduces the micronuclei formation significantly (P < 0.005, P < 0.01, P < 0.001) (R2 = 0.980). The reduction percentage of micronuclei was 41.77%, 48.43%, and 68.75% at different concentrations (100, 250, 500 mg/kg. b.wt respectively). There was no sign of toxicity in the treatment with extracts only since the values were almost near to normal groups, and they are not significantly different from the NC groups [Table 4].
Table 4.
The effect of treatment with MECC on the micronuclei induced by CP in bone marrow cells of mice
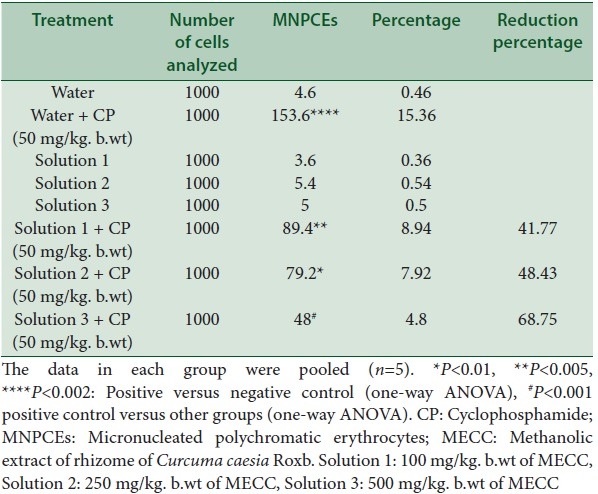
The PC group was compared with NC groups, and all other treatment groups were compared to the PC group.
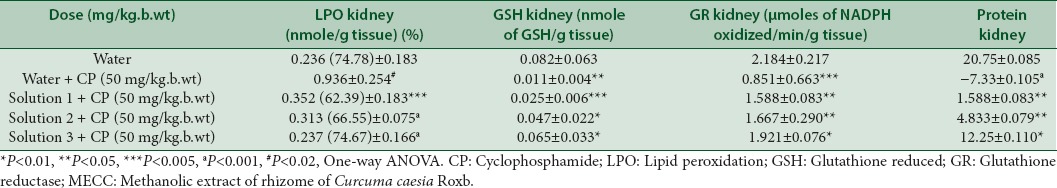
Levels of SGOT and SGPT in positive control groups were found to be significant (P < 0.01, P < 0.0001) as compared to the normal groups [Table 5]. But their amount was found to be reduced with the administration of the extract at different concentrations. Peroxidation to the lipid membranes of both liver and kidney were increased in the CP treated mice, but the increased concentration was found to be reduced by 54%, 65%, 68% in the liver and 62.39%, 66.55%, 74.67% in kidney respectively at the tested concentrations of the extract, significantly as shown in Tables 5 and 6. In vivo antioxidant enzymes such as GSH, GR, and protein were also found to be decreased in the CP treated mice, however the pretreatment with extract increased their concentration near to normal, which is an indication of the protective effect of the extract against oxidative stress produced by the reactive metabolites of CP [Tables 5 and 6].
Table 5.
Effect of MECC on biochemical parameters in CP induced hepatic toxicity
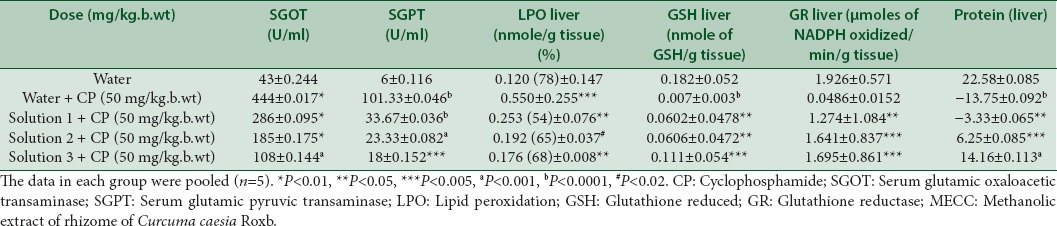
Table 6.
Effect of MECC on biochemical parameters in CP induced kidney toxicity
DISCUSSION
Medicinal plants and their derivatives have been used as an alternative to synthetic medicines in many countries. Medicinal plants play an important role in two-sided approach: One, plant-derived compounds are complex in nature which are difficult to synthesize in the laboratory and are helpful in the prevention of onset of cancer by its antioxidant activity and stimulation of the immune system,[35] second, plant-derived compounds are used for prevention and decreasing side effects of conventional cancer treatments.[36] The present study, on MECC reveals the presence of alkaloids, carbohydrates, reducing sugars, flavonoids, terpenes, steroids, and tannins in it [Table 1]. The process of respiration, cell-mediated immune functions, and other process utilizing oxygen produces free radicals as an end product, continuously in the living body.[37] Our body has enough antioxidant to defense against such free radicals, but exogenous as well as extra free radicals inside the body imbalances defense system, leading to oxidative stress.[38] Such oxidative stress is the leading cause of DNA damage and micronuclei formation in bone marrow cells.[39] In the present study, MECC was found to scavenge ABTS+ and Superoxide anion free radicals [Tables 2 and 3] and such scavenging activity is regarded as one of the most important techniques in preventing damage to DNA.[40] CP induced micronuclei in PCEs in the bone marrow cells of mice is an indication of chromosomal damage[41,42,43] similar to the present study as shown in Table 4. There was a significant increase in micronuclei (P < 0.002) in the PC group as compared to normal groups. But the formation of micronuclei was reduced significantly (P < 0.005, P < 0.01, P < 0.001) with the pretreatment of MECC at different concentrations (100, 250, 500 mg/kg. b.wt) with the percent reduction of 41.77%, 48.43% and 68.75% respectively. CP under metabolic activation by cyt p450 produces metabolic products such as, hydroxycyclophosphamide which is used for chemoprevention[44] and acrolein (ROS) that cross-links DNA, also decrease the antioxidant activity.[45] A previous study on a methanolic extract of C. caesia Roxb. found to scavenge against 2,2-diphenyl-1-picrylhydrazyl.[23,46] In addition to this, the present study also found MECC to scavenge against ABTS + and SO2-, based on these it was hypothesized that MECC being an anti-oxidative prevents the interaction of DNA and metabolic product acrolein produced by CP in the nucleus resulting in an increase reduction of micronuclei formation. Peroxidation of lipids produces an end product, malondialdehyde (MDA) which disrupts the cell membrane, thereby increasing permeability to ions.[47,48] Hepatic damage results in the leakage of SGOT and SGPT into the serum resulting in their increased concentrations.[49] SGOT and SGPT are the liver marker enzymes and elevated level of them is an indicative of loss of functional integrity of cell membranes in the liver.[50] In the present study, the MDA level of the PC group was increased in the liver significantly (P < 0.005) as compared to normal groups [Table 5]. The level of SGOT and SGPT were found to increase in the serum of the PC group significantly (P < 0.01; P < 0.0001) as compared to normal groups [Table 5] may be due to increase of MDA level. It was found that the level of peroxidation in kidney was also found to increase significantly (P < 0. 02) in PC groups as compared to normal groups. However, pretreatment with MECC at three different concentrations (100, 250, 500 mg/kg. b.wt) reduces the formation of lipid peroxidation in both kidney and liver significantly as compared to positive groups as shown in the above Tables 5 and 6. Also, the level of SGOT and SGPT were also found to reduce significantly with different concentrations of MECC tested as shown in Table 5.
Determination of GSH is regarded as one of the most important factor to show the amount of antioxidant reserve in the organism.[51,52,53] Reactive metabolites of CP (acrolein) conjugate with GSH resulting in the formation of glutathionylpropionaldehyde which induces oxidative stress and depletion of GSH.[54] The depletion of GR and protein content is also related to the production of reactive metabolites of CP.[55] In the present study, the content of GSH, GR and protein were found to decrease in the PC group significantly [Tables 5 and 6] in both liver and kidney as compared to normal groups. Moreover pretreatment with MECC increased the content of GSH, GR, and protein in both liver and kidney of mice.
CONCLUSIONS
Our present work demonstrated that methaolic extract of rhizome of C. caesia Roxb. has not shown any genotoxicity and reduces the genotoxicity caused by reactive metabolites of CP. It is shown for the first time that MECC has protective effect against genotoxicity induced by CP in bone marrow cells as well as protects against toxicity induced in the liver and kidney by CP. So it could be provided as one of the herbal supplementation in chemoprevention of CP to ameliorate the side effects of it.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Heisanam Pushparani Devi
Heisanam Pushparani Devi, She is a Research Scholar in the Department of Biotechnology, Assam University Silchar and her interest is on medicinal plant studies like antioxidant activity, antimutagenic activity, antigenotoxic activity etc.

Pranab Behari Mazumder
Pranab Behari Mazumder, Professor Pranab Behari Mazumder is a faculty of Department of Biotechnology, assam University, Silchar and his interest is on medicinal plant studies as well microbial related studies. Currently many of the Researchers is working under his guidance and many of them have awarded Ph. D degree under his Guidence.
Acknowledgments
The authors thank to the Plant Biotechnology Laboratory, Department of Biotechnology, Assam University, Silchar for providing all the equipments.
REFERENCES
- 1.USA: AICR; 2007. World Cancer Research Fund/American Institute for Cancer Research. Food, Physical Activity, and the Prevention of Cancer: A Global Perspective. [Google Scholar]
- 2.France: IARC; 2008. World Health Organization. World Cancer Report 2008. [Google Scholar]
- 3.McMillen M. 8 Common Surgery Complications. WebMD Feature. WebMD. 2013 [Google Scholar]
- 4.Johnson IT. Phytochemicals and cancer. Proc Nutr Soc. 2007;66:207–15. doi: 10.1017/S0029665107005459. [DOI] [PubMed] [Google Scholar]
- 5.Sawadogo WR, Schumacher M, Teiten MH, Dicato M, Diederich M. Traditional West African pharmacopeia, plants and derived compounds for cancer therapy. Biochem Pharmacol. 2012;84:1225–40. doi: 10.1016/j.bcp.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Takimoto CH, Calvo E, Pazdur R, Coia LR, Hoskins WJ. Cancer Management, A Multidisciplinary Approach. 9th ed. 2005. Principles of oncologic pharmacotherapy; pp. 23–42. [Google Scholar]
- 7.IARC. IARC monograph on the evaluation of carcinogenicity: An update of IARC monographs. Vol. 1-42: International Agency for Research on Cancer. 1987 [Google Scholar]
- 8.Abraham P, Sugumar E. Enhanced PON1 activity in the kidneys of cyclophosphamide treated rats may play a protective role as an antioxidant against cyclophosphamide induced oxidative stress. Arch Toxicol. 2008;82:237–8. doi: 10.1007/s00204-007-0240-3. [DOI] [PubMed] [Google Scholar]
- 9.Patel JM. Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology. 1987;45:79–91. doi: 10.1016/0300-483x(87)90116-8. [DOI] [PubMed] [Google Scholar]
- 10.Hales BF. Comparison of the mutagenicity and teratogenicity of cyclophosphamide and its active metabolites, 4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein. Cancer Res. 1982;42:3016–21. [PubMed] [Google Scholar]
- 11.Sladek NE. Metabolism of cyclophosphamide by rat hepatic microsomes. Cancer Res. 1971;31:901–8. [PubMed] [Google Scholar]
- 12.Sladek NE. Metabolism of oxazaphosphorines. Pharmacol Ther. 1988;37:301–55. doi: 10.1016/0163-7258(88)90004-6. [DOI] [PubMed] [Google Scholar]
- 13.Leishangthem S, Dinendra SL. Study of some important medicinal plants found in Imphal-East district, Manipur, India. Int J Sci Res Publ. 2014;4 [Google Scholar]
- 14.Banerjee A, Nigam SS. Antifungal activity of the essential oil of Curcuma caesia Roxb. Indian J Med Res. 1976;64:1318–21. [PubMed] [Google Scholar]
- 15.Arulmozhi DK, Sridhar N, Veeranjaneyulu A, Arora SK. Preliminary mechanistic studies on the smooth muscle relaxant effect of hydroalcoholic extract of Curcuma caesia. J Herb Pharmacother. 2006;6:117–24. doi: 10.1080/j157v06n03_06. [DOI] [PubMed] [Google Scholar]
- 16.Paliwal P, Pancholi SS, Patel RK. Pharmacognostic parameters for evaluation of the rhizomes of Curcuma caesia. J Adv Pharm Technol Res. 2011;2:56–61. doi: 10.4103/2231-4040.79811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangla M, Shuaib M, Jain J, Kashyap M. In-vitro evaluation of antioxidant activity of Curcuma caesia Roxb. Int J Pharm Sci Res. 2010;1:98–102. [Google Scholar]
- 18.Karmakar I, Saha P, Sarkar N, Bhattacharya S, Haldar PK. Neuropharmacological assessment of Curcuma caesia rhizome in experimental animal models. Orient Pharm Exp Med. 2011;11:251–5. [Google Scholar]
- 19.Gill R, Kalsi V, Singh A. Phytochemical investigation and evaluation of anthelmintic activity of Curcuma amada and Curcuma caesia - A comparative study. Inventi Impact Ethnopharmacol 2011. 2011 [Google Scholar]
- 20.Rajamma AG, Bai V, Nambisan B. Antioxidant and antibacterial activities of oleoresins isolated from nine Curcuma species. Phytopharmacology. 2012;2:312–7. [Google Scholar]
- 21.Das S, Bordoloi PK, Phukan D, Singh SR. Study of the anti-ulcerogenic activity of the ethanolic extracts of rhizome of Curcuma caesia (eecc) against gastrsic ulcers in experimental animals. Asian J Pharm Clin Res. 2012;5:200–3. [Google Scholar]
- 22.Krishnaraj M, Manibhushanrao K, Mathivanan N. A comparative study of phenol content and antioxidant activity between non conventional Curcuma caesia Roxb. and Curcuma amada Roxb. Int J Plant Prod. 2010;4:169–74. [Google Scholar]
- 23.Devi HP, Mazumder PB, Devi LP. Antioxidant and Antimutagenic activity of Curcuma caesia Roxb. rhizome extracts. Toxicol Rep. 2015;2:423–8. doi: 10.1016/j.toxrep.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandey AK, Chowdhary AR. Volatile constituents of rhizome oil of Curcuma caesia Roxb. from central India. Flavour Fragr. 2003;18:463–5. [Google Scholar]
- 25.Sofowora A. 2nd ed. Sunshine House, Ibadan, Nigeria: Spectrum Books Ltd; 1993. Screening plants for bioactive agents. Medicinal Plants and Traditional Medicinal in Africa; pp. 134–56. [Google Scholar]
- 26.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 27.Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–54. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 28.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 29.Serpeloni JM, Bisarro dos Reis M, Rodrigues J, Campaner dos Santos L, Vilegas W, Varanda EA, et al. In vivo assessment of DNA damage and protective effects of extracts from Miconia species using the comet assay and micronucleus test. Mutagenesis. 2008;23:501–7. doi: 10.1093/mutage/gen043. [DOI] [PubMed] [Google Scholar]
- 30.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. 2nd ed. Oxford, UK: Oxford University Press; 1989. The chemistry of free radicals and related reactive species; pp. 60–1. [Google Scholar]
- 32.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 33.David M, Richard JS. Methods of Enzymatic Analysis. In: Bergmeyer J, Grab M, editors. Deerfield Beach, Floride: Verlag Chemie, Wenhein; 1983. p. 358. [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 35.Mantle D, Wilkins RM. Medicinal plants in the prevention and therapy of cancer. In: Yaniv Z, Bachrach U, editors. Handbook of Medicinal Plants. New York: The Haworth Press; 2005. pp. 281–318. [Google Scholar]
- 36.Wagner H. Trends and challenges in phytomedicine: Research in the New Millennium. In: Yaniv Z, Bachrach U, editors. Handbook of Medicinal Plants. New York: The Haworth Press; 2005. p. 3. [Google Scholar]
- 37.Hussain A, Ramteke A. Flower extract of Nyctanthes arbor-tristis modulates glutathione level in hydrogen peroxide treated lymphocytes. Pharmacognosy Res. 2012;4:230–3. doi: 10.4103/0974-8490.102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gangar SC, Sandhir R, Koul A. Anti-clastogenic activity of Azadirachta indica against benzo (a) pyrene in murine forestomach tumorigenesis bioassay. Acta Pol Pharm. 2010;67:381–90. [PubMed] [Google Scholar]
- 39.Jain R, Jain SK. Effect of Buchanania lanzan Spreng. bark extract on cyclophosphamide induced genotoxicity and oxidative stress in mice. Asian Pac J Trop Med. 2012;5:187–91. doi: 10.1016/S1995-7645(12)60022-4. [DOI] [PubMed] [Google Scholar]
- 40.Hosseinimehr SJ, Karami M. Chemoprotective effects of captopril against cyclophosphamide-induced genotoxicity in mouse bone marrow cells. Arch Toxicol. 2005;79:482–6. doi: 10.1007/s00204-005-0655-7. [DOI] [PubMed] [Google Scholar]
- 41.Vijayalaxmi RRJ, Reiter RJ, Herman TS, Meltz ML. Melatonin and radioprotection from genetic damage: In vivo / in vitro studies with human volunteers. Mutat Res. 1996;371:221–8. doi: 10.1016/s0165-1218(96)90110-x. [DOI] [PubMed] [Google Scholar]
- 42.Tice RR, Erexson GL, Shelby MD. The induction of micronucleated polychromatic erythrocytes in mice using single and multiple treatments. Mutat Res. 1990;234:187–93. doi: 10.1016/0165-1161(90)90014-f. [DOI] [PubMed] [Google Scholar]
- 43.MacGregor JT, Schlegel R, Choy WN, Wehr CM. Micronuclei in circulating erythrocytes: A rapid screen for chromosomal damage during routine toxicity testing in mice. Dev Toxicol Environ Sci. 1983;11:555–8. [PubMed] [Google Scholar]
- 44.Huttunen KM, Raunio H, Rautio J. Prodrugs - from serendipity to rational design. Pharmacol Rev. 2011;63:750–71. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 45.Ray S, Pandit B, Ray SD, Das S, Chakraborty S. Cyclophosphamide induced lipid peroxidation and changes in cholesterol content: Protective role of reduced glutathione. Iran J Pharm Sci. 2011;7:255–67. [Google Scholar]
- 46.Chirangini P, Sharma GJ, Sinha SK. Sulfur free radical reactivity with curcumin as reference for evaluating antioxidant properties of medicinal Zingiberales. J Environ Pathol Toxicol Oncol. 2004;23:227–36. doi: 10.1615/jenvpathtoxoncol.v23.i3.60. [DOI] [PubMed] [Google Scholar]
- 47.Devasagayam TP, Boloor KK, Ramasarma T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J Biochem Biophys. 2003;40:300–8. [PubMed] [Google Scholar]
- 48.Anoopkumar-Dukie S, Walker RB, Daya S. A sensitive and reliable method for the detection of lipid peroxidation in biological tissues. J Pharm Pharmacol. 2001;53:263–6. doi: 10.1211/0022357011775299. [DOI] [PubMed] [Google Scholar]
- 49.Deb AC. 7th ed. Kolkata: New Central Book Agency; 1998. Fundamentals of Biochemistry. [Google Scholar]
- 50.Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 51.Lu SC, Huang ZZ, Yang JM, Tsukamoto H. Effect of ethanol and high-fat feeding on hepatic gamma-glutamylcysteine synthetase subunit expression in the rat. Hepatology. 1999;30:209–14. doi: 10.1002/hep.510300134. [DOI] [PubMed] [Google Scholar]
- 52.Odukoya OA, Inya-Agha SI, Ilori OO. Immune boosting herbs: Lipid peroxidation in liver homogenates as index of activity. J Pharmacol Toxicol. 2007;2:190–5. [Google Scholar]
- 53.Balouchzadeh A, Rahimi HR, Ebadollahi N, Minaei-Zangi AR, Sabzevari O. Aqueous extract of Iranian green tea prevents lipid peroxidation and chronic ethanol liver toxicity in rats. J Pharmacol Toxicol. 2011;6:691–700. [Google Scholar]
- 54.Pastore A, Piemonte F, Locatelli M, Lo Russo A, Gaeta LM, Tozzi G, et al. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin Chem. 2001;47:1467–9. [PubMed] [Google Scholar]
- 55.Rehman MU, Tahir M, Ali F, Qamar W, Lateef A, Khan R, et al. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: The protective effect of ellagic acid. Mol Cell Biochem. 2012;365:119–27. doi: 10.1007/s11010-012-1250-x. [DOI] [PubMed] [Google Scholar]