Abstract
The presence of cerebral lesions in patients with neurosensory alterations provides a unique window into brain function. Using a fuzzy logic based combination of morphological information about 27 olfactory-eloquent brain regions acquired with four different brain imaging techniques, patterns of brain damage were analyzed in 127 patients who displayed anosmia, i.e., complete loss of the sense of smell (n = 81), or other and mechanistically still incompletely understood olfactory dysfunctions including parosmia, i.e., distorted perceptions of olfactory stimuli (n = 50), or phantosmia, i.e., olfactory hallucinations (n = 22). A higher prevalence of parosmia, and as a tendency also phantosmia, was observed in subjects with medium overall brain damage. Further analysis showed a lower frequency of lesions in the right temporal lobe in patients with parosmia than in patients without parosmia. This negative direction of the differences was unique for parosmia. In anosmia, and also in phantosmia, lesions were more frequent in patients displaying the respective symptoms than in those without these dysfunctions. In anosmic patients, lesions in the right olfactory bulb region were much more frequent than in patients with preserved sense of smell, whereas a higher frequency of carriers of lesions in the left frontal lobe was observed for phantosmia. We conclude that anosmia, and phantosmia, are the result of lost function in relevant brain areas whereas parosmia is more complex, requiring damaged and intact brain regions at the same time.
Highlights
-
•
Brain lesion pattern were investigated by means of MRI techniques in 127 patients with a history of head trauma.
-
•
A fuzzy-logic based combination of morphological information about 27 olfactory-eloquent brain regions was analyzed.
-
•
Anosmia was associated with a negative function pattern, i.e., with damage, in particular of the olfactory bulb.
-
•
Parosmia, a distorted perception of odors, by contrast required intact brain regions along with damages.
1. Introduction
The human sense of smell is an important component of the quality of life (Croy et al., 2014, Doty et al., 1998, Hummel and Nordin, 2005, Merkonidis et al., 2015, Rinaldi, 2007) and has been recognized as an early symptom in neurological (Doty et al., 1988, Hawkes, 1996, Murphy et al., 1990) and psychiatric (Brewer et al., 2003, McCaffrey et al., 2000) diseases. Smelling involves a complex cerebral network of pathways extending from the first neuron located in the olfactory epithelium, where approximately 400 genes coding for olfactory receptors are expressed (Ache and Young, 2005, Gilad and Lancet, 2003, Verbeurgt et al., 2014), functionally concentrating in the olfactory bulb and from there spreading across the brain (Gottfried, 2006) to several regions activated by olfactory stimuli in humans (Gottfried, 2006, Savic, 2002).
Among olfaction-relevant brain regions, morphological dynamics have been observed (Gottfried, 2006). While volume losses in relevant gray matter (Bitter et al., 2010) or the olfactory bulb (Meisami, 1976, Mueller et al., 2005, Rombaux et al., 2009, Yousem et al., 1999) were accompanied by reversible anosmia, restoration of relevant brain areas such as the olfactory bulb was accompanied with the improvement of olfactory function (Gudziol et al., 2009, Royet et al., 2013). This suggests an association of brain lesion pattern with particular clinical pictures of olfactory dysfunction in humans.
In the present study, pattern of brain lesions was sought in patients who had presented with various olfactory dysfunctions which they associated with a head trauma in their medical history. The hypothesis was pursed that associations can be found among patterns of single lesions and specific olfactory dysfunctions such as anosmia, i.e., the complete loss of the sense of smell, parosmia, i.e., a distorted perception of an olfactory stimulus or phantosmia, i.e., an olfactory sensation in the absence of any physical odor.
2. Methods
2.1. Patients and study design
This cross-sectional retrospective study followed the Declaration of Helsinki; measurements were taken at the University of Louvain, Belgium. The enrolled cohort consisted of 143 patients (age 16–84 years, mean ± standard deviation 45.2 ± 14.6 years; 82 men) who had presented themselves at the Department of Otolaryngology at the Hospital Saint Luc, Brussels, Belgium, with the symptom “olfactory loss” which they associated with a head trauma which was dated back by the patients between 1 and 312 months (mean ± standard deviation 29.3 ± 36.3 months). Rhinological examination including nasal endoscopy was performed and a detailed structured history (Welge-Luessen et al., 2013) of the patients' olfactory acuity was taken. Subsequently, patients underwent a psychophysical olfactory test followed by the anatomical magnetic resonance imaging based assessment of the brain morphology with a focus on olfaction relevant.
2.2. Assessment of olfactory function
Patients were questioned about their olfactory function (Welge-Luessen et al., 2013) and particular symptoms such as parosmia or phantosmia were queried. Psychophysical olfactory testing was performed using a clinically established test battery (“Sniffin’ Sticks”; Burghart, Wedel, Germany (Hummel et al., 1997, Kobal et al., 1996)). This test is based on felt-tip pens that contain liquid odors instead of dye. The pen's cap was removed by the experimenter for approximately 3 s and the pen's tip was placed 1–2 cm in front of the nostrils. In the case of triplet pen presentation was at an interval of approximately 3 s. Specifically, odor thresholds were obtained for the rose-like odor phenyl ethyl alcohol presented in 16 successive 1:2 dilution steps starting from a 4% solution. Using a three-alternative forced-choice task and a staircase paradigm starting at low phenyl ethyl alcohol concentrations, one pen with the odorant and two blanks were presented at each dilution step. Two successive correct identifications or one incorrect identification triggered a reversal of the staircase. The odor threshold was estimated using the mean of the last four out of seven staircase reversals. The odor discrimination was determined with 16 triplets of pens, two of each triplet containing the same odor and the third a different, “target” one (for names of odorants see Hummel et al. (1997)). The discrimination performance was assessed employing a three-alternative forced-choice task (normal score > 10 correct discriminations for both sexes). The odor identification was determined with 16 odors (for names of odors see Hummel et al. (1997)) using a four-alternative forced-choice task with presentation of a list of four descriptors for each pen using different stimulus sequences for every measurement. The established evaluation of olfactory performance was based on the calculation of a composite “TDI score” (“Threshold Discrimination Identification”) as the sum of the scores from the three subtests (Wolfensberger et al., 2000). Established standard criteria of olfactory diagnosis were indicated by TDI ≤ 30.5, with the separation of hyposmia (30.5 ≥ TDI > 15.5) from functional anosmia, from here on termed “anosmia” at TDI ≤ 15.5 (Hummel et al., 2007).
2.3. Assessment of brain morphology
Cerebral lesions were analyzed bilaterally at 40 cerebral areas (Table 1) reportedly involved in the human central nervous processing of smell (Gottfried, 2006). Participants were examined on a 1.5 T MR imaging system (Signa EchoSpeed; GE Healthcare, Milwaukee, Wisconsin) using a standardized protocol including (i) a 5-mm-thick standard T2-weighted FSE image (TR = 2352 ms, TE = 80 ms). This covered the whole brain to rule out any organic brain disorder, (ii) 5-mm thick standard FSE fluid-attenuated inversion recovery images (TR = 10,000 ms, TE = 12 ms, TI = 2800 ms) covering the whole brain and 5-mm thick T2-weighted gradient echo images acquired using echo-planar imaging (TR = 1465 ms, TE = 16 ms) covering the whole brain to rule out the presence of any parenchymal or meningeal deposit, and (iii) 2-mm thick T1- and T2-weighted FSE images acquired in the coronal plane covering the anterior and middle segments of the base of the skull. The coronal T1-weighted FSE sequence was performed with parameters TR = 666 ms, TE = 10 ms, section thickness = 2 mm without intersection gap, FOV of 170 × 136 mm2, matrix of 288 × 184, resulting in a voxel size of 2 × 0.59 × 0.74 mm3. The coronal T2-weighted FSE sequence was performed with parameters TR = 1737 ms, TE = 90 ms, section thickness = 2 mm without intersection gap, FOV, 170 × 136 mm2, matrix of 376 × 299 resulting in a voxel size of 2 × 0.45 × 0.45 mm3.
Table 1.
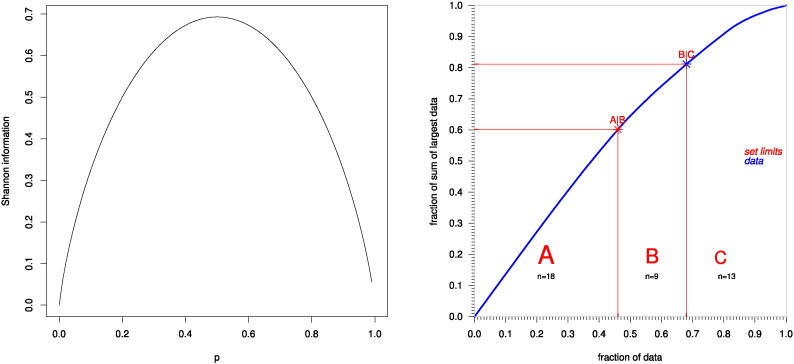
Brain regions selected based on prior knowledge (Gottfried, 2006) about their importance for the human sense of smell. The table lists the number of observations per brain region, the number of patients in whom the respective region showed lesions, and the Shannon information, calculated as Info(BRi) = - p0 , i ∙ ln (p0 , i) - p1 , i ∙ ln (p1 , i) , where p0,i and p1,i are the observed probabilities of the non-observation or observation, respectively, of a brain lesion obtained as p0,i = Ndamaged / N and p1,i = 1 − p0,i.
| Region | N | N damaged | Shannon info |
|---|---|---|---|
| Amygdala L | 127 | 11 | 0.29463346 |
| Amygdala R | 127 | 13 | 0.33024248 |
| Anterior insula L | 127 | 13 | 0.33024248 |
| Anterior insula R | 127 | 16 | 0.37868101 |
| Olfactory bulb L | 87 | 58 | 0.63651417 |
| Olfactory bulb R | 87 | 57 | 0.64418578 |
| Entorhinal cortex L | 127 | 57 | 0.68789898 |
| Entorhinal cortex R | 127 | 40 | 0.62301241 |
| Frontal cortex L | 127 | 55 | 0.68416121 |
| Frontal cortex R | 127 | 61 | 0.69237198 |
| Hypothalamus L | 127 | 18 | 0.40809566 |
| Hypothalamus R | 127 | 17 | 0.39365585 |
| Lateral orbitofrontal cortex L | 127 | 59 | 0.69063407 |
| Lateral orbitofrontal cortex R | 127 | 64 | 0.69311618 |
| Medial insula L | 127 | 14 | 0.34700893 |
| Medial insula R | 127 | 12 | 0.31280067 |
| Medial orbitofrontal cortex L | 127 | 75 | 0.67665731 |
| Medial orbitofrontal cortex R | 127 | 77 | 0.67037474 |
| Mesial temporal lobe L | 127 | 33 | 0.57289213 |
| Mesial temporal lobe R | 127 | 23 | 0.47306094 |
| Occipital cortex L | 127 | 6 | 0.19031927 |
| Occipital cortex R | 127 | 11 | 0.29463346 |
| Pallidum L | 127 | 10 | 0.27568156 |
| Pallidum R | 127 | 9 | 0.25587365 |
| Parietal cortex L | 127 | 10 | 0.27568156 |
| Parietal cortex R | 127 | 13 | 0.33024248 |
| Perirhinal cortex L | 127 | 58 | 0.68939147 |
| Perirhinal cortex R | 127 | 40 | 0.62301241 |
| Piriform cortex L | 127 | 33 | 0.57289213 |
| Piriform cortex R | 127 | 31 | 0.55575378 |
| Posterior insula L | 127 | 2 | 0.08099408 |
| Posterior insula R | 127 | 4 | 0.13990478 |
| Striatum L | 127 | 2 | 0.08099408 |
| Striatum R | 127 | 3 | 0.11181897 |
| Temporal lobe L | 127 | 27 | 0.5173791 |
| Temporal lobe R | 127 | 29 | 0.53727061 |
| Thalamus L | 127 | 1 | 0.04598614 |
| Thalamus R | 127 | 1 | 0.04598614 |
| Temporal lobe pole L | 127 | 63 | 0.69311618 |
| Temporal lobe pole R | 127 | 49 | 0.6668447 |
The degree of regional brain damage was quantified using a four-point ordinal scale [0…3]. Absent damage was attributed a value of 0, moderate damage a value of 1 (lesion clearly present; extent very small), medium damage a value of 2 and severe damage a value of 3 (lesion clearly present; large extent). Grading was done by experienced observers and inter-observer incongruences were discussed until a joint decision was reached.
2.4. Data analysis
The analyses were performed using the R software package (version 3.2.1 for Linux; http://CRAN.R-project.org/). More detailed descriptions are provided in the Supplementary Materials. In brief, neuroimaging data from 135 patients was available for the 40 different brain regions (Table 1). However, eight patients with missing data from 8 to 34 regions were excluded, so that the brain morphology data set further analyzed consisted of four matrices of dimension 127 × 40 for each of the four neuroimaging techniques, i.e., “flair”, “Epi”, “SWI” and “T2”. Each cell contained a number of 0, 1, 2 or 3 quantifying the degree of brain damage diagnosed with the respective technique. The brain lesion information was combined, per matrix cell, using the fuzzy logic “OR”. A final 127 × 40 matrix was generated that contained the maximum of the respective cell across the four original matrices. This corresponded to the clinical routine where each technique has its advantages, however, a negative finding with one but not another technique will not be diagnosed as healthy but interpreted as a lower diagnostic performance of the first technique, and the clinical interpretation will be based on the positive finding.
Damages per regions were found in 1 to 77 patients (0.8–99.2% of the cohort; Table 1). Nor-informative regions, i.e., those showing lesions in close to all or in none of the patients were excluded based on the Shannon information criterion (Shannon, 1951) computed as Info(BRi) = - p0 , i ∙ ln (p0 , i) - p1 , i ∙ ln (p1 , i) , where BRi denotes the ith brain region and p0,i and p1,i are the observed probabilities of the non-observation or observation, respectively, of a brain lesion (Fig. 1). The precise limit of the Shannon information up to which a brain region could be regarded as containing a sufficiently informative distribution of lesions among the patients was calculated by means of a computed ABC analysis (Ultsch and Lötsch, 2015) which is a categorization technique originally developed for problems in economics (Juran, 1975, Pareto, 1909) to search for a subset with minimum possible effort that gives the maximum yield, aiming at dividing a set of positive data into three disjoint subsets called “A”, “B” and “C”. Subsets “A” and “B” comprise profitable values, i.e., “the important few”, whereas subset “C” comprises non-profitable values, i.e., “the trivial many”.
Fig. 1.
Graph of the Shannon information and ABC analysis of the information content provided by the observed number of patients with lesions, relative to all observations, in a particular brain region. Left: Graph of the Shannon information (Shannon, 1951) depicting its formula as Info(BRi) = - p0 , i ∙ ln (p0 , i) - p1 , i ∙ ln (p1 , i) , where p0,i and p1,i are the observed probabilities of the non-observation or observation, respectively, of a brain lesion. Right: ABC plot of the cumulative distribution function of the Shannon information per brain region (blue line).The limits of sets A, B and C resulting from the present ABC analysis are drawn as red lines. For further details about an ABC analysis, see Ultsch and Lötsch (2015). The 13 brain regions assigned to ABC set “C” (Shannon information < 0.32, see Table 1) were not further analyzed because of providing low information judged by the ABC analysis as “trivial”. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Following ABC analysis, 27 brain regions were identified that, by precisely computed criteria, conferred sufficiently informative distributions of lesions for subsequent analysis. To this end, the 127 × 27 matrix was submitted to hierarchical cluster analysis aimed at identifying functional pattern shared among patients with similar olfactory dysfunction. The obtained cluster structure that subgrouped the patients was subsequently interpreted by extracting decision rules, in terms of the degree of lesion per brain area, from a classification and regression tree (CART) classifier (Breimann et al., 1993). The obtained tree model was cross-validated using a leave-k-out approach, where k was a randomly picked tenth of the total sample and the tree models were built 100 times with the respective remaining data. Subsequently, associations of brain lesion pattern with the presence of olfactory dysfunctions, i.e., anosmia, parosmia or phantosmia, were analyzed by means of Wilcoxon signed rank (Wilcoxon, 1945) or Kruskal-Wallis (Kruskal and Wallis, 1952) and χ2 tests.
3. Results
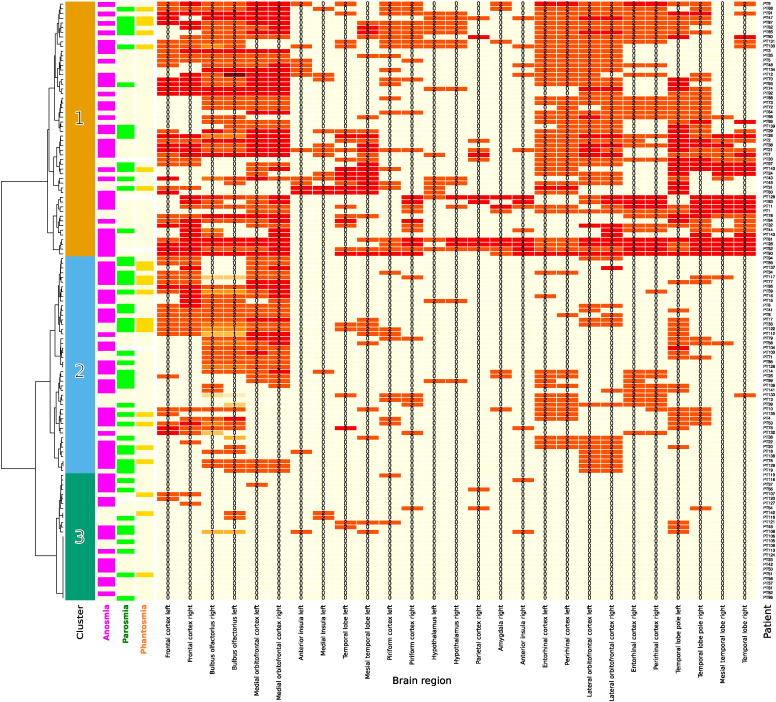
The data set resulting from a fuzzy “OR” combination of the brain lesion grading found in the brain images acquired with four different techniques, i.e., “flair”, “Epi”, “SWI” and “T2” images, consisted of a 127 patients × 40 brain regions matrix. Thalamic lesions were seen only in each one patient at the left or right sides, whereas many patients had lesions at the left and/or right medial orbitofrontal cortices (77 or 75 of 127, respectively). Values of the Shannon information below a threshold of 0.32 had been identified in a calculated ABC analysis as belonging to the subset (C) of information content, which can be considered as “trivial” (Table 1). Following exclusion of the uninformative regions, the data set submitted to further analysis consisted of a 27 × 127 matrix of combined (fuzzy “OR”) brain lesion gradings (Fig. 2). Of these 71 men and 56 women, aged 16–84 years (mean ± standard deviation: 45 ± 14.4 years), 81 patients were anosmic, 44 were hyposmic and only two were normosmic. Twenty-two patients reported symptoms of phantosmia and 50 patients had parosmia.
Fig. 2.
Matrix heat plot with hierarchical cluster dendrogram (left margin). The degree of brain damage is given as a number in each cell and as he cells' color code (from light yellow to (dark) red). The plot provides an overview of the brain lesion pattern of the present patients (rows, n = 127, patients ID codes shown at the right). The brain regions that had provided sufficient information to pass the ABC analysis of the Shannon information are shown at the abscissa (columns, n = 27). The dendrogram at the left shows the result of Ward hierarchical cluster analysis group the patients for brain lesion pattern. Three clusters emerged (color bar next right of this dendrogram; cluster numbers indicated). In addition, observations of olfactory dysfunction are shown as colored lines at the succeeding bars. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
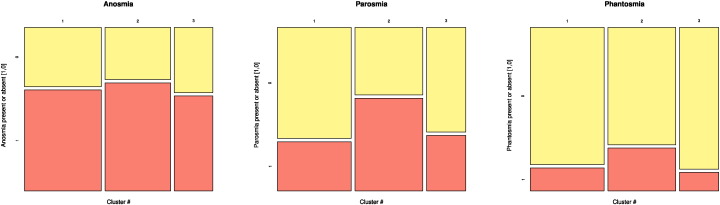
Three clusters of patients with different brain lesion pattern (dendrogram at the left of Fig. 2) were identified by a clustering approach, with cluster sizes of n = 54, 46 and 27 for clusters 1 through 3, respectively (cluster numbers arbitrarily assigned). The clusters differed with respect to the mean degree of brain lesions (Kruskal–Wallis test: χ2 = 107.4, df = 2, p < 2.2 · 10− 16). Exploration of the distribution of olfactory dysfunctions across the three clusters (Fig. 3) suggested a higher prevalence of parosmia and phantosmia in cluster #2, i.e., the cluster with medium overall brain damage (see Fig. 2). The cluster association was statistically significant for parosmia (χ2 test of the patients' cluster membership versus olfactory dysfunction: χ2 = 7.80, df = 2, p = 0.02) whereas the distributions of anosmia and phantosmia were not significantly different among clusters (χ2 = 0.51, df = 2, p = 0.77, and χ2 = 3.43, df = 2, p = 0.18, respectively).
Fig. 3.
Mosaic plot showing the distribution of olfactory dysfunctions, anosmia, parosmia, and phantosmia, among the patients' clusters. Clusters are drawn along the abscissa (1, 2, 3) while the presence (“1”) or absence (“0”) of the olfactory dysfunction are drawn along the ordinates. The width of each cell is proportional to the number of patients it comprises.
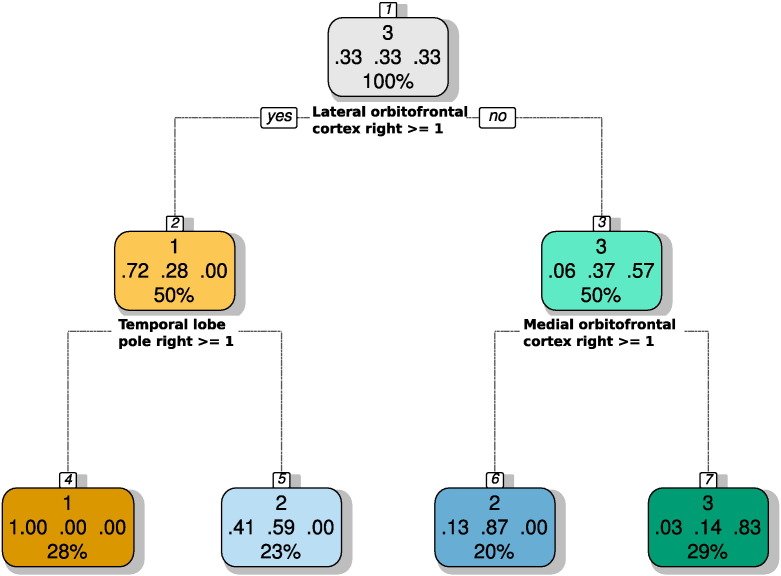
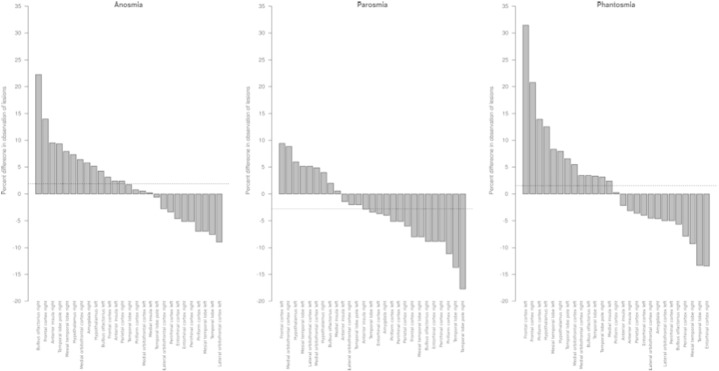
CART analysis (Fig. 4) indicated that cluster #2 comprised patients with either a lesion in the medial but not the lateral orbitofrontal cortex or a lesion in the lateral orbitofrontal cortex but not in the temporal lobe pole, all at the right hemisphere. An involvement of an intact right temporal lobe pole in parosmia was supported by an analysis of the relative percentages of lesions found in the 27 analyzed brain regions in patients with versus without olfactory dysfunctions. Specifically, in patients with parosmia, the most pronounced percent difference in carriers or non-carriers of a lesion was found for the right temporal lobe where lesions were less often found in patients with parosmia than in patients without parosmia (χ2 = 3.8717, df = 1, p = 0.0491). This negative direction of the differences was unique for parosmia. In anosmic patients (Fig. 5), the region with most pronounced differences between carriers and non-carriers was the right olfactory bulb, however, lesions in that region were much more frequent in anomics than in patients with preserved sense of smell (χ2 = 6.04, df = 1, p = 0.01). Similarly, in patients with phantosmia, the most pronounced difference was a higher frequency of lesions in the left frontal lobe (χ2 = 7.2706, df = 1, p = 0.0070091).
Fig. 4.
Classification and regression tree (CART) showing the hierarchical criteria of the patients' cluster assignment (for clusters, see Fig. 2) based on their brain lesion pattern. When the condition noted at each decision node applies, the tree is followed at the respective path down. At the end of such path, the cluster is indicated. The model provided cluster assignment at a cross-validated accuracy of 82.5%. Nodes representing patients classified by the tree as belong to the three clusters are cored in orange, blue and green analogously to the cluster coloring in Fig. 2. Three numbers in the middle of the nodes display the proportion of individuals in that node that really belonged to cluster #1, #2 or #3. Therefore, the color gradient is a visual representation of these numbers, with more intense colors with more correct classifications. At the bottom of each note the percentage of subjects belonging to this node from all subjects in the cohort is given (rounded to integer). The plot of the tree was obtained using the “fancyRpartPlot” function of the R package “rattle” (G. Williams; https://cran.r-project.org/web/packages/rattle/index.html (Williams, 2011)).
Fig. 5.
Percent differences in observations of brain damages. The columns indicate the relative percentages of patients presenting a lesion at a particular brain area versus patients not presenting a lesion, stratified (panels) for olfactory dysfunctions. Values > 0 indicate that more patients with the olfactory symptom had lesions than those without that symptom. The dotted horizontal lines indicate the mean of the differences. This significantly differed toward a negative value for parosmia (one-sided t-test versus a mean of 0: t = − 2.094, df = 26, p = 0.04616), i.e., patients with parosmia had comparatively more intact brain regions in a cohort were every member had a brain lesion in at least one of the olfaction relevant areas.
4. Discussion
The present comprehensive exploration of lesions in 27 olfaction relevant brain regions emphasized the importance of a pattern of damages for specific olfactory dysfunctions emphasizing an importance of both, the presence or the absence of regional damages in olfactory pathologies. While anosmia was a negative symptom that was associated with the damage of the olfactory bulb, the occurrence of parosmia required, by contrast, also intact parts of the brain, most pronounced but not exclusively identified to involve the temporal lobe. As the analysis employed a data-driven exploratory and deductive approach (Lötsch and Geisslinger, 2010) rather than a hypothesis driven concept, except that the selection of brain areas used prior knowledge to include a most complete set of regions associated with the processing and perception of olfactory input, its results required the judgment of their biological plausibility, which will be discussed in the following. It has also to be pointed out that the functional associations were possible with the presently observed lesion pattern that had been attributed by the patients to a specific trauma, however, the brain structure might have changed, i.e., partly recovered or additionally damaged, due to other reasons in the 1–312 moths interval between the trauma and the present olfactory/MRI data acquisition.
Firstly, the analysis clearly emphasized the highly significant role of the olfactory bulb for the sense of smell. As recently where in an analysis of the brain lesion pattern following head trauma identified an algorithm for the diagnosis of posttraumatic olfactory loss in which a lesion in the right olfactory bulb was the first and most important decisive MRT finding associated with anosmia (Lötsch et al., 2015), a damage in the right olfactory bulb was again the most prominent finding associated with complete olfactory loss. However, as previously, damage in the olfactory bulb did not completely explain anosmia, hence, further lesions contributed to anosmia in the present cohort. Indeed, while olfactory loss is frequent after brain injury (Sumner, 1964) a contribution of a rupturing of the olfactory nerves to the olfactory loss (Delank and Fechner, 1996) cannot be excluded in the present data as morphological information about the olfactory nerve was not accessible. However, other etiologies such as viral or sinu nasal diseases have been excluded by a thorough anamnestic/clinical work-up of the patients.
Secondly, parosmia emerged as a symptom requiring in addition to damages in certain brain regions the absence of lesions, specifically, a lower prevalence of damage in the temporal lobe. The requirement of a partly intact olfactory system is consistent with the definition of parosmia as an olfactory dysfunction that is characterized by the inability of the brain to properly identify an odor's “natural” smell (Bonfils et al., 2005), which implies the ability to perceive odors physically present in contrast to phantosmia where odors are perceived in the absence of an odor source. According to the present CART classifier, parosmia required both intact and damaged brain areas, specifically, either an undamaged lateral orbitofrontal cortex or an undamaged temporal lobe pole, whereas damages were needed in either the medial or lateral orbitofrontal cortices, however, not in both at the same time. This suggests the origin of the distorted odor perception in a partly intact olfactory system that displays a damage pattern compatible with the hypothesis of hampered interplay among its components. The main role seems to play an undamaged right temporal lobe pole, which characterized a narrow majority of the patients belonging to the brain lesion pattern cluster with the highest prevalence of parosmia. The temporal lobe pole plays a not yet completely but probably important role in the central processing of olfactory information. A role of the temporal lobe was seen as “linking odor object representations to transmodal networks, given its anatomical proximity to olfactory and visual object processing areas” (Kasai et al., 2003, Olofsson et al., 2013, Olson et al., 2007). Lesions induced by radiation have been found to be associated with decreased olfactory function (Leyrer et al., 2014) and deficits of the temporal lobe pole in primary progressive aphasia were related to severe deficits in odor naming and matching, which would be a compatible association provided the hypothesis that distorted input from a damaged lateral orbitofrontal cortex results in the report of a wrong perception.
Thirdly, phantosmia was the consequence of, mainly, damages in the frontal lobe, which is long known to be involved in the conscious perception of odors (Bowman et al., 2012, Wilson et al., 2014). However, without reaching statistical significance, the slightly higher prevalence of phantosmia in the cluster where also parosmia was more frequent suggests a rather complex generation of olfactory phantoms in the human cortical olfactory system. Finally, present findings reproduce the predominant role of the right brain side as critically involved in olfaction. This agrees with the idea that the right hemisphere, in general, is of high significance in the processing of olfactory information (Daniels et al., 2001, Hudry et al., 2014, Hummel et al., 1995, Zatorre et al., 1992).
5. Conclusions
This work used a bioinformatics based approach to the relevance of particular brain regions for an olfactory dysfunction, which following structure recognition in a matrix of patient versus brain damage data identified regions or pattern of regions associated with anosmia or parosmia. The procedure resulted in a verification of the main role of the olfactory bulb for the perception of odors, whereas it emphasized that the symptom of parosmia is more complex, requiring damaged and intact brain regions at the same time. The results are biologically plausible based on prior knowledge about the role of specific brain regions in human olfaction. Future long-term follow-up of patient with posttraumatic parosmia, and possibly phantosmia, will show whether current findings bear prognostic information.
Funding
The work has been supported by the Landesoffensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz (LOEWE, JL), LOEWE-Zentrum: “Translational Medicine and Pharmacology” and by the Deutsche Forschungsgemeinschaft (grant number DFG HU 441/18–1, TH). The funders had no role in method design, data selection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement
The authors have declared that no competing interests exist.
References
- Ache B.W., Young J.M. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Bitter T., Gudziol H., Burmeister H.P., Mentzel H.-J., Guntinas-Lichius O., Gaser C. Anosmia leads to a loss of gray matter in cortical brain areas. Chem. Senses. 2010;35:407–415. doi: 10.1093/chemse/bjq028. [DOI] [PubMed] [Google Scholar]
- Bonfils P., Avan P., Faulcon P., Malinvaud D. Distorted odorant perception: analysis of a series of 56 patients with parosmia. Arch. Otolaryngol. Head Neck Surg. 2005;131:107–112. doi: 10.1001/archotol.131.2.107. [DOI] [PubMed] [Google Scholar]
- Bowman N.E., Kording K.P., Gottfried J.A. Temporal integration of olfactory perceptual evidence in human orbitofrontal cortex. Neuron. 2012;75:916–927. doi: 10.1016/j.neuron.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breimann L., Friedman J.H., Olshen R.A., Stone C.J. Chapman and Hall; Boca Raton: 1993. Classification and Regression Trees. [Google Scholar]
- Brewer W.J., Wood S.J., McGorry P.D., Francey S.M., Phillips L.J., Yung A.R., Anderson V., Copolov D.L., Singh B., Velakoulis D., Pantelis C. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am. J. Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Croy I., Nordin S., Hummel T. Olfactory disorders and quality of life—an updated review. Chem. Senses. 2014;39:185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- Daniels C., Gottwald B., Pause B.M., Sojka B., Mehdorn H.M., Ferstl R. Olfactory event-related potentials in patients with brain tumors. Clin. Neurophysiol. 2001;112:1523–1530. doi: 10.1016/s1388-2457(01)00590-9. [DOI] [PubMed] [Google Scholar]
- Delank K.W., Fechner G. Pathophysiology of post-traumatic anosmia. Laryngo-Rhino-Otologie. 1996;75:154–159. doi: 10.1055/s-2007-997554. [DOI] [PubMed] [Google Scholar]
- Doty R.L., Deems D.A., Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty R.L., Genow A., Hummel T. Scratch density differentiates microsmic from normosmic and anosmic subjects on the University of Pennsylvania Smell Identification Test. Percept. Mot. Skills. 1998;86:211–216. doi: 10.2466/pms.1998.86.1.211. [DOI] [PubMed] [Google Scholar]
- Gilad Y., Lancet D. Population differences in the human functional olfactory repertoire. Mol. Biol. Evol. 2003;20:307–314. doi: 10.1093/molbev/msg013. [DOI] [PubMed] [Google Scholar]
- Gottfried J.A. Smell: central nervous processing. Adv. Otorhinolaryngol. 2006;63:44–69. doi: 10.1159/000093750. [DOI] [PubMed] [Google Scholar]
- Gudziol V., Buschhuter D., Abolmaali N., Gerber J., Rombaux P., Hummel T. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—a longitudinal study. Brain. 2009;132:3096–3101. doi: 10.1093/brain/awp243. [DOI] [PubMed] [Google Scholar]
- Hawkes C.H. Assessment of olfaction in multiple sclerosis. Chem. Senses. 1996;21:486. [Google Scholar]
- Hudry J., Ryvlin P., Saive A.-L., Ravel N., Plailly J., Royet J.-P. Lateralization of olfactory processing: differential impact of right and left temporal lobe epilepsies. Epilepsy Behav. 2014;37:184–190. doi: 10.1016/j.yebeh.2014.06.034. [DOI] [PubMed] [Google Scholar]
- Hummel T., Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125:116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- Hummel T., Pauli E., Schuler P., Kettenmann B., Stefan H., Kobal G. Chemosensory event-related potentials in patients with temporal lobe epilepsy. Epilepsia. 1995;36:79–85. doi: 10.1111/j.1528-1157.1995.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Hummel T., Sekinger B., Wolf S., Pauli E., Kobal G. “Sniffin' sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Hummel T., Kobal G., Gudziol H., Mackay-Sim A. Normative data for the “sniffin' sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur. Arch. Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- Juran J.M. The non-Pareto principle; Mea culpa. Qual. Prog. 1975;8:8–9. [Google Scholar]
- Kasai K., Shenton M.E., Salisbury D.F., Onitsuka T., Toner S.K., Yurgelun-Todd D., Kikinis R., Jolesz F.A., McCarley R.W. Differences and similarities in insular and temporal pole MRI gray matter volume abnormalities in first-episode schizophrenia and affective psychosis. Arch. Gen. Psychiatry. 2003;60:1069–1077. doi: 10.1001/archpsyc.60.11.1069. [DOI] [PubMed] [Google Scholar]
- Kobal G., Hummel T., Sekinger B., Barz S., Roscher S., Wolf S.R. “Sniffin' sticks”: screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- Kruskal W.H., Wallis W.A. Use of ranks in one-criterion variance anaylsis. J. Am. Stat. Assoc. 1952;47:583–621. [Google Scholar]
- Leyrer C.M., Chan M.D., Peiffer A.M., Horne E., Harmon M., Carter A.F., Hinson W.H., Mirlohi S., Duncan S.E., Dietrich A.M., Lesser G.J. Taste and smell disturbances after brain irradiation: a dose–volume histogram analysis of a prospective observational study. Pract. Radiat. Oncol. 2014;4:130–135. doi: 10.1016/j.prro.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J., Geisslinger G. Bedside-to-bench pharmacology: a complementary concept to translational pharmacology. Clin. Pharmacol. Ther. 2010;87:647–649. doi: 10.1038/clpt.2010.18. [DOI] [PubMed] [Google Scholar]
- Lötsch J., Reither N., Bogdanov V., Hähner A., Ultsch A., Hill K., Hummel T. A brain-lesion pattern based algorithm for the diagnosis of posttraumatic olfactory loss. Rhinology. 2015;53:365–370. doi: 10.4193/Rhino15.010. [DOI] [PubMed] [Google Scholar]
- McCaffrey R.J., Duff K., Solomon G.S. Olfactory dysfunction discriminates probable Alzheimer's dementia from major depression: a cross-validation and extension. J. Neuropsychiatry Clin. Neurosci. 2000;12:29–33. doi: 10.1176/jnp.12.1.29. [DOI] [PubMed] [Google Scholar]
- Meisami E. Effects of olfactory deprivation on postnatal growth of the rat olfactory bulb utilizing a new method for production of neonatal unilateral anosmia. Brain Res. 1976;107:437–444. doi: 10.1016/0006-8993(76)90243-2. [DOI] [PubMed] [Google Scholar]
- Merkonidis C., Grosse F., Ninh T., Hummel C., Haehner A., Hummel T. Characteristics of chemosensory disorders—results from a survey. Eur. Arch. Otorhinolaryngol. 2015;272(6):1403–1416. doi: 10.1007/s00405-014-3210-4. [DOI] [PubMed] [Google Scholar]
- Mueller A., Abolmaali N.D., Hakimi A.R., Gloeckler T., Herting B., Reichmann H., Hummel T. Olfactory bulb volumes in patients with idiopathic Parkinson's disease a pilot study. J Neural Transm (Vienna) 2005;112(10):1363–1370. doi: 10.1007/s00702-005-0280-x. [DOI] [PubMed] [Google Scholar]
- Murphy C., Gilmore M.M., Seery C.S., Salmon D.P., Lasker B.R. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol. Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Olofsson J.K., Rogalski E., Harrison T., Mesulam M.M., Gottfried J.A. A cortical pathway to olfactory naming: evidence from primary progressive aphasia. Brain. 2013;136:1245–1259. doi: 10.1093/brain/awt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Pareto V. Giard et Briére; Paris: 1909. Manuale di economia politica, Milan: Società editrice libraria. revised and translated into French as Manuel d’économie politique. [Google Scholar]
- Rinaldi A. The scent of life. The exquisite complexity of the sense of smell in animals and humans. EMBO Rep. 2007;8:629–633. doi: 10.1038/sj.embor.7401029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombaux P., Duprez T., Hummel T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology. 2009;47:3–9. [PubMed] [Google Scholar]
- Royet J.P., Plailly J., Saive A.L., Veyrac A., Delon-Martin C. The impact of expertise in olfaction. Front. Psychol. 2013;4:928. doi: 10.3389/fpsyg.2013.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I. Imaging of brain activation by odorants in humans. Curr. Opin. Neurobiol. 2002;12:455–461. doi: 10.1016/s0959-4388(02)00346-x. [DOI] [PubMed] [Google Scholar]
- Shannon C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1951;30:50–64. [Google Scholar]
- Sumner D. Post-traumatic anosmia. Brain. 1964;87:107–120. doi: 10.1093/brain/87.1.107. [DOI] [PubMed] [Google Scholar]
- Ultsch A., Lötsch J. Computed ABC Analysis for Rational Selection of Most Informative Variables in Multivariate Data. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeurgt C., Wilkin F., Tarabichi M., Gregoire F., Dumont J.E., Chatelain P. Profiling of olfactory receptor gene expression in whole human olfactory mucosa. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Luessen A., Leopold D.A., Miwa T. Smell and Taste Disorders - Diagnostic and Clinical Work-Up. In: Welge-Luessen A., Hummel T., editors. Management of Smell and Taste Disorders - A Practical Guide for Clinicians. Thieme; Stuttgart: 2013. pp. 49–57. [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- Williams G.J. Springer; 2011. Data Mining with Rattle and R: The Art of Excavating Data for Knowledge Discovery. [Google Scholar]
- Wilson D.A., Xu W., Sadrian B., Courtiol E., Cohen Y., Barnes D.C. Cortical odor processing in health and disease. Prog. Brain Res. 2014;208:275–305. doi: 10.1016/B978-0-444-63350-7.00011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensberger M., Schnieper I., Welge-Lussen A. “Sniffin' sticks”: a new olfactory test battery. Acta Otolaryngol. 2000;120:303–306. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- Yousem D.M., Geckle R.J., Bilker W.B., Kroger H., Doty R.L. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad. Radiol. 1999;6:264–272. doi: 10.1016/s1076-6332(99)80449-8. [DOI] [PubMed] [Google Scholar]
- Zatorre R.J., Jones-Gotman M., Evans A.C., Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]