Abstract
Impaired automatic emotion regulation (AER) is closely related to major depressive disorder. Our research in adults has identified two AER-related components, Go N2 and NoGo P3, in an implicit emotional Go/NoGo paradigm. However, it is unclear whether Go N2 and NoGo P3 reflect the development of AER in adolescents and the relationship of these components with subclinical depressive symptoms and trait anhedonia. We collected EEG data from 55 adolescents while they completed the implicit emotional Go/NoGo task. After the experiment, the subjects completed the Chinese version of the Temporal Experience of Pleasure Scale and the Beck Depression Inventory. Consistent with results in adults, we determined that Go N2 represents automatic top-down attention to emotions in Go trials, whereas NoGo P3 represents automatic response inhibition in NoGo trials. These AER components exhibited age-dependent improvement during adolescence. Additionally, NoGo P3 amplitudes elicited by viewing positive faces were positively correlated with trait anhedonia, whereas NoGo P3 amplitudes elicited by viewing negative faces were negatively correlated with depressive symptoms. Our observations provide further understanding of the neurodevelopmental mechanism of AER and yield new insight into dissociable impairments in AER in adolescents with major depressive disorder during positive and negative implicit processing.
Keywords: Adolescence, Anhedonia, Automatic emotion regulation, Depression, Go/NoGo
Highlights
-
•
We studied the development of automatic emotion regulation in adolescents.
-
•
Go N2 reflects automatic top-down attention to emotions in Go trials.
-
•
NoGo P3 reflects automatic response inhibition in NoGo trials.
-
•
NoGo P3 amplitudes of positive faces correlate positively with anhedonia.
-
•
NoGo P3 amplitudes of negative faces correlate negatively with depressive symptoms.
1. Introduction
Automatic emotion regulation (AER; Jackson et al., 2003), also called implicit (Koole and Rothermund, 2011) or unconscious (Williams et al., 2009) emotion regulation is pervasively used to control emotional responses in daily life, and its dysfunction has been implicated in the development of psychopathologies such as mood disorders or depression in adolescents (Rive et al., 2013). In the process model of emotion regulation (Gross and Thompson, 2007), AER can be conceptualized as the modification of any aspect of one's emotional response without conscious intent, without being aware of the emotion regulatory process and without attempting to deliberately control emotions (Mauss et al., 2007). However, measuring AER in experiments is very challenging for researchers. Fortunately, there have been several experimental strategies to garner evidence for AER. Specifically, the implicit paradigm presents emotional stimuli but requires subjects to execute a cognitive task that is not related to emotions. Thus, the experiment allows for inferences about AER-related processes by comparing emotional conditions with a non-emotional (neutral) condition (Koole and Rothermund, 2011).
Based on neuroimaging studies, Phillips et al. (2008) described a neural model of voluntary and automatic emotion regulation, which determined that AER mainly depends on the function of the ventromedial network, including the anterior cingulate cortex (ACC) (Phillips et al., 2008). However, neuroimaging studies did not reveal the accurate temporal course of AER. In contrast, event-related potential (ERP) studies with good temporal resolution can capture all levels of emotion-generation processes. By comparing the time course of different emotional stimuli, the researchers can isolate the AER-related components (Koole and Rothermund, 2011). For example, AER modulates the late positive potentials (Mocaiber et al., 2010, Zhang and Zhou, 2014) and alters two earlier ERP components, Go N2 and NoGo P3, during an implicit emotional facial Go/NoGo task (Zhang and Lu, 2012). However, these studies in adults did not reveal the developmental mechanism of AER in adolescents. In this study, we employed Go N2 and NoGo P3 as electrophysiological indicators to investigate the development of AER during adolescence and their links with depressive symptoms and anhedonia (Downar et al., 2014).
The Go/NoGo paradigm has been traditionally used to evaluate response inhibition related to the ACC function. However, in the experiment with an event-related design, participants need to switch between Go trials and NoGo trials. Therefore, the Go/NoGo task has possible response-switching components corresponding to different stages of information processing. In NoGo trials, frontal NoGo N2 and NoGo P3 amplitudes are greater than Go N2 and Go P3, respectively (Albert et al., 2010, Lamm et al., 2006). NoGo N2 is a negative shift with a peak between 200 and 400 ms following NoGo stimuli. NoGo P3 is a positive-going component that was evaluated in a time frame from 300 to 700 ms. Moreover, NoGo P3 is thought to directly reflect the inhibitory process itself (Albert et al., 2010, Spronk et al., 2008).
Particularly during the implicit emotional Go/NoGo task, NoGo P3 was modulated by affective facial valence in our adult study (Zhang and Lu, 2012), and the emotional valence was sufficient to evoke response tendencies (Chiu et al., 2008). In the NoGo task, emotional- and motor-response inhibition have been shown to coexist and coactivate some brain areas, including the ACC, that are associated with the interaction between emotional processing and motor inhibition (Goldstein et al., 2007, Berkman et al., 2009, Albert et al., 2011), which is observed in the P3 (but not in the N2) time range (Albert et al., 2011). Both voluntary inhibition of the motor response (button press) and implicit emotion regulation are additive and simultaneously reflected in the NoGo P3 waveform. Thus, NoGo P3 superimposes with automatic response inhibition of emotions (Zhang and Lu, 2012). In contrast, NoGo N2 amplitudes do not vary with emotional valence and represent only cognitive conflict monitoring (Albert et al., 2011). Therefore, NoGo N2 might not be linked to AER (Zhang and Lu, 2012).
The Go/NoGo paradigms with affect-loaded stimuli involve the interaction of emotion and attention (Blair et al., 2007) and provide new insight into the emotion-modulated executive attentional process (Albert et al., 2010, Albert et al., 2011, Hum et al., 2013). The motivated attention model proposes that affectively salient stimuli attract more attention resources than neutral stimuli (Lang et al., 1997). However, the resource theory assumes that the attention capacity is limited (Kahneman, 1973). With limited attention resources, participants have to intentionally or unintentionally exert attentional control over affective stimuli so that they can perform goal-directed behaviors (Blair et al., 2007). Thus, in the Go trials, there exists a process that is linked to the attentional control of emotions, which depends on the level of cognitive load (Pessoa et al., 2002). For instance, Go N2 is suggested to index the degree to which attentional control is demanded (Dennis and Chen, 2007). Specifically, during the implicit emotional Go/NoGo task, Go N2 amplitudes evoked by viewing emotional faces were significantly lower than those evoked by viewing neutral faces (Zhang and Lu, 2012). Generally, a stimulus-driven bottom-up process occurs within 200 ms. However, in the present study, Go N2 occurs after 200 ms. In addition, stimulus-driven attention cannot explain why Go N2 amplitudes for positive and negative faces are less negative than those for neutral faces. If Go N2 is driven by stimulus-driven attention, Go N2 amplitudes evoked by positive and negative faces should be more negative than those evoked by neutral faces. Therefore, we propose that Go N2 is altered by emotions and represents automatic top-down attention toward emotions (Zhang and Lu, 2012). In contrast, increased Go P3 amplitudes in response to positive and negative faces are in line with the viewpoint that Go P3 mirrors motivated attention (Knyazev et al., 2009, Schupp et al., 2004).
The affective Go/NoGo task can evaluate emotional response inhibition and may be useful for assessing major depressive disorder (MDD), characterized by abnormalities in deliberate or automatic emotion regulation (Chiu et al., 2008). Some findings stress the hyperactive negativity in MDD during negative affect processing (Joormann, 2010). For instance, previous studies employed explicit emotional Go/NoGo tasks where facial expressions of emotion had to be actively identified for successful performance (Han et al., 2012, Ladouceur et al., 2006). These studies found that adolescents with MDD exhibited faster reaction times to sad face Go trials embedded in neutral face NoGo trials in the moderate probability condition (Ladouceur et al., 2006). There is also an inverse correlation between depressive symptoms and reaction time to negative (angry) faces in the NoGo trials (Han et al., 2012). Undergraduates displaying a high degree of depressive symptoms exhibit larger NoGo P3 responses to negative than to positive stimuli (Krompinger and Simons, 2009). Although these results from the explicit emotional tasks disclosed negativity bias in volitional emotion regulation in depressed individuals, they did not unveil AER-related deficits in depression during implicit emotional processing.
Other studies propose that depression might originate from a reduction in positive affectivity (Davey et al., 2008, Treadway and Zald, 2011). Specifically, anhedonia is associated with a deficit in the reward-processing dopamine system, which increases the risk for depression (Liu et al., 2014). For example, using an oddball task with standard stimuli (O′s) and a deviant stimulus (X) at random times, Franken et al. (2006) found that early (70–125 ms), middle (125–175 ms) and late (300–500 ms) ERP components of subjects with a low hedonic tone were attenuated relative to subjects with a high hedonic tone. Particularly, depressed individuals exhibit reduced activation in the right ACC and caudate during reward anticipation compared to controls (Smoski et al., 2009). The positive bias in depressed individuals reduces as severity of anhedonia increases (Dunn et al., 2009). To our knowledge, however, only one ERP study has indicated that the amplitude of feedback negativity to gain feedback is related to the severity of anhedonia in depressed participants (Liu et al., 2014). Therefore, it remains unclear whether anhedonia is associated with AER in the implicit emotional Go/NoGo task.
During adolescence, continual pruning and myelination of the prefrontal cortex (Cloak et al., 2010) contribute to an increase in cortical efficiency later in the developmental process (Casey et al., 2000), including top-down mechanisms that are involved in AER, such as response inhibition (Rive et al., 2013). Thus, adolescents progressively improve their automatic inhibitory capacity for emotional processing (Wiers et al., 2007). However, the cortical function in adolescents is not fully mature because the limbic reward systems develop earlier than the prefrontal control regions (Casey et al., 2008). For example, adolescents exhibited exaggerated amygdala response to emotional expressions relative to children and adults during an emotional Go/NoGo task (Hare et al., 2008). Teenagers also had greater between-subjects ventral-dorsal striatal coactivation than children and adults for happy NoGo versus Go trials (Somerville et al., 2011), which implicates exaggerated ventral striatal representation of appetitive cues in adolescents relative to an intermediary cognitive control response (Casey et al., 2008).
The triadic model of motivated behaviors in adolescence assumes an immature supervisory role of the medial/ventral prefrontal cortex (implicated in AER) in orchestrating the contributions of the amygdala (the avoidant system) and ventral striatum (the approach system) in response to affective stimuli (Ernst et al., 2006). Thus, the development of the regulatory mechanisms lags behind the development of affective brain systems so that adolescents are more sensitive to affective stimuli and are more vulnerable to clinical depression than children and adults (Davey et al., 2008). For example, in an emotional Go/NoGo task, response inhibition is more readily disrupted by negative emotional distraction in early adolescence than at other ages (Cohen-Gilbert & Thomas, 2013). These results support the delayed development of inhibitory control. Moreover, adolescents with MDD exhibit enhanced subgenual ACC activation to fearful faces (versus neutral faces) during an implicit emotional face task (Tao et al., 2012). Therefore, clarifying developmental mechanisms in AER is of potential value for the clinical treatment of adolescent depression.
The present study aimed to examine the temporal course of AER and the association with subclinical depressive symptoms and trait anhedonia during adolescence. We collected ERP data of 55 adolescents as they performed the implicit emotional Go/NoGo task, in which they judged the gender of faces but ignored facial emotions. After the experiment, they completed the Chinese version of the Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006, Chan et al., 2012) and the Beck Depression Inventory (BDI; Beck et al., 1961, Liu et al., 2011). Our experiment was not a typical Go/NoGo task because we used an equal number of Go and NoGo trials to reduce novelty effects.
Based on AER results in adults (Zhang and Lu, 2012), we predicted that in the Go trials, N2 amplitudes evoked by seeing emotional faces would be reduced relative to amplitudes elicited by neutral faces (Dennis and Chen, 2007) and that in the NoGo trials, P3 amplitudes evoked by emotional faces would be increased compared with those evoked by neutral faces (Albert et al., 2010, Spronk et al., 2008). Considering increased cortical efficiency during adolescence (Casey et al., 2000, Wiers et al., 2007), we predicted that Go N2 amplitudes would decline with age and that NoGo P3 amplitudes would increase with age. According to previous results (Dunn et al., 2009, Han et al., 2012), we predicted that NoGo P3 amplitudes elicited by seeing positive faces would be positively correlated with anhedonia and that NoGo P3 amplitudes elicited by negative faces would be negatively correlated with subclinical depressive symptoms.
2. Methods
2.1. Participants
Fifty-five right-handed adolescent students participated in the experiment. They had normal or corrected-to-normal visual acuity and had no history of head injury or neurological disorders. They did not take any medication within two weeks. They were also required to report the grade point average (M = 3.62, SD = 0.43) to exclude any intellectual delays. The cut-off value of grade point average was set to 1.7, which is the equivalent of grade D. Pubertal status was measured by the Chinese self-report Pubertal Development Scale with good reliability and validity (Chan et al., 2010, Petersen et al., 1988). All adolescents, on average, were categorized into Tanner pubertal stage II-IV (from early to late adolescence) (Petersen et al., 1988). Data from one male and one female were ruled out due to the low-quality of the EEG recording. The final sample was divided into three age groups: younger adolescents (7 female and 11 male, ages 11.65 to 13.07 years), middle adolescents (6 female and 12 male, ages 13.78 to 14.82 years), and older adolescents (6 female and 11 male, ages 15.56 to 16.74 years). We first obtained verbal assent from each adolescent participant and then obtained written consent from their parents or guardians on behalf of the minors enrolled in our study. All participants were paid ~$10 for their participation. The ethics committee of the key laboratory of psychology at Shanghai Normal University approved the study protocol.
2.2. Psychological measure
2.2.1. Anhedonia
This study used a 20-item TEPS to measure individual trait disposition in anhedonia, i.e., anticipatory and consummatory pleasurable experiences in the long term (Gard et al., 2006). A lower total score on the scale indicates a higher level of anhedonia. The Chinese version of the TEPS has demonstrated adequate reliability in previous studies (Chan et al., 2012, Liu et al., 2014). The internal consistency of the TEPS in our study was good (Cronbach's alpha was 0.82).
2.2.2. Depressive symptoms
The BDI is a 21-item scale that evaluates the severity of depression (Beck et al., 1961). Recent research has indicated that the BDI is valid for adolescents (Ramezani et al., 2014) and the Chinese version used for the current study has been validated in Chinese samples (Liu et al., 2011). The Cronbach's alpha in the present sample was 0.83.
2.3. Stimuli
One hundred and sixty-two face pictures were selected from the Chinese Face Affective Picture System (CFAPS; Wang and Luo, 2005), with 54 positive (happy), 54 negative (angry and fearful) and 54 neutral (calm) faces. The stimuli consisted of equal numbers of female and male faces. Because there were only 20 angry faces and 20 fearful faces per gender in the CFAPS, we selected both angry and fearful faces as negative stimuli to match the number of positive and neutral stimuli. Although there was some neurophysiological evidence of the differential processing of fear and anger, we were interested in only the valence effect and not in specific affective processing. Three picture categories had no significant differences in arousal (M ± SD: Positive = 5.62 ± 0.74; Negative = 5.60 ± 0.71; Neutral = 5.26 ± 0.59; p > 0.05). However, they differed significantly from one another in the normative valence rating by the college students (M ± SD: Positive = 6.45 ± 0.28; Negative = 2.91 ± 0.27; Neutral = 4.57 ± .21; p < 0.001). No significant gender differences in arousal or valence were detected (p > 0.05). Participants viewed pictures presented at a visual angle of approximately 12 degrees in the center of a desktop screen. E-prime 2.0 (Psychology Software Tools Inc., Pittsburgh, USA) was used for both stimulus presentation and recording responses.
2.4. Procedure
After the participants became familiar with the experimental procedure, the sensors were attached to the participants in an electrically shielded room. The participants then executed an implicit emotional Go/NoGo task. The task contained equal numbers of Go and NoGo trials to control the novelty of the NoGo cues. Each trial began with a fixation (+) that appeared for 500 ms, followed by the Chinese cue “Female” or “Male” presented for 1000 ms. Then, a facial picture was randomly presented between 100 and 250 ms and the participants were asked to judge the facial gender. In the Go trial, where the facial gender matches the preceding cue, participants pushed the SPACE key using their index fingers. Participants were instructed to respond to the face as soon as possible but to ensure accuracy. In the NoGo trial, where the facial gender did not match the preceding cue, participants viewed the picture and did not need to press any buttons. The screen then went blank for a random period ranging between 800 and 1200 ms before the next trial began. The experiment consisted of 360 trials split into 30 randomized blocks. Each block covered 12 randomized trials [2 (trial category: Go and NoGo) × 2 (facial gender: female and male) × 3 (emotional valence: positive, negative, and neutral)]. The experiment included a 20 s resting baseline every 9 blocks. The first three blocks were used as practice and were excluded from data analysis.
2.5. Data processing and statistical analysis
Scalp EEG data were obtained from 64 Ag/AgCl electrodes (NeuroScan Inc., USA) mounted in a Quick-cap (conforming to the International 10–10 System) and referenced to the left mastoid. Vertical electro-occulogram was recorded from two electrodes fixed above and below the left eye, and the horizontal electro-occulogram was recorded from two electrodes fixed at the outer canthus of each eye. The impedance of EEG electrodes was kept at less than 5 kΩ using electrode gel. All EEG signals were sampled at 1 kHz and were band-pass filtered from 0.01 to 100 Hz. The data were visually inspected to remove eye movement and muscle artifacts. The continuous EEG signals were corrected for blink artifact using an ocular artifact reduction procedure (Semlitsch et al., 1986), re-referenced an average mastoid, and low-pass filtered at 30 Hz (24 dB/octave). The EEG data were segmented for each trial, spanning 200 ms prior to each picture onset to 1000 ms after the presentation of the face stimuli and were baseline corrected using the pre-stimulus period. Epochs with the amplitude over ± 100 μV at any site were excluded from averaging.
Averaged ERPs were individually calculated from correct trials for trial category, facial gender, and emotional valence. In agreement with the methods and previous research in adults (Rousselet and Pernet, 2011), we selected the following nine sites where the N2 and P3 are most pronounced (Zhang et al., 2006): three frontal sites, F3, Fz, and F4; three frontocentral sites, FC3, FCz, and FC4; and three central sites, C3, Cz, and C4. The N2 amplitude was evaluated as the mean activity from 200 to 350 ms after the stimulus onset. The P3 amplitude was analyzed as the mean activity in the time window from 350 to 700 ms (Zhang and Lu, 2012).
Initially, N2 and P3 amplitudes were evaluated separately with five-way repeated measures ANOVAs with trial category (Go and NoGo), emotional valence (positive, negative, and neutral), facial gender (male and female) and topographical site (frontal, frontocentral, and central) as within-subject factors and age (younger, middle, and older adolescents) as the between-subject factor. However, because no effect of facial gender or facial gender-related interactions was detected, we omitted the facial gender factor from subsequent ERP analyses for conciseness. We then focused our analyses on four-way repeated measures ANOVAs with trial category (Go and NoGo), topographical site (frontal, frontocentral, and central) and emotional valence (positive, negative, and neutral) as within-subject factors and age (younger, middle, and older adolescents) as the between-subject factor.
Three-way repeated measures mixed ANOVA with emotional valence × facial gender × age was conducted to analyze the response times of the correct Go trials. The accuracy (i.e., button presses in the Go trials and no responses in the NoGo trials) were investigated using a four-way repeated measures mixed ANOVA with trial category × facial gender × emotional valence × age. SPSS Version 20.0 General Linear Model software was used for statistical analysis of behavioral and ERP data. Statistical analyses were adjusted for variance nonsphericity using the Greenhouse–Geisser correction. All post-hoc analyses used the Bonferroni adjustment.
3. Results
3.1. Behavioral data
3.1.1. Response time
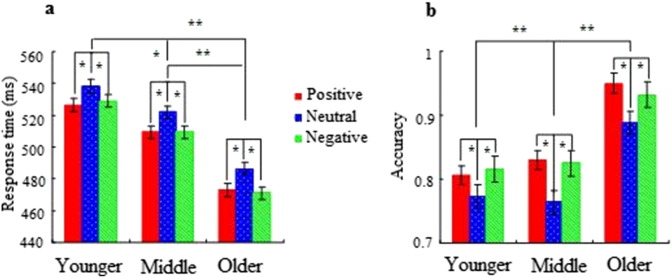
A three-way repeated measures mixed ANOVA revealed a significant main effect of emotional valence on the response time [F (2, 49) = 7.42, p < 0.01]. The post-hoc comparisons indicated that the mean response times to positive and negative faces were shorter than those to neutral faces (p < 0.05); however, no significant difference between positive and negative faces was present (p > 0.05). Furthermore, there was a significant main effect of age [F (2, 49) = 10.21, p < 0.001; Fig. 1a]. Post-hoc tests demonstrated that older adolescents responded to the three facial categories faster than middle adolescents (p < 0.05) and that middle adolescents responded to the three facial categories faster than younger adolescents (p < 0.01). No other main or interaction effects were observed (all p > 0.05).
Fig. 1.
Mean response time (RT) (a) and accuracy (b) elicited by viewing positive, neutral, and negative faces in correct Go trials in younger, middle, and older adolescents. Error bars denote the standard error. *p < 0.05, **p < 0.01.
3.1.2. Accuracy
The main effect of trial category revealed that all adolescents made more accurate responses in the NoGo trials than in the Go trials [F (1, 48) = 9.45, p < 0.001]. The four-way ANOVA analysis also revealed a significant interaction between trial category and emotional valence [F (2, 48) = 5.38, p < 0.01; Fig. 1b]. Post-hoc comparisons indicated that presentation of positive and negative faces resulted in higher mean accuracies than presentation of neutral faces in the Go trials (p < 0.05), although no significant difference was observed between positive and negative faces (p > 0.05). Furthermore, no significant difference in valence was observed in the NoGo trials (p > 0.05). Additionally, there was a significant interaction between trial category and age [F (2, 48) = 9.57, p < 0.001]. Post-hoc analyses revealed that older adolescents responded to the three facial categories more accurately than middle and younger adolescents in the Go trials (p < 0.01). However, no significant difference between different age groups was observed in the NoGo trials (p > 0.05).
3.2. ERP data
3.2.1. N2 amplitudes
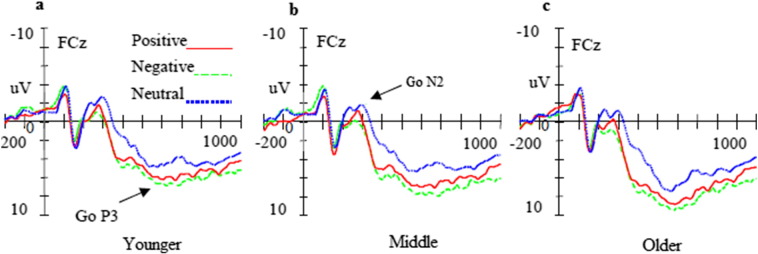
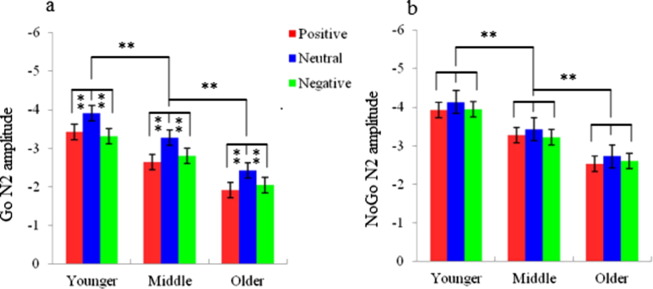
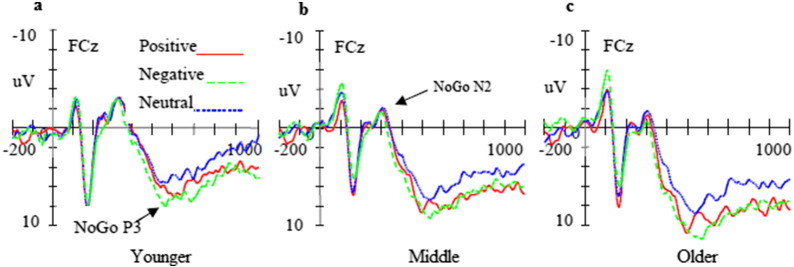
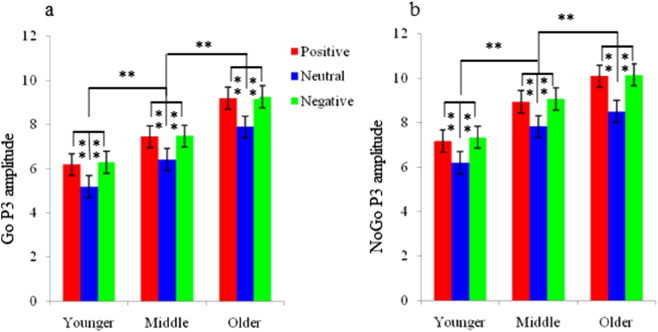
The main effect of trial category indicated that N2 amplitudes were larger in the NoGo trials than in the Go trials [F (1, 48) = 10.58, p < 0.001]. The main effect of topographical site showed that N2 amplitudes were largest at the frontocentral sites (for example FCz) [F (2, 48) = 11.63, p < 0.001]. Furthermore, the four-way repeated measures ANOVA demonstrated a significant main effect of age [F (2, 48) = 9.89, p < 0.001]. The post-hoc comparison indicated that N2 amplitudes in younger adolescents were more negative than those in middle adolescents (p < 0.01) and N2 amplitudes in middle adolescents were more negative than those in older adolescents (p < 0.01). Finally, a significant interaction between trial category and valence was observed [F (2, 48) = 9.69, p < 0.001]. Post-hoc tests demonstrated that presentation of positive and negative faces resulted in smaller N2 amplitudes than presentation of neutral faces in the Go trials (p < 0.01; Fig. 2, Fig. 4a). No differences in N2 amplitudes to the three emotional valences reached significance in the NoGo trials (p > 0.05; Fig. 3, Fig. 4b).
Fig. 2.
Averaged waveforms (μV) for neutral (blue dotted lines), positive (red solid lines), and negative pictures (green dashed lines) in Go trials in younger (a), middle (b), and older (c) adolescents. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Statistical analysis of N2 amplitudes elicited by viewing positive (red), neutral (blue) and negative (green) faces in Go (a) and NoGo (b) trials in younger, middle and older adolescents. Error bars denote the standard error. *p < 0.05, **p < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Averaged waveforms (μV) for neutral (blue dotted lines), positive (red solid lines), and negative pictures (green dashed lines) in NoGo trials in younger (a), middle (b), and older (c) adolescents. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.2. P3 amplitudes
The main effect of trial category demonstrated that P3 amplitudes in the NoGo trials were larger than those in the Go trials [F (1, 48) = 10.81, p < 0.001; Fig. 2, Fig. 3]. Moreover, ANOVA of P3 amplitudes revealed a significant main effect of emotional valence [F (2, 48) = 10.32, p < 0.001; Fig. 5a and b]. Post-hoc comparisons indicated that P3 amplitudes elicited by presentation of positive and negative faces were larger than those elicited by neutral faces (p < 0.01). Additionally, the main effect of topographical site revealed the largest P3 amplitudes at frontocentral sites [F (2, 48) = 10.63, p < 0.001]. We also observed a significant interaction between age and topographical site [F (4, 48) = 5.86, p < 0.01]. Post-hoc analyses indicated that P3 amplitudes at the frontal and frontocentral sites of older adolescents were larger than those of middle adolescents (p < 0.01), which, in turn, were larger than those of younger adolescents (p < 0.01). At the central sites, P3 amplitudes of middle and older adolescents were larger than those of younger adolescents (p < 0.01).
Fig. 5.
Statistical analysis of P3 amplitudes elicited by viewing positive (red), neutral (blue) and negative (green) faces in Go (a) and NoGo (b) trials in younger, middle and older adolescents. Error bars denote the standard error. *p < .05, **p < .01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Correlation analysis
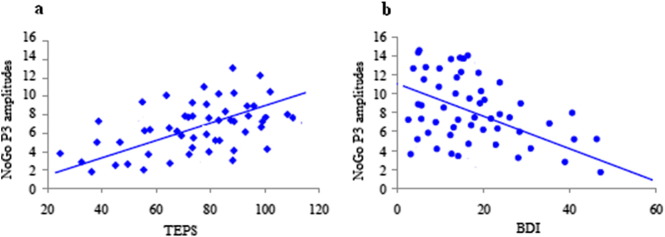
Pearson's correlation analyses revealed a negative correlation between Go P3 amplitudes and mean response time in the Go trials (r = −.47, p < .01). Particularly, response time was negatively correlated with the Go P3 amplitudes elicited by positive (r = − 0.49, p < 0.01) and negative (r = − 0.52, p < 0.01) faces but not those elicited by neutral faces (r = − 0.07, p > 0.05). When using Fisher z transformation, we did not find any significant differences in the correlation coefficient between positive and negative faces (p > .05). Furthermore, trait anhedonia measured by TEPS was positively correlated with NoGo P3 amplitudes elicited by positive faces (r = 0.38, p < 0.05; Fig. 6a) but not those elicited by negative and neutral faces (p > 0.05). Depressive symptoms were negatively correlated with NoGo P3 amplitudes elicited by negative faces (r = − 0.41, p < 0.01; Fig. 6b) but not those elicited by positive and neutral faces (p > 0.05). Specifically, after controlling for age, these correlation coefficients were still significant (all ps < 0.05). No other correlations were observed (all ps > 0.05).
Fig. 6.
Scatter plots depicting Pearson's correlation analyses. Panel a shows the positive correlation between NoGo P3 amplitudes elicited by viewing positive faces and anhedonia measured by the Temporal Experience of Pleasure Scale (TEPS). Panel b shows the negative correlation between NoGo P3 amplitudes elicited by viewing negative faces and depressive symptoms measured by the Beck Depression Inventory (BDI). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We applied the implicit emotional Go/NoGo paradigm to focus on the developmental electrophysiological indicators of AER in adolescents. Like the general non-affective Go/NoGo tasks, our results revealed greater NoGo N2 and NoGo P3 activation than Go N2 and Go P3 activation, respectively (Albert et al., 2010). Consistent with results in adults (Zhang and Lu, 2012), our findings in adolescents further confirmed two AER-related processes, Go N2 and NoGo P3, which varied as a function of age. The behavioral data also supported the ERP results. NoGo P3 amplitudes elicited by the presentation of positive and negative faces exhibited dissociable correlational patterns with trait anhedonia and subclinical depressive symptoms. Taken together, these results suggest that the implicit emotional Go/NoGo task is sensible to the development of AER, which might be closely associated with adolescent depression.
Consistent with results in adults (Zhang and Lu, 2012), we observed that emotional faces exhibited lower Go N2 amplitudes than neutral faces, although adolescents were required to judge the gender of emotional faces but to ignore facial emotions. On the basis of the motivated attention model that proposes that motivated stimuli attract more attention (Lang et al., 1997), researchers predicted that Go N2 amplitudes elicited by positive and negative faces should be more negative than those elicited by neutral faces. However, our Go N2 results did not support this prediction. Although it is possible that aspects of facial affect were coded to some degree consciously in our study, automatic processing of emotional information cannot fully rule out conscious awareness but requires minimal attentional resources because four features of automaticity, namely efficient, unintentional, uncontrollable, and unconscious, are not always tightly linked or activated in an all-or-none fashion (Teachman et al., 2012). In the implicit emotional Go/NoGo task, the ACC that projects extensively to other brain regions automatically presets the emotional processing network to carry out the instructed task so that emotional faces automatically consume less top-down attentional resources than neutral faces (Eimer and Holmes, 2007, Brown et al., 2007). Therefore, our results in adolescents further demonstrate that Go N2 reflects automatic top-down attention of the ACC to emotions. This explanation, however, should be considered with caution. Future studies are necessary to confirm these results using source analysis or the combination of fMRI and ERP. Moreover, consistent with increased cortical efficiency during adolescence (Casey et al., 2000, Wiers et al., 2007), Go N2 amplitudes exhibited age-dependent changes during adolescence. These observations suggest that Go N2 is a useful electrophysiological indicator for assessing the development of automatic top-down attention.
In contrast, subsequent Go P3 amplitudes elicited by viewing positive and negative faces were larger than those elicited by neutral faces. Furthermore, Go P3 amplitudes were negatively correlated with mean response time. Furthermore, the negative correlation between Go P3 amplitudes and reaction time was driven by the valence effect. Behaviorally, all adolescents made faster and more accurate responses to positive and negative faces than to neutral faces, which disrupts the speed-accuracy trade-off. In the explicit emotional Go/NoGo task, Tottenham et al. (2011) also found that adolescents were the fastest group for the emotional Go trials but not for neutral Go trials. Although we used the implicit emotional Go/NoGo paradigm, emotional stimuli were fully visible (i.e., not masked) to the subjects so that adolescents used emotional information to guide their responses somewhat consciously. However, our results were different from those of the prior explicit emotional Go/NoGo tasks (Tottenham et al., 2011) because the disruption of the speed/accuracy trade-off did not emerge in the NoGo trials in our study. Therefore, we did not fully rule out conscious leakage of affective information in our implicit study. According to the motivated attention model (Lang et al., 1997), Go P3 mirrors motivated attention to emotions and is not related to AER. Future studies should employ the implicit emotional Go/NoGo task with masked affective stimuli to confirm our results.
In NoGo trials, NoGo N2 was not influenced by affective valence, suggesting that NoGo N2 is not directly linked with AER. However, NoGo N2 might play a role in cognitive conflict monitoring (Albert et al., 2011). In our study, we expected to observe a response competition conflict but not an emotional conflict when participants were instructed to make a facial gender judgment and ignore facial emotions. In agreement with the non-emotional Go/NoGo paradigm (Jonkman, 2006), this conflict monitoring effect of NoGo N2 improves with age. In contrast, subsequent NoGo P3 was altered by emotional valence and thus superimposed automatic response inhibition, which is in line with NoGo P3 reflecting response inhibition during explicit emotional Go/NoGo tasks (Albert et al., 2010, Albert et al., 2011, Spronk et al., 2008). Moreover, this automatic response inhibition exhibited distinct interactions between age and topographical site. As age increased, automatic response inhibition of NoGo P3 was less dependent on the prefrontal function in adolescents. These findings are consistent with a protracted development of inhibitory control that continues well into adulthood (Tottenham et al., 2011). Additionally, neuroimaging studies have determined the importance of the ACC in response inhibition (Chiu et al., 2008). Taken together, these results suggest that NoGo P3 is an AER marker to evaluate the development of the automatic response inhibitory function of the ACC in adolescents.
Our observations support the dual process model of emotion regulation that determines an imbalance between heightened emotional processing in subcortical regions and less-effective prefrontal regulation during adolescence (Casey et al., 2008). Thus, the regulatory system (prefrontal cortex) in adolescents is immature and still develop into adulthood so that adolescents are more easily controlled by stimulus responses than adults (Casey et al., 2008, Ernst et al., 2006). However, studies in adolescents and adults did not find an interaction between emotional valence and age, inconsistent with the other Go/NoGo studies in adolescents (Cohen-Gilbert and Thomas, 2013, Tottenham et al., 2011). Our study used the equal Go/NoGo ratio, which reduces novelty effect on ERPs but also might reduce the inhibitory demands of the task where a high Go/NoGo ratio is used to induce a prepotent tendency. As a result, accuracy was higher in the NoGo trials, and the interaction between emotion and age did not emerge in our study.
During adolescence, exaggerated emotional reactivity, for example heightened activation of the amygdala, might increase the need for top-down control and put adolescents at a greater risk (Hare et al., 2008). Depressed adolescents exhibited impaired inhibitory control of the ACC during negative stimulus processing (Maalouf et al., 2012). Neuroimaging studies have reported greater subgenual ACC of depressed adolescents in response to fearful faces during an implicit emotional face task (Tao et al., 2012). In the present study, subclinical depressive symptoms were negatively associated with NoGo P3 elicited by negative faces. We propose that NoGo P3 amplitudes reflect automatic response inhibition. This finding implies that participants with higher BDI scores have more difficulty to automatically inhibit negative emotional response. Thus, hypoactive automatic response inhibition might result in the hyperactive negativity bias in participants with MDD (Joormann and Gotlib, 2010). In contrast, anhedonia is associated with abnormality in the reward system, for example in the ventral tegmental area, which projects densely to the ACC (Treadway and Zald, 2011). Decreased hedonic tone is associated with reductions in both automatic and effortful cognitive processing of relevant stimuli (Somerville et al., 2011). The reduction in positive affect is frequently observed in depressed adolescents (Davey et al., 2008). In line with these results, adolescents in our study exhibited a positive correlation between trait anhedonia and NoGo P3 amplitudes elicited by seeing positive faces, indicating that participants with higher anhedonia have more hyperactive automatic response inhibition, which might lead to reduction in positive affect.
Our results suggest that the different aspects of the task were differently related to depressive symptoms and anhedonia in our subclinical sample. Our sample is subclinical and their depressed states were probably temporary and did not reach the degree of clinical psychopathology. However, the depressed state in this population could worsen if the subjects were exposed to frustrating environmental stimuli or events, such as rejection by their peers (Davey et al., 2008). Taken together, our results suggest that depression in adolescents might be associated with impairment in AER-related positive and negative emotion regulation. Future studies should use clinical adolescent samples to further investigate this possibility.
In summary, our implicit emotional study extended AER-related results in adults into adolescents. Go N2 is modified by affective valence and represents automatic top-down control of emotions, whereas NoGo P3 has a direct link with automatic response inhibition. These AER-related ERP components show age-dependent improvement during adolescence. Moreover, adolescents exhibit the positive association between trait anhedonia and NoGo P3 amplitudes elicited by positive faces, whereas adolescents exhibit the negative association between depressive symptoms and NoGo P3 amplitudes elicited by seeing negative faces. These observations provide important insight into AER-related impairment mechanism in MDD during adolescence.
Acknowledgment
This study was granted by the National Natural Science Foundation of China (31470997 and 81171289).
References
- Albert J., Lopez-Martin S., Carretie L. Emotional context modulates response inhibition. Neural and behavioral data. NeuroImage. 2010;49:914–921. doi: 10.1016/j.neuroimage.2009.08.045. [DOI] [PubMed] [Google Scholar]
- Albert J., Lopez-Martin S., Tapia M., Montoya D., Carretie L. The role of the anterior cingulate cortex in emotional response inhibition. Hum. Brain Mapp. 2011;33:2147–2160. doi: 10.1002/hbm.21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Burklund L., Lieberman M.D. Inhibitory spillover: intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. NeuroImage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair K.S., Smith B.W., Mitchell D.G.V., Morton J., Vythilingam M., Pessoa L., Fridberg D., Zametkin A., Nelson E.E., Drevets W.C., Pine D.S., Martin A., Blair R.J.R. Modulation of emotion by cognition and cognition by emotion. NeuroImage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.R.G., Vilis T., Everling S. Frontoparietal activation with preparation for antisaccades. J. Neurophysiol. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan N.P.T., Sung R.Y.T., Nelson E.A.S., So H.K., Tse Y.K., Kong A.P.S. Measurement of pubertal status with a Chinese self-report pubertal development scale. Matern. Child Health J. 2010;14:466–473. doi: 10.1007/s10995-009-0481-2. [DOI] [PubMed] [Google Scholar]
- Chan R.C.K., Wang Y., Yan C., Zhao Q., McGrath J., His X., William S.S. A study of trait anhedonia in non-clinical Chinese samples: evidence from the Chapman Scales for physical and social anhedonia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P.H., Holmes A.J., Pizzagalli D.A. Dissociable recruitment of rostral anterior cingulated and inferior frontal cortex in emotional response inhibition. NeuroImage. 2008;42:988–997. doi: 10.1016/j.neuroimage.2008.04.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloak C., King G., Chang L., Alicata D., Sadino J., Ernst T. Imaging peri-adolescent pruning of the frontal cortex and cognitive maturation. Int. J. Dev. Neurosci. 2010;28:658–659. [Google Scholar]
- Cohen-Gilbert J.E., Thomas K.M. Inhibitory control during emotional distraction across adolescence and early adulthood. Child Dev. 2013;84:1954–1966. doi: 10.1111/cdev.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Yucel M., Allen N.B. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci. Biobehav. Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Dennis T.A., Chen C.C. Neurophysiological mechanisms in the emotional modulation of attention: the interplay between threat sensitivity and attentional control. Biol. Psychol. 2007;76:1–10. doi: 10.1016/j.biopsycho.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J., Geraci J., Salomons T.V., Dunlop K., Wheeler S., McAndrews M.P., Bakker N., Blumberger D.M., Daskalakis Z.J., Kennedy S.H., Flint A.J., Giacobbe P. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol. Psychiatry. 2014;76:176–185. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Dunn B.D., Stefanovitch I., Buchan K., Lawrence A.D., Dalgleish T. A reduction in positive self-judgment bias is uniquely related to the anhedonic symptoms of depression. Behav. Res. Ther. 2009;47:374–381. doi: 10.1016/j.brat.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M., Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken I.H.A., Van Strien J.W., Nijs H.M.T. Effect of hedonic tone on event-related potential measures of cognitive processing. Psychiatry Res. 2006;142:233–239. doi: 10.1016/j.psychres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Gard D.E., Gard M.G., Kring A.M., John O.P. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers. 2006;40:1086–1102. [Google Scholar]
- Goldstein M. Neural substrates of the interaction of emotional stimulus processing and motor inhibition control: an emotional linguistic go/nogo fMRI study. NeuroImage. 2007;36:1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- Gross J.J., Thompson R.A. Emotion regulation: Conceptual foundations. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 3–24. [Google Scholar]
- Han G., Klimes-Dougan B., Jepsen S., Ballard K., Nelson M., Houri A., Kumra S., Cullen K. Selective neurocognitive impairments in adolescents with major depressive disorder. J. Adolesc. 2012;35:11–20. doi: 10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional Go–NoGo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hum K.M., Manassis K., Lewis M.D. Neural mechanisms of emotion regulation in childhood anxiety. J. Child Psychol. Psychiatry. 2013;54:552–564. doi: 10.1111/j.1469-7610.2012.02609.x. [DOI] [PubMed] [Google Scholar]
- Jackson D.C., Mueller C.J., Dolski I., Dalton K.M., Nitschke J.B., Urry H.L., Rosenkranz M.A., Ryff C.D., Singer B.H., Davidson R.J. Now you feel it, now you don't: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol. Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Jonkman L.M. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood: a Go/Nogo ERP study. Brain Res. 2006;1097:181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Joormann J. Cognitive inhibition and emotion regulation in depression. Curr. Dir. Psychol. Sci. 2010;19:161–166. [Google Scholar]
- Joormann J., Gotlib I.H. Emotion regulation in depression: relation to cognitive inhibition. Cognition and Emotion. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D. Prentice-Hall; Englewood Cliffs, NJ: 1973. Attention and Effort. [Google Scholar]
- Knyazev G.G., Slobodskoj-Plusnin J.Y., Bocharov A.V. Event-related delta and theta synchronization during explicit and implicit emotion processing. Neuroscience. 2009;164:1588–1600. doi: 10.1016/j.neuroscience.2009.09.057. [DOI] [PubMed] [Google Scholar]
- Koole S.L., Rothermund K. “I feel better but I don't know why”: the psychology of implicit emotion regulation. Cognition and Emotion. 2011;25:389–399. doi: 10.1080/02699931.2010.550505. [DOI] [PubMed] [Google Scholar]
- Krompinger J.W., Simons R.F. Electrophysiological indicators of emotion processing biases in depressed undergraduates. Biol. Psychol. 2009;81:153–163. doi: 10.1016/j.biopsycho.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Williamson D.E., Birmaher B., Axelson D.A., Ryan N.D., Casey B.J. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. J. Child Psychol. Psychiatry. 2006;47:1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Lamm C., Zelazo P.D., Lewis M.D. Neural correlates of cognitive control in childhood and adolescence: disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B. Motivated attention: affect, activation, and action. In: Lang P.J., Simons R.F., Balaban M., editors. Motivated Attention: Affect, Activation, and Action. Erlbaum; Mahwah, NJ: 1997. pp. 97–135. [Google Scholar]
- Liu W., Chan R.C.K., Wang L., Huang J., Cheung E.F.C., Gong Q., Gollan J.K. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1045–1052. doi: 10.1016/j.pnpbp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Liu W., Wang L., Shang H., Shen Y., Li Z., Cheung E.F.C., Raymond C.K.C. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Maalouf F.T., Clark L., Tavitian L., Sahakian B.J., Brent D., Phillips M.L. Bias to negative emotions: a depression state-dependent marker in adolescent major depressive disorder. Psychiatry Res. 2012;198:28–33. doi: 10.1016/j.psychres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Mauss I.B., Bunge S.A., Gross J.J. Automatic emotion regulation. Personal. Soc. Psychol. Rev. 2007;8:220–247. [Google Scholar]
- Mocaiber I., Pereira M.G., Erthal F.S., Machado-Pinheiro W., David I.A., Cagy M., Maurício C., Eliane V., Letícia O. Fact or fiction? An event-related potential study of implicit emotion regulation. Neurosci. Lett. 2010;476:84–88. doi: 10.1016/j.neulet.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Pessoa L., McKenna M., Gutierrez E., Ungerleider L.G. Neural processing of emotional faces requires attention. PNAS. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13:829–833. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramezani M., Johnsrude I., Rasoulian A., Bosma R., Tong R., Hollenstein T., Harkness K., Abolmaesumi P. Temporal-lobe morphology differs between healthy adolescents and those with early-onset of depression. NeuroImage: Clinical. 2014;6:145–155. doi: 10.1016/j.nicl.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive M.M., Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhé H.G. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rousselet G.A., Pernet C.R. Quantifying the time course of visual object processing using ERPs: It's time to up the game. Front. Psychol. 2011;2:1–6. doi: 10.3389/fpsyg.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Cuhbert B.N., Bradley M.M., Hilllman C.H., Hanmm A.O., Lang P.J. Brain processes in emotional perception: motivated attention. Cognition and Emotion. 2004;18:593–611. [Google Scholar]
- Semlitsch P.A., Schuster P., Presslisch O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smoski M.J., Felder J., Bizzell J., Green S.R., Ernst M., Lynch T.R., Dichter G.S. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J. Affect. Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk M., Jonkman L.M., Kemner C. Response inhibition and attention processing in 5- to 7-year-old children with and without symptoms of ADHD: an ERP study. Clin. Neurophysiol. 2008;119:2738–2752. doi: 10.1016/j.clinph.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Tao R., Calley C.S., Hart J., Mayes T.L., Nakonezny P.A., Lu H., Kennard B.D., Tamminga C.A., Emslie G.J. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. Am. J. Psychiatr. 2012;169:381–388. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachman B.A., Joormann J., Steinman S.A., Gotlib I.H. Automaticity in anxiety disorders and major depressive disorder. Clin. Psychol. Rev. 2012;32:575–603. doi: 10.1016/j.cpr.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Casey B.J. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front. Psychol. 2011;2:1–9. doi: 10.3389/fpsyg.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Zald D.H. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Luo Y.J. Standardization and assessment of college students' facial expression of emotion. Chin. J. Clin. Psychol. 2005;13:396–398. [Google Scholar]
- Wiers R.W., Bartholow B.D., van den Wildenberg E., Thush C., Engels R.C.M.E., Sher K.J., Grenard J., Ames S.L., Stacy A.W. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol. Biochem. Behav. 2007;86:263–283. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Williams L.E., Bargh J.A., Nocera C.C., Gray J.R. The unconscious regulation of emotion: nonconscious reappraisal goals modulate emotional reactivity. Emotion. 2009;9:847–854. doi: 10.1037/a0017745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Lu J. Time course of automatic emotion regulation during a facial Go/Nogo task. Biol. Psychol. 2012;89:444–449. doi: 10.1016/j.biopsycho.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhou R. Individual differences in automatic emotion regulation affect the asymmetry of the LPP component. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.W., Xu J., Zhao L. Emotion regulation deficit in patients of late-onset depression revealed by ERP study. Space Medicine & Medical Engineering. 2006;19:150–153. [Google Scholar]