Functional imaging by [18-F] fluorodeoxyglucose positron emission tomography (FDG PET) is an accurate modality in detecting residual viable lymphoma and is often used to evaluate response to therapy.(1) However, the data on utility of PET imaging in assessing disease response to salvage therapy in the setting of allogeneic hematopoietic transplantation (alloHCT) for non-Hodgkin lymphoma (NHL) is limited.(2–4) We evaluated 73 patients with NHL treated between 2004–2010. Transplantation protocols and study design were approved by the Institutional Review Board and informed consent was obtained prior to treatment. Patients underwent PET/computed tomography (CT) imaging less than 30 days before and at 100 days after allogeneic donor transplantation using the Siemens Biograph 16 PET scanner. Imaging scans were reviewed centrally by a nuclear medicine radiologist (JF, AKK). Follicular lymphoma was the most common lymphoma subset, followed by diffuse large B cell lymphoma (DLBCL), T cell lymphoma, and mantle cell lymphoma (Table 1). The median age was 50 years, and 60% were males. Most patients received reduced intensity conditioning which consisted of fludarabine, cyclophosphamide and total body irradiation as previously reported.(5) Myeloablative conditioning (MAC) consisted of cyclophosphamide with fractionated total body irradiation (1320 cGy). All patients received cyclosporine from day −3 for a minimum of 100 days, followed by a taper through day 180. In addition, MAC patients received methotrexate 15 mg/m2 IV on day +1 followed by 10 mg/m2 IV on days +3, +6 and +11. Mycophenolate mofetil (1–1.5 gm IV or orally twice a day for the first 30 days) was used after RIC and in all umbilical cord blood (UCB) recipients after MAC. All patients received supportive care as previously reported.(5) Graft sources included UCB (45%) or HLA-matched related donor (55%). Median follow-up was 3.3 years (range 1–6.7 years).
Table 1.
Patient and transplant characteristics
| Factor | Total patients | PETpos | PETneg | p-value |
|---|---|---|---|---|
| Total Patients | 73 | 44 | 29 | |
| Age in years | ||||
| Median (range) | 50 (18–69) | 49 (26–68) | 52 (18–69) | 0.99 |
| Gender | 0.81 | |||
| Male | 44 | 27 (61%) | 17 (59%) | |
| Karnofsky performance score | ||||
| ≥90 | 35 | 22 (50%) | 14 (51%) | 0.92 |
| <90 | 33 | 22 (50%) | 12 (46%) | |
| Comorbidity score | ||||
| 0–2 | 38 | 23 (52%) | 15 (52%) | 0.34 |
| ≥3 | 35 | 21 (48%) | 14 (48%) | |
| Diagnosis | 0.18 | |||
| Follicular lymphoma (+indolent) | 30 | 22 (42%) | 8 (22%) | |
| Diffuse large B cell lympoma | 17 | 10 (23%) | 7 (24%) | |
| Mantle cell lymphoma | 11 | 7 (16%) | 4 (14%) | |
| T cell lymphoma | 15 | 5 (11%) | 10 (31%) | |
| Prior chemo regimens | 0.47 | |||
| 1–2 | 29 | 17 (38%) | 12 (33%) | |
| ≥3 | 43 | 27 (61%) | 17 (67%) | |
| Prior autologous HCT | 7 | 5 (11%) | 2 (7%) | 0.53 |
| Disease Status at transplant | <0.01 | |||
| Complete remission by CT scan | 22 | 3 (8%) | 19 (66%) | |
| Partial remission by CT scan | 42 | 34 (76%) | 10 (34%) | |
| Refractory | 9 | 7 (16%) | 0 (0%) | |
| B symptoms at any time pre-HCT | 44 | 28 (64%) | 16 (57%) | 0.58 |
| Bone Marrow involved pre-HCT | 9 | 7 (16%) | 2 (7%) | 0.30 |
| Disease stage at diagnosis | ||||
| 2–3 | 12 | 5 (12%) | 7 (25%) | |
| 4 | 60 | 39 (89%) | 21 (75%) | |
| Nodal groups on pre-HCT CT scan none | 14 | 6 (12%) | 8 (23%) | 0.78 |
| 1–2 | 49 | 31 (72%) | 18 (62%) | |
| ≥3 | 10 | 7 (16%) | 3 (15%) | HR |
| Extra-nodal disease pre-HCT | 0.01 | |||
| Yes | 20 | 18 (41%) | 2 (10%)* | |
| No | 45 | 26 (59%) | 19 (90%) | |
| Conditioning | ||||
| Myeloablative | 25 | 15 (34%) | 10 (34%) | 0.07 |
| Reduced intensity | 48 | 29 (66%) | 19 (66%) | |
| Donor Source | 0.01 | |||
| Matched related donor | 39 | 23 (52%) | 16 (55%) | |
| Matched unrelated donor | 1 | 0 | 1 (3%) | |
| Umbilical cord blood | 33 | 21 (48%) | 12 (41%) | |
| Years of follow-up | ||||
| Median (range) | 3.33 (1.00–6.74) | 3.55 (1.00–6.74) | 3.05 (1.00–6.28) | 0.22 |
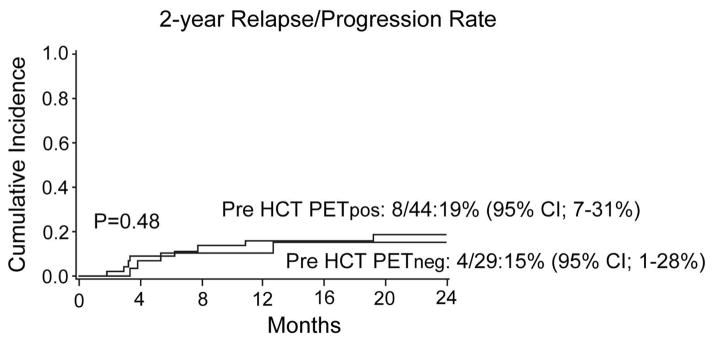
Pre-transplantation PET performed at median of 17 days (range 4–39 days) detected lymphoma in 44 patients (PETpos 57%) and 29 patients had no detectable lymphoma by PET (PETneg 43%). Most PETpos patients (77%) were in partial remission (PR) by CT, and 8% were in complete remission by PET/CT scan; 7% had chemorefractory disease (all had indolent histology). Most PETpos patients (88%) had > 1 site of nodal involvement, and 40% had extranodal disease at the time of transplantation. Two PETneg patients had low-level marrow involvement at the time of transplantation. Only four patients had lymphadenopathy over 5 cm in largest diameter and all were FDG-PET positive. Seven patients underwent prior autologous HCT. Other patient, disease, and transplantation characteristics were balanced in both PETpos and PETneg groups. The cumulative incidence of relapse/progression at two years was 17% and was similar in patients who were PETpos and PETneg pre-transplantation (19% [95% CI 7–31%] vs 15% [95% CI 1–28%]; p=0.48; Figure). Non-relapse mortality at 1 year was 11% (95% CI 4–18%) and was not affected by pre-transplant PET. A period of more than two years from diagnosis to transplantation was the only variable favorably associated with a lower relapse/progression rate (HR 0.28 (95%CI 0.08–1.0; p=0.05) with no influence on progression free survival (PFS) and overall survival (OS). There was no difference in PFS according to age (>50 years HR 0.87; 95%CI 0.28–2.64), gender (female HR 1.1; 95%CI 0.35–3.47), histology (DLBCL HR 2.14; Mantle cell HR 2.15 and T cell NHL HR 1.68 compared to Follicular lymphoma, overall p=0.73); RIC conditioning (HR 1.75; 95%CI 0.45–5.75), donor type (UCB HR 0.38; 95%CI 0.12–1.23), or marrow involvement at the time of transplant (HR 0.81; 95%CI 0.24–2.66). At three years, PETpos group had had similar PFS and OS compared to PETneg group (PFS; 55% [95%CI 39–69) vs 62% [95%CI 38–79]; p=0.27; OS; 69% [95% CI 53–81%] vs 73% [95% CI 48–87]; p=0.39). PFS for follicular lymphoma was 72% (95% CI 46–87%) in PETpos and 60% (95% CI 20–85%) in PETneg patients (p=0.69).
Figure.
Impact of pre-transplant PET status on cumulative risk of relapse
Post-transplantation PET imaging was performed in 62 patients (85% of entire cohort) at day 100 after allogeneic transplantation. Of 44 patients with PET detectable lymphoma immediately pre-transplantation, half converted to PETneg remission (n=24, 52%) 100 days after transplantation. Notably, three out of four patients with large >5cm PETpos lymphadenopathy attained PETneg remission. Patients with no FDG-PET uptake 100 days post transplantation had 3-year PFS 73% (95% CI 58–86%) compared to 25% (95% CI 4–55%); p=0.01) in patients who were FDG-PET positive imaging.
Consistent with recent report from Memorial Sloan-Kettering Cancer Center and University College London, our study supports the conclusion that FDG PET imaging performed prior to allogeneic transplantation does not have a high prognostic impact on post-transplant relapse and survival and does not provide additional value in patients with NHL who attained chemosensitivity by conventional CT-based criteria.(2,4) These findings are in contrast to high-dose chemotherapy and autologous HCT where FDG-PET is proven to be significantly prognostic for survival.(6) We demonstrated that patients with FDG-avid residual lymphoma enjoyed favorable outcomes and a relatively low relapse rate of 19%. The immune-mediated graft-versus-lymphoma (GVL) effect of allogeneic donor transplantation (7–10) may overcome the presence of active NHL in patients with abnormal FDG uptake prior to transplant, remarkably even in some patients with larger tumor bulk indolent NHL. Limitations of most series include retrospective design, patient and transplant procedure heterogeneity and limited size of each lymphoma subset with potentially lower power of detecting differences in the primary end-points within these groups (2–4). Future investigations of the prognostic role of PET prior to alloHCT are needed to validate these results and prospective trials designed for specific lymphoma subsets should incorporate PET/CT imaging as a testable prognostic biomarker. Until such data becomes available, the reliance on PET imaging and routine FDG-PET for monitoring NHL responses prior to alloHCT should be discouraged.
Acknowledgments
This work was supported in part by NIH P30 CA77598 utilizing the biostatistics and informatics core, Masonic Cancer Center, University of Minnesota shared resource, and the Janie Lymphoma Fund (LJB). Research reported in this publication was also in part supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
Footnotes
Financial Disclosure Statement
Authors declare no competing financial interests.
References
- 1.Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 2.Sauter CS, Lechner L, Scordo M, Zheng J, Devlin SM, Fleming SE, et al. Pre-Transplant FDG-PET Scan Lacks Prognostic Value in Chemosensitive B-Cell Non-Hodgkin Lymphoma Patients Undergoing Non-Myeloablative Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2014;20:881–4. doi: 10.1016/j.bbmt.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodero A, Crocchiolo R, Patriarca F, et al. Pretransplantation [18-F] fluorodeoxyglucose positron emission tomography scan predicts outcome in patients with recurrent Hodgkin lymphoma or aggressive non-Hodgkin lymphoma undergoing reduced-intensity conditioning followed by allogeneic stem cell transplantation. Cancer. 2010;116:5001–5011. doi: 10.1002/cncr.25357. [DOI] [PubMed] [Google Scholar]
- 4.Lambert JR, Bomanji JB, Peggs KS, et al. Prognostic role of PET scanning before and after reduced-intensity allogeneic stem cell transplantation for lymphoma. Blood. 2010;115:2763–2768. doi: 10.1182/blood-2009-11-255182. [DOI] [PubMed] [Google Scholar]
- 5.Tomblyn M, Brunstein C, Burns LJ, Miller JS, MacMillan M, DeFor TE, et al. Similar and promising outcomes in lymphoma patients treated with myeloablative or nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:538–545. doi: 10.1016/j.bbmt.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Besien K, Loberiza FR, Jr, Bajorunaite R, et al. Comparison of autologous and allogeneic hematopoietic stem cell transplantation for follicular lymphoma. Blood. 2003;102:3521–3529. doi: 10.1182/blood-2003-04-1205. [DOI] [PubMed] [Google Scholar]
- 7.Peniket AJ, Ruiz de Elvira MC, Taghipour G, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31:667–678. doi: 10.1038/sj.bmt.1703891. [DOI] [PubMed] [Google Scholar]
- 8.Robinson SP, Canals C, Luang JJ, Tilly H, Crawley C, Cahn JY, et al. The outcome of reduced intensity allogeneic stem cell transplantation and autologous stem cell transplantation when performed as a first transplant strategy in relapsed follicular lymphoma: an analysis from the Lymphoma Working Party of the EBMT. Bone Marrow Transplant. 2013;48:1409–1414. doi: 10.1038/bmt.2013.83. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimi A, Izutsu K, Takahashi M, et al. Conventional allogeneic hematopoietic stem cell transplantation for lymphoma may overcome the poor prognosis associated with a positive FDG-PET scan before transplantation. Am J Hematol. 2008;83:477–481. doi: 10.1002/ajh.21158. [DOI] [PubMed] [Google Scholar]
- 10.Ram R, Gooley TA, Maloney DG, Press OW, Pagel JM, Petersdorf SH, et al. Histology and time to progression predict survival for lymphoma recurring after reduced-intensity conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1537–1545. doi: 10.1016/j.bbmt.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]