Supplemental Digital Content is available in the text.
Keywords: angioplasty, drug-eluting stents, ischemia, peripheral arterial disease
Abstract
Background—
Endovascular infrapopliteal treatment of patients with critical limb ischemia using percutaneous transluminal angioplasty (PTA) and bail-out bare metal stenting (BMS) is hampered by restenosis. In interventional cardiology, drug-eluting stents (DES) have shown better patency rates and are standard practice nowadays. An investigator-initiated, multicenter, randomized trial was conducted to assess whether DES also improve patency and clinical outcome of infrapopliteal lesions.
Methods and Results—
Adults with critical limb ischemia (Rutherford category ≥4) and infrapopliteal lesions were randomized to receive PTA±BMS or DES with paclitaxel. Primary end point was 6-month primary binary patency of treated lesions, defined as ≤50% stenosis on computed tomographic angiography. Stenosis >50%, retreatment, major amputation, and critical limb ischemia–related death were regarded as treatment failure. Severity of failure was assessed with an ordinal score, ranging from vessel stenosis through occlusion to the clinical failures. Seventy-four limbs (73 patients) were treated with DES and 66 limbs (64 patients) received PTA±BMS. Six-month patency rates were 48.0% for DES and 35.1% for PTA±BMS (P=0.096) in the modified-intention-to-treat and 51.9% and 35.1% (P=0.037) in the per-protocol analysis. The ordinal score showed significantly worse treatment failure for PTA±BMS versus DES (P=0.041). The observed major amputation rate remained lower in the DES group until 2 years post-treatment, with a trend toward significance (P=0.066). Less minor amputations occurred after DES until 6 months post-treatment (P=0.03).
Conclusions—
In patients with critical limb ischemia caused by infrapopliteal lesions, DES provide better 6-month patency rates and less amputations after 6 and 12 months compared with PTA±BMS.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00471289.
WHAT IS KNOWN
Drug-eluting stents (DES) reduce neointimal hyperplasia and are nowadays the reference treatment in coronary lesions.
In the similarly sized infrapopliteal arteries, DES yield higher primary patency rates at 1 year compared with percutaneous transluminal angioplasty or bare metal stents.
Studies thus far have failed to show a difference in clinical outcomes.
WHAT THE STUDY ADDS
DES provide better clinical outcomes with less amputations and less serious treatment failures in patients with critical limb ischemia and infrapopliteal lesions when compared with percutaneous transluminal angioplasty±bare metal stents.
Infrapopliteal paclitaxel-eluting DES show higher 6-month patency rates compared with the current reference treatment, percutaneous transluminal angioplasty with bail-out bare metal stents, in patients with critical limb ischemia caused by infrapopliteal lesions.
A treatment strategy with DES should therefore be considered in patients with critical limb ischemia caused by infrapopliteal lesions.
Critical limb ischemia (CLI) manifests with chronic ischemic rest pain, tissue loss, or both. At present, the incidence of CLI, the final stage of peripheral arterial disease, in the Western world is ≈500 to 1000 cases per 1 million inhabitants every year.1
Major risk factors in the development of CLI are diabetes mellitus, increased age, and smoking.1,2 With an aging Western population and an increasing prevalence of diabetes mellitus, the burden of CLI and its costs are likely to increase in the near future.3
Preventing major amputation is of particular concern in the treatment of CLI as amputation is associated with a high periprocedural morbidity and mortality and functional outcome is often poor.4
To avoid amputation and relieve pain, revascularization techniques attempt to restore unobstructed arterial blood flow into the affected foot.1,5,6 The selected method of revascularization, either endovascular or surgical, depends on the local anatomic situation, condition of the patient, estimated risk, and expected patency of the reconstruction.1,5
Although the patency of infrapopliteal percutaneous transluminal angioplasty (PTA) and bail-out bare metal stenting (BMS), the current standard endovascular treatment, may be less than of bypass surgery, limb salvage rates are equivalent for at least middle-term outcome.3,5,7–11 The major advantage of endovascular treatment is the lower periprocedural morbidity and mortality, which is of particular concern in typical patients with CLI, that is, older and fragile patients with systemic atherosclerosis and diabetes mellitus who are at high risk for cardiovascular events.1
Vascular restenosis, caused by intimal hyperplasia due to vessel injury during PTA, remains the main limitation of infrapopliteal PTA and BMS with clinical relapse and reinterventions.7,8,10–13 Drug-eluting stents (DES) are considered a possible solution to the problem of restenosis by reducing neointimal hyperplasia, after promising results in coronary arteries.12 There is a limited number of randomized studies concerning the use of DES in infrapopliteal arteries to date and these studies either included patients with intermittent claudication (≤68%)13–15 or had limited follow-up (12-month angiography available in 46% of patients).16
The PTA and Drug Eluting Stents for Infrapopliteal Lesions in Critical Limb Ischemia (PADI) trial, an investigator-initiated multicenter randomized study, was conducted to determine whether DES reduce restenosis and occlusion in infrapopliteal arteries in patients with CLI and may thus decrease amputation. In this trial, 6-month patency rates and clinical outcomes at 6 and 12 months after endovascular treatment of infrapopliteal lesions in CLI are compared using either paclitaxel-eluting DES or PTA with bail-out BMS.
Methods
Study Design and Study Population
The PADI trial is an investigator-initiated, multicenter, randomized, controlled, nonblinded, double-arm study conducted in 3 major vascular centers in the Netherlands. The protocol was approved by the medical ethical boards of the participating centers and all enrolled patients gave written informed consent. The trial was registered at http://www.clinicaltrials.gov (identifier NCT00471289).
Adult patients were eligible for enrollment if they have CLI (defined as Rutherford category ≥4)17 caused by infrapopliteal lesions.
Lesions were considered for inclusion if there was >50% luminal loss, lesion length of ≤90 mm, and reference vessel diameter 2 to 6 mm, estimated by pretreatment imaging.18 Inflow had to be unobstructed, possibly after revascularization in the femoropopliteal segment during the same session. Outflow distal to target lesions should consist of ≥1 crural vessel with expected unobstructed runoff until the level of the ankle joint.
Detailed inclusion and exclusion criteria are available in Table I in the Data Supplement.
Randomization and Masking
After target lesions were successfully crossed with a guidewire, patient’s limbs were randomly allocated to 1 of the 2 treatment strategies, PTA±BMS or DES. The attending radiological technician opened the sealed, opaque envelope containing a computer-generated random sequence on a 1:1 basis. Randomization was per limb and stratified in blocks per center. The block size (n=4) was known only to the statistician. Patients, operators, and investigators were not blinded to treatment assignment.
Study Procedures
Endovascular procedures were mainly performed by an antegrade approach using 6F sheaths. In case of failure, a contralateral retrograde transfemoral approach was used. Lesions were crossed under fluoroscopic guidance with the combination of a catheter and guidewire according to the choice of the operator, usually transluminally. Subintimal routes were used when transluminal recanalization failed.
After randomization, patients were treated according to 1 of the 2 treatment strategies.
In the DES arm, target lesions were treated with balloon expandable paclitaxel-eluting stainless steel coronary stents (TAXUS Liberté; Boston Scientific, Natick, MA). Paclitaxel, an extract derived from the Taxus brevifolia (Pacific Yew) tree, inhibits smooth muscle cell proliferation by affecting mitosis.12 If necessary, according to the operator, mainly in cases of occlusion, lesions were predilated. The premounted DES was advanced over the guidewire and deployed at the site of the target lesion, according to the manufacturer’s manual. The full length of lesions was covered, if necessary with overlapping stents. A maximum of 3 stents were allowed with a 3 to 5 mm overlap.
Patients in the PTA±BMS arm received PTA according to the normal practice of the operator. A balloon with a diameter matching the target vessel was advanced over the guidewire and inflated at the target lesion site. Average inflation time was 1 minute. If bail-out stenting was required caused by post-PTA occlusion or flow-limiting dissection, only nondrug-eluting BMS were allowed.
A maximum of 3 lesions per limb were included. When a patient was included for both limbs, each limb was randomized separately.
After the procedure, the access site was closed with a sealing device or with manual compression.
During the procedure, 5000 international units of heparin were administered intra-arterially. Post-procedure all patients were prescribed 100 mg of carbasalate calcium daily indefinitely and 75 mg of clopidogrel daily (with 300 mg loading dose) orally for ≥6 months.
Follow-Up
Patient assessments were planned before intervention, at discharge, after 3, 6, and 12 months and annually until 5 years. Assessments included medical history, physical examination including severity scoring of limb ischemia according to the Rutherford classification,17 ankle-brachial index, toe pressure, and duplex sonography of the treated limb. Computed tomographic angiography (CTA) of the pelvis and lower extremities was performed at 6 months follow-up. Patency of treated sites on CTA was scored independently by 2 board-certified interventional radiologists (J.M.M. and H.O.), who were unaware of the treatment. The degree of restenosis was scored from 0% to 50.0%, 50.0% to 99.9%, or occluded. In case of discordance, lesions were reassessed simultaneously to reach consensus.
Study End Points
Primary end point of the PADI trial was primary binary patency per treated lesion at 6 months, defined as ≤50% loss of luminal diameter on CTA without reintervention in interim. If CTA was not available but digital subtraction angiography or duplex sonography was available, patency of treated sites was scored by those techniques. Loss of >50% of luminal diameter on CT, treatment in interim by means of infrapopliteal bypass or endovascular reintervention, major amputation, and death related to CLI were considered as treatment failure. An ordinal score was used to grade the severity of treatment failure from vessel restenosis, through vessel occlusion to treatment in interim, major amputation, or CLI-related death.
Additional secondary end points were ischemic categorization of the treated leg by means of Rutherford classification, minor and major amputation (at or below versus above ankle level, respectively) of the trial leg, and periprocedural (within 30 days) complications, serious adverse events, and death.
Statistical Analysis
On the basis of published data, a patency rate of 50% was assumed in the PTA±BMS arm at 6 months.19 The study was designed to have a power of 80% to detect an elevation of the patency rate by DES to 75% with a 2-sided P<0.05. Taking into account a 15% loss to follow-up rate, the required sample rate had to be ≥136 patients.18
To evaluate difference in primary end point between PTA±BMS and DES, logistic regression with adjustment for multiple lesions within 1 limb was applied, assuming compound symmetry of the covariance matrix. For the ordinal composite end point a weighted χ2 test was used, with weights equal to the inverse number of lesions per limb. Limbs in patients included for both limbs were considered independent for the analysis.
The observed rate of amputation, death, and the combined end point amputation or death was estimated with the Kaplan–Meier method. Patients were censored at the end of follow-up. Differences between the treatment strategies were assessed with the log-rank test. Differences in mean Rutherford category were analyzed with t test.
All end points were evaluated in the modified-intention-to-treat (MITT) analysis, excluding patients who did not fulfill all inclusion criteria or complete follow-up and who had incorrectly been included. Furthermore, patients who had died because of causes unrelated to CLI were censored. In addition, all end points were repeated in the per-protocol (PP) analysis considering analyses per lesion, in which only lesions were included which were technically treated according to randomization.
In the PTA±BMS arm all lesions were treated according to protocol and included in PP analysis. Twenty-two lesions in the DES arm were treated with PTA only and excluded from PP analysis. Eight lesions were located near a joint, 5 lesions at a bifurcation, technical failure precluded stenting in 1 lesion, and in 8 lesions only PTA was performed because of operator preference.
Analyses were performed in SPSS version 21 and SAS System 9.3 for Windows by B.E.H. and M.I.S.
Results
Patient and Lesion Characteristics
From October 2007 through February 2013, 75 limbs in 74 patients were randomly assigned to DES and 69 limbs in 67 patients to PTA±BMS (Figure 1). In the DES arm, 1 patient (1 limb) and in the PTA arm 3 patients (3 limbs) were excluded from the MITT analysis.
Figure 1.
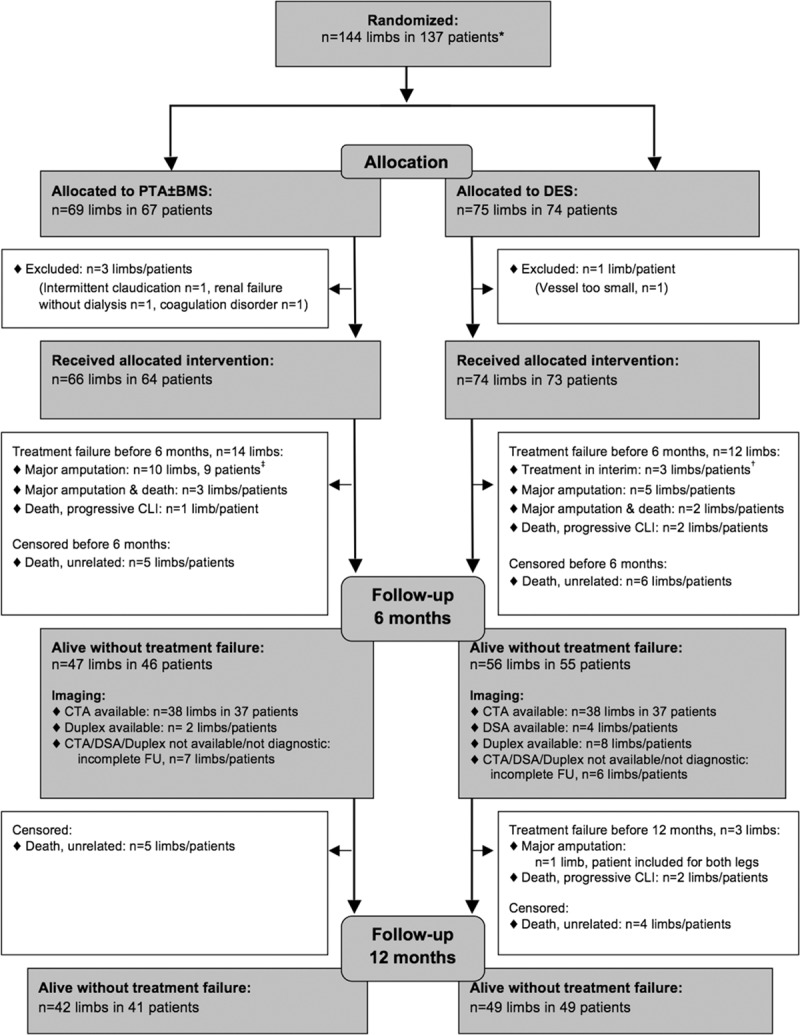
Flow chart, modified-intention-to-treat population. BMS indicates bare metal stent; CLI, critical limb ischemia; CTA, computed tomographic angiography; DES, drug-eluting stent; DSA, digital subtraction angiography; FU, follow-up; and PTA, percutaneous transluminal angioplasty. *Four patients included for 2 limbs with 1 limb in each arm. †One of these patients died before 1 year FU. ‡Two of these patients died before 1 year FU.
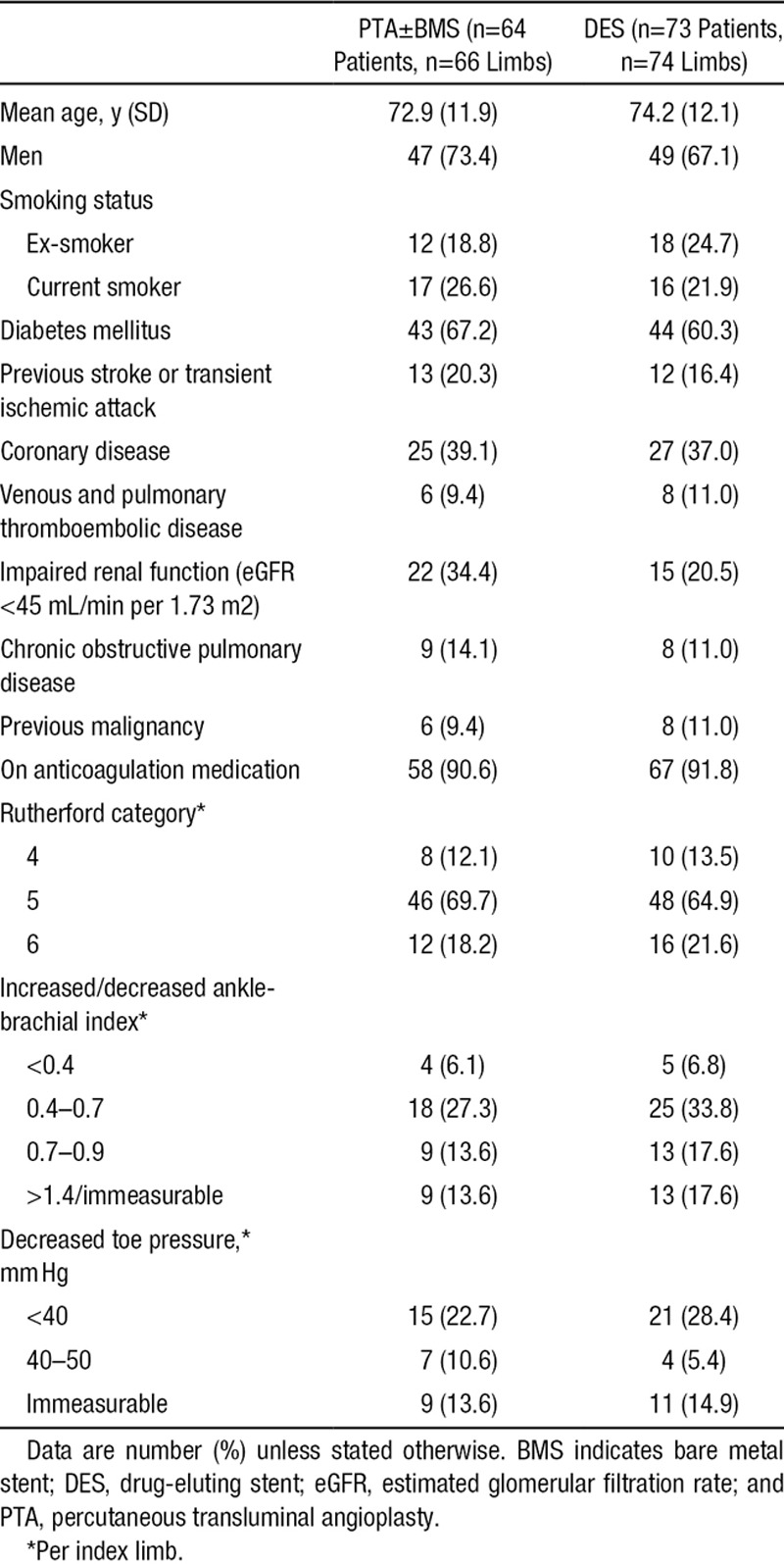
Both arms had similar baseline characteristics (Table 1). Diabetes mellitus was a common comorbidity. The overall mean baseline Rutherford category was 5.1 with a range of 4 to 6.
Table 1.
Baseline Characteristics
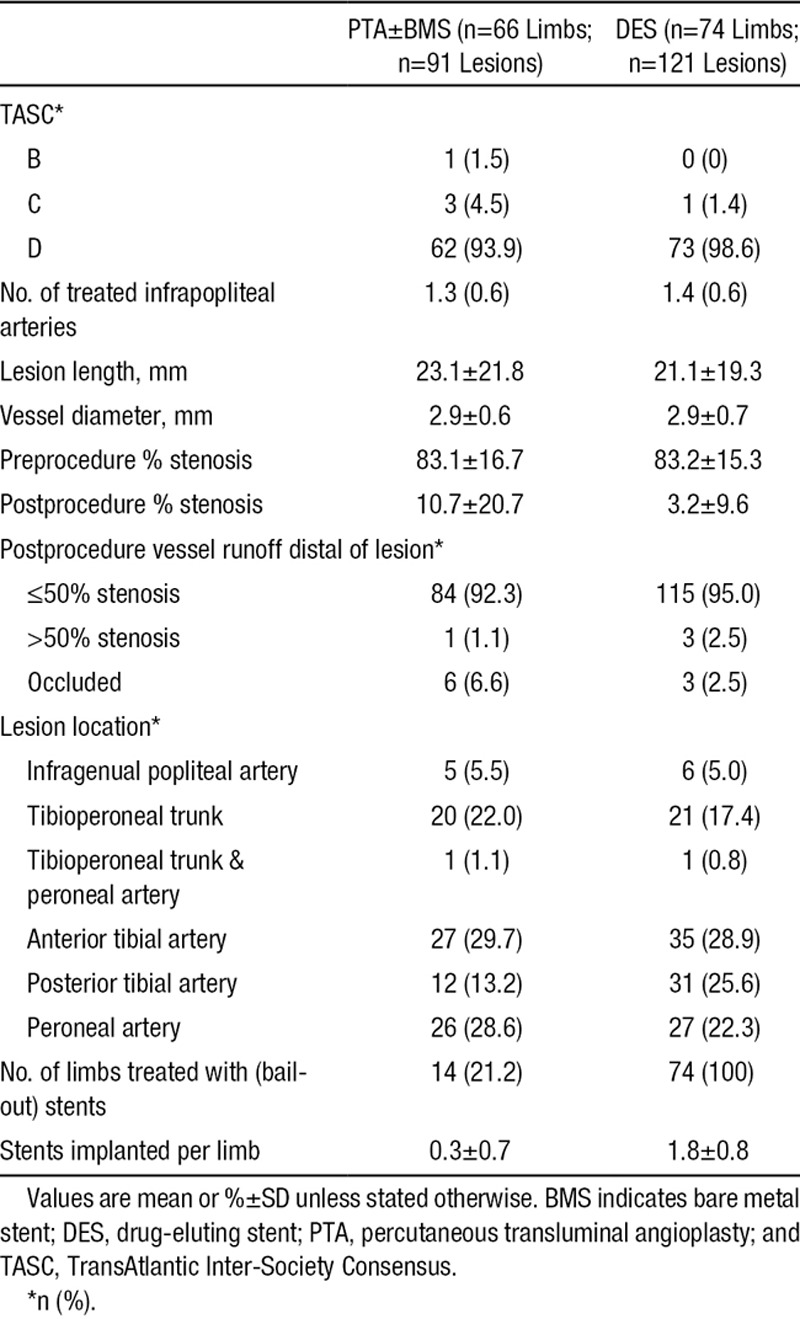
Almost all limbs showed extensive peripheral arterial disease; 93.9% of limbs in the PTA±BMS arm and 98.6% in the DES arm were classified as category D, according to the TransAtlantic Inter-Society Consensus (Table 2).20 Ninety-one lesions were treated in the PTA±BMS arm and 121 lesions in the DES arm, an average of 1.4 and 1.6 lesions per limb, respectively. Lesion characteristics were similar in both arms (Table 2). Residual stenosis after treatment was significantly less in the DES than in the PTA±BMS arm (3.2% versus 10.7%; P=0.002). An average of 1.8 stents was implanted per limb randomized for DES. In the PTA±BMS arm, a mean of 0.3 bail-out BMS were placed per limb.
Table 2.
Lesion Characteristics
End Points
The MITT analysis showed a 6-month patency rate of 48.0% in the DES arm versus 35.1% in the PTA±BMS arm (P=0.096). In the PP analysis, this difference was statistically significant; 51.9% in the DES arm and 35.1% in the PTA±BMS arm (P=0.037; Table 3).
Table 3.
Primary End Point Per Lesion After 6 Months, MITT, and PP Analysis
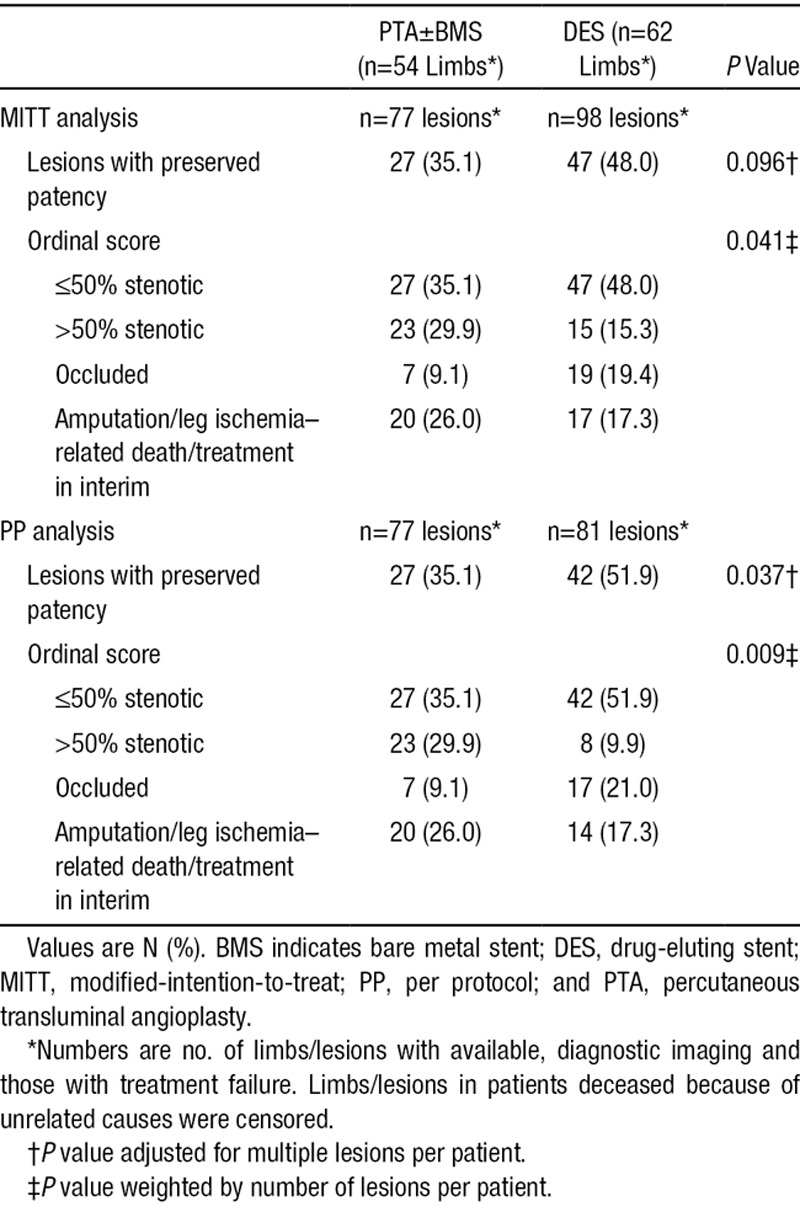
Lesions in the DES arm showed a more favorable composite clinical and morphological outcome than those in the PTA arm after 6 months in both the MITT (P=0.041) and PP analysis (P=0.009; Table 3).
During the first 6 months after treatment, there were 7 major amputations of the index limb (9.8%; 95% CI, 2.9%–16.7%) in the DES arm, versus 13 (20.5%; 95% CI, 10.5%–30.5%) in the PTA±BMS arm (P=0.10; Table 4). The major amputation rate after 1 year was 11.4% (95% CI, 4.0%–18.8%) in the DES arm and 20.5% (95% CI, 10.5%–30.5%) in the PTA±BMS arm. The Kaplan–Meier curves during the 2-year follow-up period diverged after 2 months in advantage of DES (P=0.066; Figure 2).
Table 4.
Clinical Outcomes After 6 and 12 Months
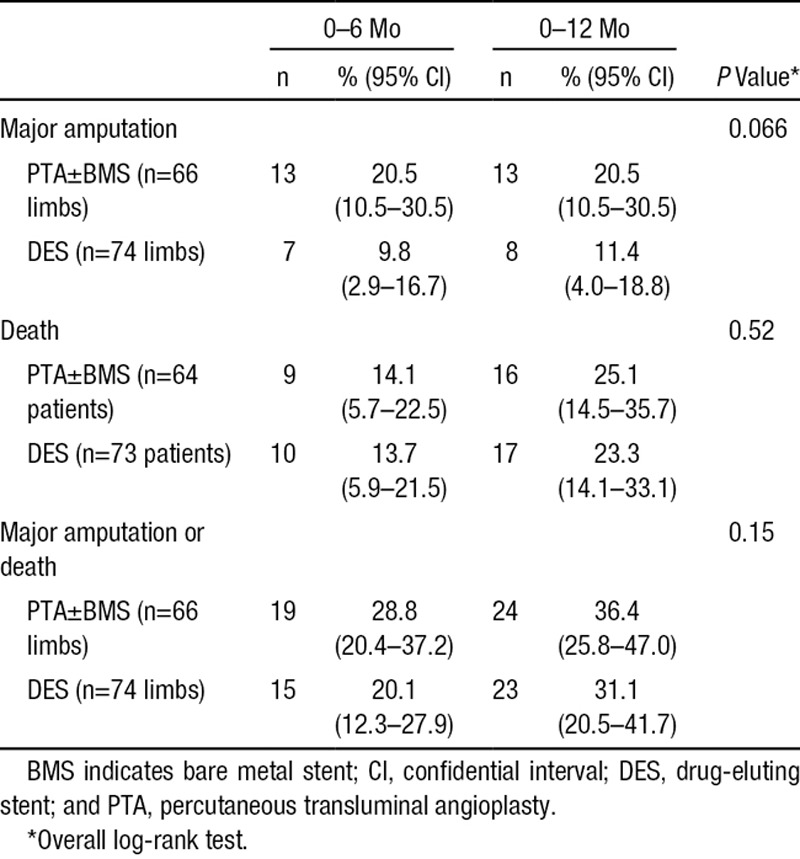
Figure 2.
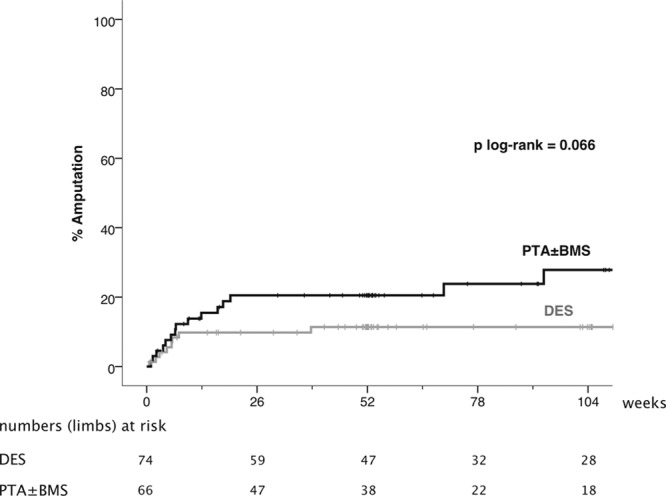
Kaplan–Meier curves representing the estimated 2-year cumulative incidence rates of major amputation per limb after percutaneous transluminal angioplasty (PTA)±bare metal stent (BMS) and drug-eluting stent (DES).
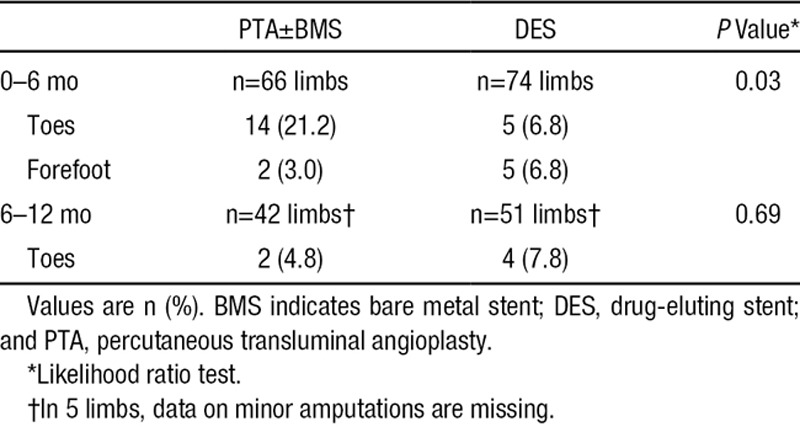
Significantly less minor amputations occurred in the DES arm during the first 6 months after treatment (P=0.03), but not during the second 6-month interval (Table 5).
Table 5.
Worst Minor Amputations
During the first post-treatment year, 17 patients in the DES arm and 16 patients in the PTA±BMS arm died, corresponding to a survival rate of 76.7% (95% CI, 66.9%–85.9%) versus 74.9% (95% CI, 64.3%–85.5%), respectively (Table 4). The Kaplan–Meier curves of survival and death or amputation until 2-year follow-up showed no significant difference between the treatment arms (P=0.52 and 0.15, respectively; Figures 3 and 4).
Figure 3.
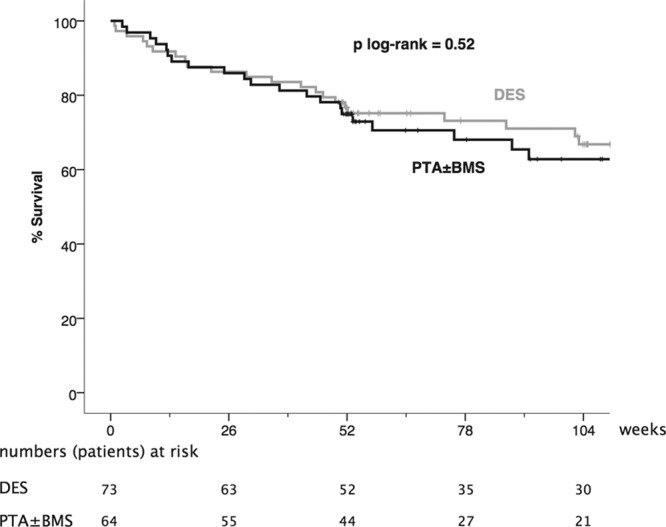
Kaplan–Meier curves representing the estimated 2-year cumulative incidence rates of survival per patient after percutaneous transluminal angioplasty (PTA)±bare metal stent (BMS) and drug-eluting stent (DES).
Figure 4.
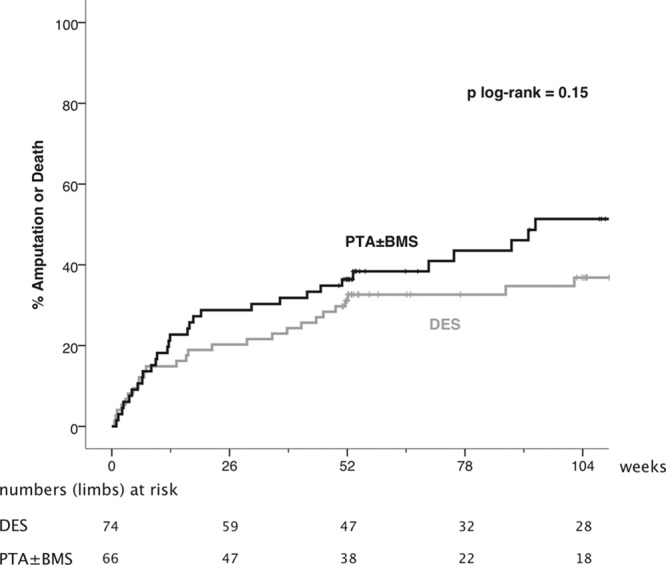
Kaplan–Meier curves representing the estimated 2-year cumulative incidence rates of major amputation or death per limb after percutaneous transluminal angioplasty (PTA)±bare metal stent (BMS) and drug-eluting stent (DES).
Three patients (4.1%) in the DES arm underwent retreatment of the affected leg within 6-month follow-up, against none in the PTA±BMS arm (P=0.098).
The mean Rutherford category,17 ankle-brachial index, and toe pressure after 6 and 12 months improved significantly in the survivors of both groups compared with baseline (P≤0.005; Figures 5–7; Table II in the Data Supplement). These improvements were comparable in both groups.
Figure 5.
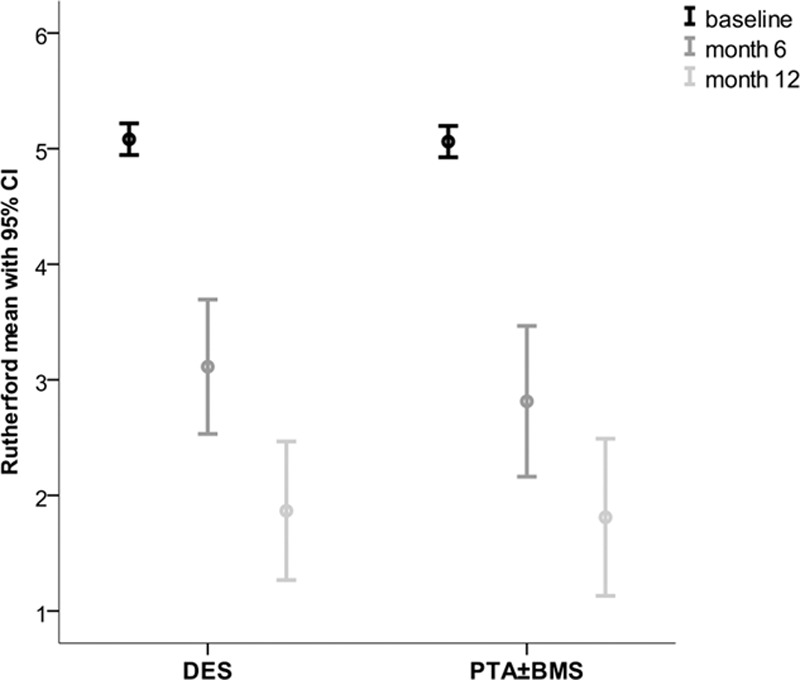
Mean Rutherford categories at baseline, 6 and 12 months. BMS indicates bare metal stent; CI, confidential interval; DES, drug-eluting stent; and PTA, percutaneous transluminal angioplasty.
Figure 7.
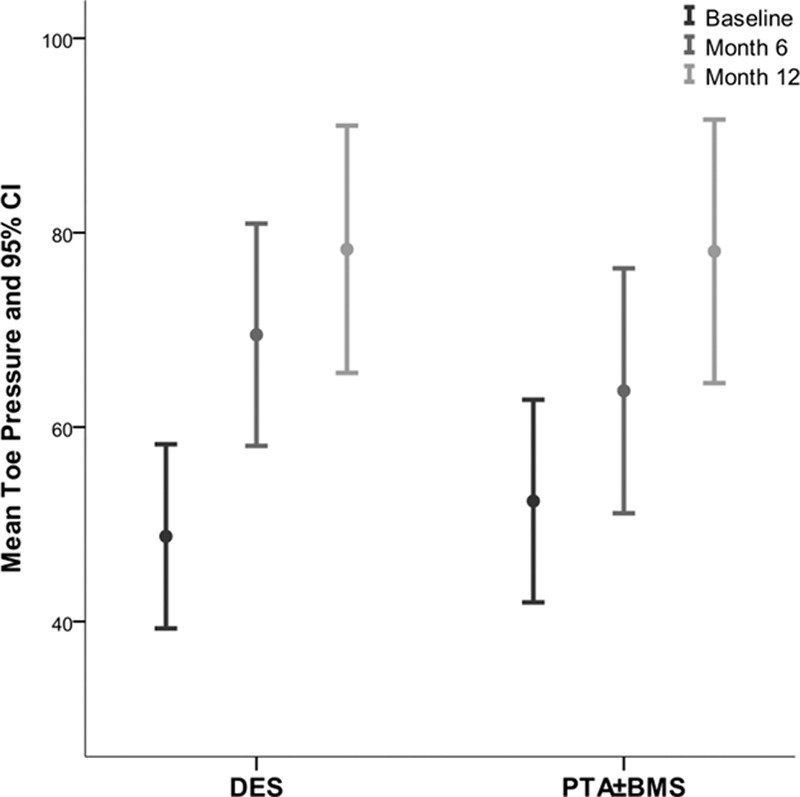
Mean toe pressure at baseline, 6 and 12 months. BMS indicates bare metal stent; CI, confidential interval; DES, drug-eluting stent; and PTA, percutaneous transluminal angioplasty.
Figure 6.
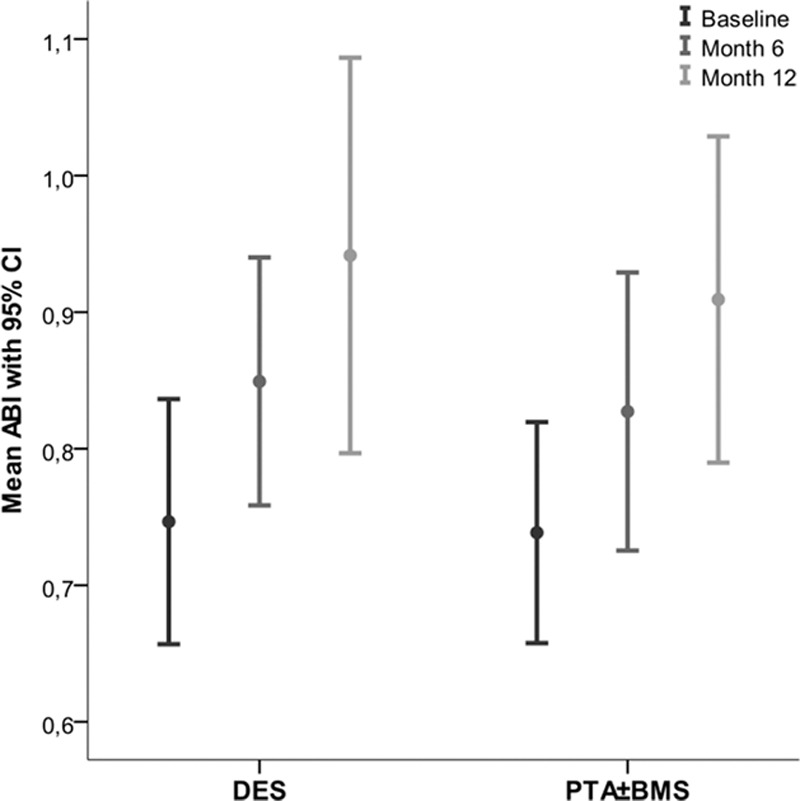
Mean ankle-brachial index at baseline, 6 and 12 months. BMS indicates bare metal stent; CI, confidential interval; DES, drug-eluting stent; and PTA, percutaneous transluminal angioplasty.
Complications and Adverse Events
Interim analysis for safety reasons at 6-week follow-up of the first 40 patients showed an acute thrombosis rate of 5% in both arms. The incidence of periprocedural complications, complications during treatment admission, and late complications did not differ significantly between the 2 arms; neither did the number of serious adverse events. A table with complications and serious adverse events is available in Table III in the Data Supplement.
Discussion
Our study shows that paclitaxel-eluting DES provide higher patency rates at 6 months in infrapopliteal stenotic or occluded lesions in patients with CLI when compared with the current reference treatment, PTA±BMS.5
Treatment failure was observed significantly more often in the latter group and was more severe, as is shown by the composite results. Such a composite end point better reflects the overall performance of DES compared with PTA±BMS because it combines the results of the morphological, local, and general clinical situation in patients.
This is the first study that demonstrates a difference in limb amputation rate using DES in CLI. The Kaplan–Meier curves of major amputations diverge at 2 months post-treatment with a trend toward significance at 2 years. In addition, there were less minor amputations.
Survival was comparable in both groups. The majority of deaths was not related to progression of limb ischemia, which can be explained by the fact that 25% of patients presenting with CLI are known to die within a year of onset of the symptoms, often caused by coronary heart disease.1,2,21,22
Surviving nonamputated patients showed improved Rutherford categories during follow-up. The decrease of Rutherford categories from 4 to 6 to stages 3 and 2 after 6- and 12-month follow up show that all these patients actually benefit from their medical care.
In interventional cardiology, DES were developed to decrease in-stent neointimal hyperplasia, which mainly occurs until 6 months post procedure.12,23,24 Nowadays, DES are standard treatment in coronary lesions because several meta-analyses proved their superior performance on restenosis and ischemia-driven repeat revascularization rates in comparison with BMS.25
Given these positive results of DES in coronary arteries, expectations arose that these stents might be efficacious in the similarly sized infrapopliteal arteries, what is supported by the results of our study. A recent meta-analysis of 3 randomized controlled trials to assess the efficacy of DES for the management of infrapopliteal arterial disease reported a significantly higher primary patency rate for DES compared with PTA or BMS at 1 year.26 There was no difference in major amputation rates; however, this is probably because of the fact that the individual studies included patients with intermittent claudication,13,14 in whom amputations are rare, or had follow-up in less than half of the cases.16 We observed a lower major amputation rate in the DES group, with a trend toward significance. Furthermore, our study demonstrates that DES reduce minor amputations. In a CLI population, major amputation is a serious threat.1,3 The severity and extensiveness of vascular disease in our patients are further reflected by the lower patency rates than anticipated in the sample size calculations. The patency and reintervention rates in our study are lower compared with studies that include patients with intermittent claudication.13,14
Till now, the experience with infrapopliteal paclitaxel-eluting stents is confined to small nonrandomized series that often included the use of various DES.27–31 Although some authors report higher infrapopliteal patency rates of sirolimus-eluting than paclitaxel-eluting stents, there are no randomized studies on this issue.13–16,26–29,32
Considering our relatively low complication rate, revascularization by means of endovascular techniques seems a justifiable treatment strategy in patients with CLI, who are in general elderly with substantial comorbidities and therefore less suitable for surgery.
Our study has some limitations.
Not all of our patients showed decreased ankle-brachial index and toe pressures, thereby not strictly meeting the hemodynamic criteria for CLI.1 This is probably because of the fact that >63% of our patients have diabetes mellitus, in whom ankle-brachial index values are known to be relatively elevated.1 Toe pressures have been thought to represent a more reliable parameter in CLI but a recent study has failed to confirm this. Optimal cut-off values remain to be established in diagnosing CLI.33 We think that the overall clinical picture of patients presenting with rest pain or tissue loss, and excluding patients with intermittent claudication, is the most important factor in properly selecting patients who need revascularization.
In 8 limbs, randomized for DES, 8 additional lesions were treated with PTA only, after treating the main lesion with DES. As a result, there is a difference between the primary end point in the MITT analysis and additional PP analysis.
Our primary end point was scored by means of CTA instead of digital subtraction angiography. CTA has been reported as an adequate tool for the assessment of arterial obstructions despite the fact that calcifications may hinder the assessment of stenosis rate.34 Because there were no lesions excluded from treatment in either group in our study because of severe calcifications, it is unlikely that treatment results have been influenced by unequal distribution of calcified lesions. In addition, digital subtraction angiography was considered too invasive and burdening for our elder and vulnerable study population.
In those cases when the index limb had been amputated or the patient had died because of progressive ischemia before 6-month follow-up, lesions were scored as treatment failures. In these cases, amputation or related death may also have been related to progression of disease elsewhere in the arteries supporting the affected limb. Patients who died because of unrelated causes were censored. Because these numbers of deceased patients are almost equal in both groups and consistent with previously reported high mortality rates among patients with CLI, it is unlikely that this has caused any bias.
In 13 patients, imaging at 6-month follow-up is not available. Patients with lower limb ischemia show high rates of loss to follow-up and low survival rates in longitudinal studies.1,16 Despite this challenging population, clinical follow-up was obtained in all our patients.
Finally, because this study is an investigator-initiated study, financial means were insufficient to provide patency analysis by means of a core laboratory. This was solved by assessing patency analysis independently by 2 board certified interventional radiologists.
Conclusions
In patients with CLI caused by infrapopliteal lesions, a treatment strategy with DES should be considered because they are associated with better patency and less amputations when compared with PTA±BMS, which is the current standard endovascular treatment.
Acknowledgments
We appreciate the effort of the clinical research coordinators in the participating centers.
Sources of Funding
The PADI trial received an unrestricted grant during the conduct of the study from The Netherlands Society for Interventional Radiology, who had no role in study design, data collection, analysis, interpretation, or writing of the report.
Disclosures
Dr Overhagen has received speaker’s fees from Cordis Corporation, Fremont, CA; Cook Medical, Bloomington, IN; and AngioDynamics, Latham, NY. The above are unrelated to the submitted work. The other authors report no conflicts.
Supplementary Material
Footnotes
Current address for J.M.M.: Department of Radiology, Rijnstate Hospital, Arnhem, The Netherlands.
The Data Supplement is available at http://circinterventions.ahajournals.org/lookup/suppl/doi:10.1161/CIRCINTERVENTIONS.114.002376/-/DC1.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American Association for Vascular Surgery/Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines. ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Associations for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease)–summary of recommendations. J Vasc Interv Radiol. 2006;17:1383–1397, quiz 1398. doi: 10.1097/01.RVI.0000240426.53079.46. doi: 10.1097/01.RVI.0000240426.53079.46. [DOI] [PubMed] [Google Scholar]
- 3.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H BASIL Trial Participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 4.Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, Strecker PK, Anderson MW, Jones DN, Whitehill TA, Moskowitz S, Krupski WC. Functional outcome in a contemporary series of major lower extremity amputations. J Vasc Surg. 2003;38:7–14. doi: 10.1016/s0741-5214(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 5.van Overhagen H, Spiliopoulos S, Tsetis D. Below-the-knee interventions. Cardiovasc Intervent Radiol. 2013;36:302–311. doi: 10.1007/s00270-013-0550-1. doi: 10.1007/s00270-013-0550-1. [DOI] [PubMed] [Google Scholar]
- 6.Peregrin JH, Koznar B, Kovác J, Lastovicková J, Novotný J, Vedlich D, Skibová J. PTA of infrapopliteal arteries: long-term clinical follow-up and analysis of factors influencing clinical outcome. Cardiovasc Intervent Radiol. 2010;33:720–725. doi: 10.1007/s00270-010-9881-3. doi: 10.1007/s00270-010-9881-3. [DOI] [PubMed] [Google Scholar]
- 7.Albers M, Romiti M, Brochado-Neto FC, De Luccia N, Pereira CA. Meta-analysis of popliteal-to-distal vein bypass grafts for critical ischemia. J Vasc Surg. 2006;43:498–503. doi: 10.1016/j.jvs.2005.11.025. doi: 10.1016/j.jvs.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Romiti M, Albers M, Brochado-Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975–981. doi: 10.1016/j.jvs.2008.01.005. doi: 10.1016/j.jvs.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Söderström MI, Arvela EM, Korhonen M, Halmesmäki KH, Albäck AN, Biancari F, Lepäntalo MJ, Venermo MA. Infrapopliteal percutaneous transluminal angioplasty versus bypass surgery as first-line strategies in critical leg ischemia: a propensity score analysis. Ann Surg. 2010;252:765–773. doi: 10.1097/SLA.0b013e3181fc3c73. doi: 10.1097/SLA.0b013e3181fc3c73. [DOI] [PubMed] [Google Scholar]
- 10.Haider SN, Kavanagh EG, Forlee M, Colgan MP, Madhavan P, Moore DJ, Shanik GD. Two-year outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43:504–512. doi: 10.1016/j.jvs.2005.11.016. doi: 10.1016/j.jvs.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Iida O, Soga Y, Kawasaki D, Hirano K, Yamaoka T, Suzuki K, Miyashita Y, Yokoi H, Takahara M, Uematsu M. Angiographic restenosis and its clinical impact after infrapopliteal angioplasty. Eur J Vasc Endovasc Surg. 2012;44:425–431. doi: 10.1016/j.ejvs.2012.07.017. doi: 10.1016/j.ejvs.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(suppl 10):S1–S42. doi: 10.1016/j.jacc.2010.06.007. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Rastan A, Tepe G, Krankenberg H, Zahorsky R, Beschorner U, Noory E, Sixt S, Schwarz T, Brechtel K, Böhme C, Neumann FJ, Zeller T. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274–2281. doi: 10.1093/eurheartj/ehr144. doi: 10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- 14.Scheinert D, Katsanos K, Zeller T, Koppensteiner R, Commeau P, Bosiers M, Krankenberg H, Baumgartner I, Siablis D, Lammer J, Van Ransbeeck M, Qureshi AC, Stoll HP ACHILLES Investigators. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290–2295. doi: 10.1016/j.jacc.2012.08.989. doi: 10.1016/j.jacc.2012.08.989. [DOI] [PubMed] [Google Scholar]
- 15.Falkowski A, Poncyljusz W, Wilk G, Szczerbo-Trojanowska M. The evaluation of primary stenting of sirolimus-eluting versus bare-metal stents in the treatment of atherosclerotic lesions of crural arteries. Eur Radiol. 2009;19:966–974. doi: 10.1007/s00330-008-1225-1. doi: 10.1007/s00330-008-1225-1. [DOI] [PubMed] [Google Scholar]
- 16.Bosiers M, Scheinert D, Peeters P, Torsello G, Zeller T, Deloose K, Schmidt A, Tessarek J, Vinck E, Schwartz LB. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–398. doi: 10.1016/j.jvs.2011.07.099. doi: 10.1016/j.jvs.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 17.Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, Jones DN. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 18.Martens JM, Knippenberg B, Vos JA, de Vries JP, Hansen BE, van Overhagen H PADI Trial Group. Update on PADI trial: percutaneous transluminal angioplasty and drug-eluting stents for infrapopliteal lesions in critical limb ischemia. J Vasc Surg. 2009;50:687–689. doi: 10.1016/j.jvs.2009.04.073. doi: 10.1016/j.jvs.2009.04.073. [DOI] [PubMed] [Google Scholar]
- 19.Tsetis D, Belli AM. The role of infrapopliteal angioplasty. Br J Radiol. 2004;77:1007–1015. doi: 10.1259/bjr/97382129. doi: 10.1259/bjr/97382129. [DOI] [PubMed] [Google Scholar]
- 20.Management of peripheral arterial disease (PAD). TransAtlantic Inter-Society Consensus (TASC). Section D: chronic critical limb ischaemia. Eur J Vasc Endovasc Surg. 2000;19(suppl A):S144–S243. [PubMed] [Google Scholar]
- 21.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 22.Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg. 1999;12:142–147. [PubMed] [Google Scholar]
- 23.Cwikiel W. Restenosis after balloon angioplasty and/or stent insertion - origin and prevention. Acta Radiol. 2002;43:442–454. doi: 10.1034/j.1600-0455.2002.430502.x. [DOI] [PubMed] [Google Scholar]
- 24.Drachman DE, Edelman ER, Seifert P, Groothuis AR, Bornstein DA, Kamath KR, Palasis M, Yang D, Nott SH, Rogers C. Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol. 2000;36:2325–2332. doi: 10.1016/s0735-1097(00)01020-2. [DOI] [PubMed] [Google Scholar]
- 25.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 26.Katsanos K, Spiliopoulos S, Diamantopoulos A, Karnabatidis D, Sabharwal T, Siablis D. Systematic review of infrapopliteal drug-eluting stents: a meta-analysis of randomized controlled trials. Cardiovasc Intervent Radiol. 2013;36:645–658. doi: 10.1007/s00270-013-0578-2. doi: 10.1007/s00270-013-0578-2. [DOI] [PubMed] [Google Scholar]
- 27.Siablis D, Karnabatidis D, Katsanos K, Diamantopoulos A, Christeas N, Kagadis GC. Infrapopliteal application of paclitaxel-eluting stents for critical limb ischemia: midterm angiographic and clinical results. J Vasc Interv Radiol. 2007;18:1351–1361. doi: 10.1016/j.jvir.2007.07.018. doi: 10.1016/j.jvir.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Feiring AJ, Krahn M, Nelson L, Wesolowski A, Eastwood D, Szabo A. Preventing leg amputations in critical limb ischemia with below-the-knee drug-eluting stents: the PaRADISE (PReventing Amputations using Drug eluting StEnts) trial. J Am Coll Cardiol. 2010;55:1580–1589. doi: 10.1016/j.jacc.2009.11.072. doi: 10.1016/j.jacc.2009.11.072. [DOI] [PubMed] [Google Scholar]
- 29.Rosales OR, Mathewkutty S, Gnaim C. Drug eluting stents for below the knee lesions in patients with critical limb ischemia: long-term follow-up. Catheter Cardiovasc Interv. 2008;72:112–115. doi: 10.1002/ccd.21557. doi: 10.1002/ccd.21557. [DOI] [PubMed] [Google Scholar]
- 30.Grant AG, White CJ, Collins TJ, Jenkins JS, Reilly JP, Ramee SR. Infrapopliteal drug-eluting stents for chronic limb ischemia. Catheter Cardiovasc Interv. 2008;71:108–111. doi: 10.1002/ccd.21367. doi: 10.1002/ccd.21367. [DOI] [PubMed] [Google Scholar]
- 31.Lookstein R, Ward T, Kim E, Fischman A, Nowakowksi F, Ellozy S, Teodorescu V, Vouyouka A, Faries P, Weintraub J. Value of drug-eluting stents after failed percutaneous transluminal angioplasty in the infrapopliteal vessels for the treatment of critical limb ischemia: favorable mid-term patency and limb salvage results. J Cardiovasc Surg (Torino) 2011;52:461–466. [PubMed] [Google Scholar]
- 32.Biondi-Zoccai GG, Sangiorgi G, Lotrionte M, Feiring A, Commeau P, Fusaro M, Agostoni P, Bosiers M, Peregrin J, Rosales O, Cotroneo AR, Rand T, Sheiban I. Infragenicular stent implantation for below-the-knee atherosclerotic disease: clinical evidence from an international collaborative meta-analysis on 640 patients. J Endovasc Ther. 2009;16:251–260. doi: 10.1583/09-2691.1. doi: 10.1583/09-2691.1. [DOI] [PubMed] [Google Scholar]
- 33.Stoekenbroek RM, Ubbink DT, Reekers JA, Koelemay MJ. Hide and seek: does the toe-brachial index allow for earlier recognition of peripheral arterial disease in diabetic patients? Eur J Vasc Endovasc Surg. 2015;49:192–198. doi: 10.1016/j.ejvs.2014.10.020. doi: 10.1016/j.ejvs.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 34.Schernthaner R, Stadler A, Lomoschitz F, Weber M, Fleischmann D, Lammer J, Loewe Ch. Multidetector CT angiography in the assessment of peripheral arterial occlusive disease: accuracy in detecting the severity, number, and length of stenoses. Eur Radiol. 2008;18:665–671. doi: 10.1007/s00330-007-0822-8. doi: 10.1007/s00330-007-0822-8. [DOI] [PubMed] [Google Scholar]


