Abstract
Introduction
reoperative sentinel lymph node biopsy (SLNB) is feasible in patients with local recurrence (LR) of invasive breast cancer, but it remains unclear if this procedure affects either treatment or outcome. Here we ask whether axillary restaging (versus none) at the time of LR affects the rate of subsequent events: axillary failure, non-axillary recurrence, distant metastasis or death.
Methods
we queried our institutional database to identify patients treated surgically for invasive breast cancer with a negative SLNB (1997–2000) who developed ipsilateral breast or chest wall recurrence as a first event. We excluded those with gross nodal disease at the time of LR. The cumulative incidence of subsequent events was estimated using competing risks methodology.
Results
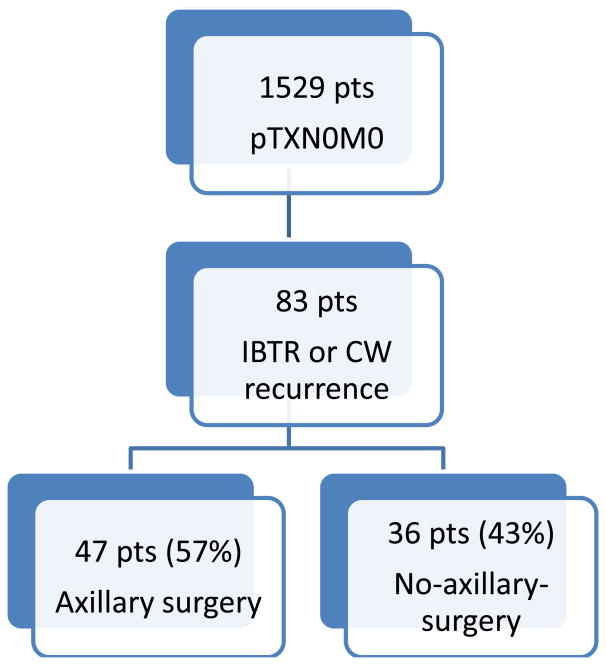
of 1527 patients with negative SLN at initial surgery, 83 had an ipsilateral breast (79) or chest wall recurrence (4) with clinically negative regional nodes. 47 (57%) were treated with and 36 (43%) without axillary surgery. Primary tumor characteristics were similar between groups, although time to LR was shorter in the no-axillary-surgery group (median 3.4 versus 6.5 years, p<0.05). All patients in the axillary surgery group and 94% of patients in the no-axillary–surgery group had surgical excision of their LR, and the use of subsequent radiation and systemic therapy was similar between groups. At a median follow-up of 4.2 years from the time of LR, the rates of axillary failure, non-axillary failure, distant metastasis and death were low and did not differ between groups.
Conclusions
among breast cancer patients with LR and clinically negative nodes, our results question the value of axillary restaging but invite confirmation in larger patient cohorts. Since randomized trials support the value of systemic therapy for all patients with invasive LR, reoperative SLNB, although feasible, may not be necessary.
Keywords: reoperative sentinel lymph node biopsy, local recurrence, breast cancer, axillary restaging
Introduction
Although management of the axilla in patients with isolated local recurrence (LR) of invasive breast cancer is not standardized, the rationale for axillary re-staging is the same as for the primary disease (prognostication, local control, and the possibility of a survival benefit) and the advantages of sentinel lymph node biopsy (SLNB) over ALND should be the same as well. We first reported “reoperative SLNB” in 20021 at a time when the putative contraindications to SLNB included previous axillary surgery; among 32 patients with prior axillary surgery (69% for breast cancer, 22% for failed SLNB or inadequate ALND, and 9% for prior axillary surgery unrelated to cancer) we found that reoperative SLNB was feasible, and was more likely to succeed if fewer than 10 nodes had been removed initially (87% success) vs 10 or more nodes (44% success). Reoperative SLNB for LR is now the subject of 25 additional reports (2004-2011)2, all with comparable results. For patients with LR as a first event, the promise of reoperative SLNB is that it might allow more accurate prognostication, more appropriate choice of local/systemic therapy, improved local control and better survival. Here we ask whether these promises have been fulfilled, by comparing two cohorts of patients with first-event LR, those who had axillary restaging vs those who did not. Clearly, reoperative axillary staging by SLNB is feasible but is it worthwhile?
Methods
Under a Waiver of Authorization from the Memorial Sloan Kettering Cancer Center Institutional Review Board, we reviewed a prospectively maintained database, identifying 1527 patients treated surgically for invasive breast cancer (January 1997 to December 2000) with a negative SLNB and with a median follow-up of 12 years. From these we identified 83 patients with invasive recurrence as a first event (79 in the ipsilateral breast and 4 in the postmastectomy chest wall) and clinically negative regional nodes; we compare the 47 patients (57%) who had reoperative SLNB with the remaining 36 (43%) who did not. We excluded patients with concomitant distant disease at the time of LR. SLNB was done following our standard protocol3, with preoperative lymphoscintigraphy and combined dye-isotope mapping.
Outcome measures were axillary failure (AF), non-axillary recurrence (NAR), distant metastasis, and death. AF was defined as gross nodal disease appearing subsequent to treatment for the LR. NAR was defined as recurrence in the remaining breast, CW, and non-axillary regional (including supraclavicular) nodes.
Statistical Analysis
Patient and treatment characteristics were summarized using median and range for continuous covariates, and frequency and percent for categorical covariates. We compared patients whose axillary LR were treated with and without axillary surgery using the Wilcoxon rank-sum and Fisher's exact tests. We used competing risks methodology to estimate the cumulative incidence of AF, NAR, distant metastasis and death. Time to event was defined as the time from LR to the first of AF, NAR, distant metastasis or death, censoring patients still alive and disease free at last follow-up. All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC) and R 3.1.1 (R Foundation, Vienna, Austria) using the survival and cmprsk packages. All p-values were two-sided and p-values <0.05 were considered significant.
Results
Comparing the 47 patients (57%) treated with axillary surgery to the 36 (43%) treated without axillary surgery (Fig. 1), there were no significant differences in median age (52 vs 52), primary tumor size (1.2 (0.2-2.5) vs 0.8 (0.1-3.7)), T stage (T1mi: 0% vs 6%, T1: 91% vs 89%, T2: 9% vs 6%), N stage (all were pN0), or tumor subtype (ER positive: 74% vs 74%, PR positive: 61% vs 65%, her2 positive: 22% vs 14%).
Fig. 1. Study population.
IBTR = ipsilateral breast tumor recurrence
CW = chest wall
The initial surgery was breast-conserving in 96% of patients with axillary restaging and 92% of those without axillary restaging, and the median times to LR were 6.5 years (1.1-15.4) and 3.4 years (0.6-14.6), respectively.
Treatment of the LR was comparable between groups (Table 1). There were no significant differences in the rates of surgical excision, chest wall RT, supraclavicular RT, chemotherapy, hormonal therapy, or anti-her2 therapy.
Table 1. Comparison of treatment given for invasive LR between patients treated with vs without axillary surgery.
| Treatment for invasive LR | Axillary procedure at time of LR (n=47) | No axillary procedure at time of LR (n=36) | |
|---|---|---|---|
| # (%) | # (%) | p-value | |
| Surgical excision | 47 (100%) | 34 (96%) | - |
| RT to chest wall | 0 (0%) | 2 (6%) | 0.18 |
| RT to supraclavicular nodes | 0 (0%) | 1 (3%) | 0.41 |
| Chemotherapy | 16 (36%) | 8 (24%) | 0.29 |
| Hormonal therapy | 22 (49%) | 13 (39%) | 0.40 |
| Anti-HER2 therapy | 1 (2%) | 1 (3%) | 1.000 |
LR = local recurrence
RT = radiotherapy
Event rates subsequent to the treatment of LR and with a median followup of 4.2 years were low (Table 2), with only 3 subsequent axillary LR, 4 NAR, 8 distant metastasis, and 6 deaths. Of 3 patients with subsequent axillary LR, 1 was in the axillary surgery group, relapsing at 6.6 years, and 2 were in the no axillary surgery group, relapsing at 0.7 and 1.1 years. Although 6 of 7 locoregional events were in the patients treated without axillary surgery, distant events and deaths were equally distributed between groups.
Table 2.
Comparison of subsequent event rates between patients treated for LR with vs without axillary surgery
| Event | Total events (n=83) | Axillary procedure at time of LR (n=47) | No axillary procedure at time of LR (n=36) | |||
|---|---|---|---|---|---|---|
| # events | 5-year post-LR event rate (95% CI) | # events | 5-year post-LR event rate (95% CI) | # events | 5-year post-LR event rate (95% CIl) | |
| Axillary failure | 3 | 2.6% (0.5-8.2) | 1* | 0.0 | 2 | 5.9% (1.0-17.4) |
| Non-axillary recurrence | 4 | 5.5% (1.8-12.6) | 0 | 0.0 | 4 | 12.8% (3.9-27.2) |
| Distant metastasis | 8 | 12.5% (5.7-22.1) | 5 | 14.7% (5.1–29.1) | 3 | 10.1% (2.4-24.1) |
| Death | 6 | 3.9% (0.7-12.1) | 2 | 4.3% (0.3–18.4) | 4 | 3.8% (0.3-16.5) |
this patient recurred at 6.6 years
Discussion
Locoregional recurrence affects 5–10% of patients treated for primary operable breast cancer4, and more than three-quarters of these occur in the ipsilateral breast/CW with clinically negative axillary nodes5. While management of the breast or CW is straightforward and typically includes surgical resection of the recurrence with or without RT, management of the axilla is not standardized and is largely unguided by current literature. Historically, patients with LR who had previously not undergone ALND were usually treated with resection of the recurrence and ALND. For primary breast cancer, an extensive literature has established SLNB as therapeutically equivalent and less morbid than ALND. Many reports now address the feasibility of reoperative SLNB in managing LR, where the advantages of SLNB over ALND should be the same.
A recent systematic review and meta-analysis by Maaskant-Braat et.al.2 of 25 studies (2004-2011) summarizes the experience with SLNB in 692 patients with LR (301 with a prior SLNB, 361 with prior ALND, and 30 with no prior axillary treatment), with results quite similar to our initial 2002 report. First, SLN were identified more often after SLNB than after ALND (81% vs 52%), for an overall success rate of 65%. Second, although a “backup” ALND was not done in all patients with negative SLN, there were no false-negatives among 63 SLN-negative procedures validated by a planned ALND, among whom a later axillary LR was reported in only one patient (0.2%). Third, the lymphatic drainage of the breast was altered by the prior axillary surgery; aberrant drainage to sites other than the ipsilateral axilla was observed more often after ALND than after SLNB (69% vs 17% among patients with successful lymphatic mapping, and 33% vs 14% among all patients). SLN were positive in 19% of patients, and of these 28% were in non-axillary sites. Finally, 9 studies reported that a reoperative SLNB changed treatment strategies in 25 of 239 patients (11%); the changes were specified in 15 patients and included contralateral ALND (n=6), altered systemic therapy (n=7) and RT to lymph node fields (n=2).
Although the above results imply that reoperative SLNB is worthwhile in all patients with invasive LR and clinically negative nodes, other lines of evidence suggest that it is not. First, in two reports summarizing NSABP trials of breast conserving surgery with systemic therapy, the 10-year rates of ipsilateral breast tumor recurrence (IBTR) were 6% for node-negative (B-13, 14, 19, 20, 23)6 and 9% for node-positive disease (B-15, 16, 18, 22, 25)7, and the 5-year overall survival rates from time of IBTR for node-negative and node-positive disease were 77% and 51%, respectively; this risk of mortality following IBTR appears sufficient to justify systemic therapy (or a change in systemic therapy) for all patients with LRR regardless of their node status at the time. Second, two randomized trials support systemic therapy following LRR. The Swiss SAKK trial (1982-1991)8 randomized 167 patients with “good risk” (largely ER-positive) isolated postmastectomy LRR, all of whom had excision and RT, to tamoxifen vs observation. They found a clear benefit for tamoxifen in 5 year disease free survival (DFS 59% vs 36%), and observed that median DFS in the tamoxifen arm was prolonged by 4.5 years. The more recent CALOR trial (2003-2010)9 randomized 162 patients with isolated postmastectomy or postlumpectomy LRR to chemotherapy vs no chemotherapy; all patients had excision of their LRR, RT was recommended for positive excision margins, and endocrine therapy was recommended for all patients with ER-positive disease. Both 5 year DFS and overall survival were significantly better in the chemotherapy arm (69% vs 57% and 88% vs 76%, respectively). The main benefit in 5 year DFS was for patients with ER-negative (67% vs 35%) rather than ER-positive disease (70% vs 69%), although the CALOR investigators appropriately cautioned that 5 year follow-up may have been insufficient to exclude a benefit in ER-positive disease as well. Finally, our own study is unable to demonstrate that initial tumor characteristics, treatment of LR or outcomes significantly differed between those patients who had axillary staging at the time of invasive LR and those who did not.
Our study has a number of limitations, including a retrospective design, disagreement among our surgeons regarding the benefit of reoperative SLNB, a low rate of LR overall and a low rate of subsequent events (80% of them non-axillary or distant) among our study population of 83 patients with invasive LR. We cannot completely explain the difference in time to LR between the patients who had axillary restaging and those who did not (6.5 vs 3.4 years). Although our study is among the largest to report on reoperative SLNB, our sample size is insufficient to exclude small differences, particularly in nodal recurrence.
These limitations also suggest the inherent difficulty of designing a randomized trial to study the role of axillary restaging at the time of invasive LR. The promise of reoperative SLN biopsy is that it might allow us to stratify risk among patients with invasive LR, tailoring the use of surgery, RT, and systemic therapy to the risk of subsequent local and distant events. In an era when rates of LR continue to decline and when treatment is increasingly dictated by tumor subtype rather than by nodal status, proof of benefit for reoperative SLNB will be ever more elusive. Going forward, it is no longer enough to show that we can restage the axilla in our patients with invasive LR (clearly, we can) or even to show that restaging changed treatment for a few patients with LR, unless we can also show that it changed outcomes. We welcome further study in larger patient cohorts but, on the basis of present evidence and our own experience, must conclude that restaging of clinically negative regional nodes in patients with invasive LR is of limited value, if any.
Synopsis.
Here we ask whether axillary re-staging for locally recurrent breast cancer is worthwhile and find comparable outcomes between patients who had restaging vs those who did not.
Footnotes
Disclosures: The authors have no disclosures to report. This study was presented in poster format at The American Society of Breast Surgeons' 16th annual meeting, 2015 , and was funded in part by the Breast Cancer Alliance and NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- 1.Port ER, Fey J, Gemignani ML, et al. Reoperative sentinel lymph node biopsy: a new option for patients with primary or locally recurrent breast carcinoma. J Am Coll Surg. 2002;195(2):167–172. doi: 10.1016/s1072-7515(02)01268-1. [DOI] [PubMed] [Google Scholar]
- 2.Maaskant-Braat AJ, Voogd AC, Roumen RM, et al. Repeat sentinel node biopsy in patients with locally recurrent breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2013;138(1):13–20. doi: 10.1007/s10549-013-2409-1. [DOI] [PubMed] [Google Scholar]
- 3.Cody HS, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol. 1999;8:85–91. doi: 10.1016/s0960-7404(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 4.Darby S, McGale P, et al. Early Breast Cancer Trialists' Collaborative G. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J.Clin. Oncol. 2009;27(15):2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis After Ipsilateral Breast Tumor Recurrence and Locoregional Recurrences in Five National Surgical Adjuvant Breast and Bowel Project Node-Positive Adjuvant Breast Cancer Trials. Journal of Clinical Oncology. 2006;24(13):2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 8.Borner M, Bacchi M, Goldhirsch A, et al. First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase III multicenter study comparing systemic treatment with observation after excision and radiation. Swiss Group for Clinical Cancer Research J Clin Oncol. 1994;12(10):2071–7. doi: 10.1200/JCO.1994.12.10.2071. [DOI] [PubMed] [Google Scholar]
- 9.Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;15(2):156–63. doi: 10.1016/S1470-2045(13)70589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]