Summary
Multidrug resistant pathogens are a widespread problem in the hospital setting especially on intensive care units (ICU). This study evaluated the susceptibility of clinical isolates of gramnegative extensively drug resistant organisms (XDR), methicillinresistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus (VRE) to a proprietary chlorhexidine digluconate (CHG) formulation used in one brand of CHG-impregnated cloths. Ten isolates each of XDR Pseudomonas aeruginosa, XDR Acinetobacter baumannii, XDR Klebsiella pneumoniae, XDR Escherichia coli, MRSA, and vancomycin-resistant Enterococcus faecium from our hospital were tested. All isolates were susceptible to the proprietary CHG formulation (0.5%, 1%, 2%), with 99% to 100% suppression of growth at the earliest time point in time kill assays (1 minute for gram-positive and 15 seconds for gram-negative organisms). Minimum inhibitory concentrations ranged from 1 : 4096 to 1 : 65536 for MRSA, 1 : 1024 to 1 : 2048 for VRE, 1 : 2048 to 1 : 4096 for XDR E. coli, 1 : 512 to 1 : 2048 for XDR A. baumannii, 1 : 512 to 1 : 1024 for XDR P. aeruginosa, and 1 : 512 to 1 : 1024 for XDR K. pneumoniae. Cloths impregnated with this CHG formulation provide effective protection against colonization and infection by many pathogens. This study provides in vitro evidence that the proprietary CHG formulation used in one brand of CHG-impregnated cloths is effective against XDR gram-negative organisms, MRSA, and VRE.
Key words: Chlorhexidine, Drug resistant, Gram-negative, Hospital-acquired infection, MRSA, VRE
Introduction
The rapid emergence and spread of multidrug resistant organisms (MDROs) in hospitals is a growing problem worldwide [1, 2]. Hospital-acquired infections, particularly those caused by MDROs, are associated with excess mortality and morbidity as well as increased hospital costs [3-7]. Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and multidrug resistant (MDR) gram-negative pathogens commonly cause hospital-acquired infections [8]. Managing hospital-acquired infections and MDROs is a daily challenge in hospitals, especially from the perspective of critical care.
Universal decolonization using topical antiseptic agents that reduce the population of microorganisms on patients' skin represents a simple, cost-effective way to prevent healthcare-associated infections [9, 10]. Universal decolonization by daily bathing of ICU patients with chlorhexidine digluconate (CHG)-impregnated cloths resulted in a substantial reduction in bloodstream infections and MRSA acquisition [9, 11-13]. CHG-impregnated cloths have also proven effective in reducing the skin burden of MRSA and VRE in ICU patients [9, 14, 15].
Evidence suggests that skin colonization with gramnegative bacterial pathogens may be a root cause of hospital- acquired infections. ICU patients who have diarrhea can be particularly at risk of gram-negative bacteria dissemination from feces to skin areas on distant parts of the body [16]. There is less information concerning the utility of CHG on antibiotic-resistant gram-negative bacteria [15], although a recent study showed that a proprietary CHG formulation reduced hospital-acquired infections caused by gram-negative bacteria [17]. The objective of this study was to quantify in vitro the antimicrobial effectiveness of a proprietary CHG formulation used in one brand of CHG-impregnated cloths against gram-negative MDR and extensively drug resistant (XDR) clinical isolates (e.g., Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii), as well as against clinical isolates of MRSA and VRE.
Materials and methods
BACTERIAL ISOLATES
Clinical isolates of P. aeruginosa, K. pneumoniae, E. coli, and A. baumannii selected for testing were classified as MDR or XDR as described elsewhere [18]. Clinical isolates of MRSA and vancomycin-resistant E. faecium were also tested. Ten consecutive isolates per species were collected from patients who were diagnosed with an infection cause by a MDRO during their stay in at the Heidelberg University Hospital, Germany, in 2014.
FORMULATIONS AND NEUTRALIZER
A proprietary 2% CHG formulation (Sage Products LLC, Cary, Illinois, US) was tested. The solution used to neutralize the antimicrobial properties of the CHG was composed of Caso-bouillon and LTHTh (Heipha, Eppelheim, Germany).
TIME KILL ASSAYS
The antimicrobial properties of the proprietary CHG formulation at concentrations of 2% (20 mg/ml, the original proprietary formulation concentration), 1% (10 mg/mL), and 0.5% (5 mg/mL) were determined using the quantitative suspension methods described by Gebel et al. [19]. Briefly, bactericidal efficacy was determined without organic load. The CHG formulation was diluted in water of standardized hardness. One milliliter of the test organism suspension and 1 mL of sterile water of standardized hardness were mixed and incubated for 2 minutes, after which the test substance was added. The resulting solutions were incubated for 1, 3, and 9 minutes, respectively; gram-negative organisms were also incubated for 15 seconds, based on the test procedures outlined in the FDA Tentative Final Monograph for Topical Antimicrobial Drug Products for Over-The-Counter Human Use (59 FR, 31444, June 17, 1994). At the end of the incubation time, 1 mL of the test solution was transferred to 10 mL of Caso-bouillon and LTHTh and neutralized for 5 minutes. Thereafter, 100 μl and 500 μl of the neutralized test solution was spread onto two agar plates. After incubation for 24 hours at 37 °C the colony forming units (CFU) were counted. The log 10 reduction was determined as the logarithm to the base 10 of the difference between the number of cells in the test solution at the beginning of the contact time and at the end.
DETERMINATION OF THE MINIMUM INHIBITORY CONCENTRATION (MIC)
MIC testing procedures were adapted based on those outlined in the FDA Tentative Final Monograph for Topical Antimicrobial Drug Products for Over-The-Counter Human Use (59 FR, 31444, June 17, 1994). Briefly, a 96-well microtiter plate (Sarstedt, Nümbrecht, Germany) containing doubling dilutions of the proprietary CHG in RPMI medium (Gibco, Darmstadt, Germany) was set up. CHG concentrations started at 2% and were then diluted by half. Concentrations for MIC testing were diluted down to 1 : 65,536. Wells containing only RPMI were used as growth controls. An overnight broth culture of each isolate was standardized to 1 x 108 CFU/mL and 50 μL volumes of this were added to the microtiter plate. Serial dilutions were done and CFUs counted on the plates to achieve a concentration of 1 x 105. Plates were incubated for 24 hours at 37 °C. The MIC was defined as the lowest concentration of CHG at which no bacterial growth was observed visually on the microtiter plate. Conversion of resazurin to resarafin (Sigma-Aldrich, Hamburg, Germany) was used as a visual indicator.
Results
ISOLATES
Ten isolates each of VRE, MRSA, XDR P. aeruginosa, XDR K. pneumoniae, and E. coli were collected and tested. Nine isolates of MDR A. baumannii and one of XDR A. baumannii were collected and tested.
IN VITRO TIME KILL STUDIES
All isolates were highly susceptible to the proprietary CHG formulation (Fig. 1). Suppression rates were 99% to 100% for all isolates, at all concentrations including the lower concentrations of 1% (10 mg/mL) and 0.5% (5 mg/mL). The 0.5% CHG formulation provided a 99.9% reduction of XDR P. aeruginosa, XDR K. pneumoniae, XDR E. coli, and XDR and MDR A. baumannii from the earliest 15-second time point, and for MRSA and VRE at the earliest 1 minute time point.
Fig. 1.
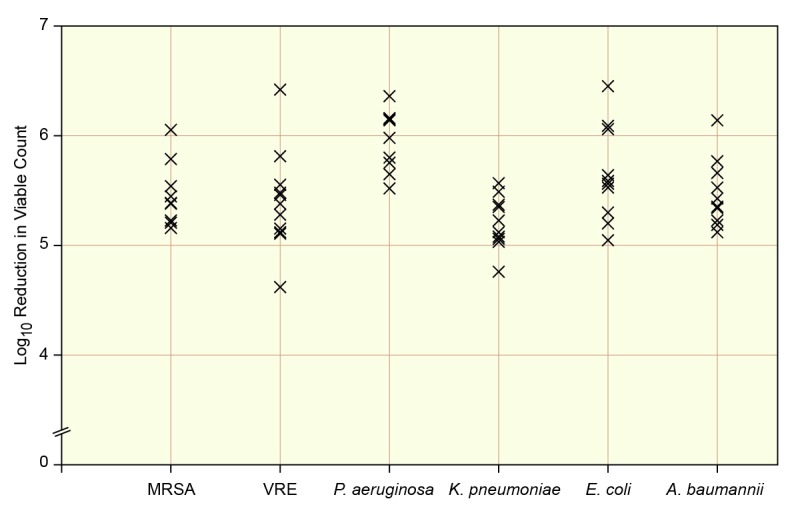
Time kill at 1 minute with a 2% CHG proprietary solution of clinical isolates of MRSA, vancomycin-resistant E. faecium, and the XDR gram-negative pathogens P. aeruginosa, K. pneumoniae, E. coli, and A. baumannii.
MIC DETERMINATIONS
MICs for the proprietary CHG solution against MRSA ranged from 1 : 4096 for a single isolate to 1 : 65536 (Tab. I). Nine out of ten XDR E. coli isolates had an MIC of 1 : 4096 with the CHG formulations. XDR A. baumannii showed relatively low MICs, with an MIC of 1 : 1024. Vancomycin-resistant E. faecium most commonly demonstrated MICs of 1 : 2048. XDR K. pneumonia and XDR P. aeruginosa had slightly higher MICs. The MICs for P. aeruginosa MICs ranged from 1 : 512 to 1 : 1024. MICs for XDR K. pneumoniae ranged from 1 : 512 to 1 : 4096.
Tab. I.
MICs of a proprietary CHG formulation against ten clinical isolates of MRSA, vancomycin-resistant E. faecium, and the XDR gramnegative pathogens P. aeruginosa, K. pneumoniae, E. coli, and A. baumannii demonstrate the susceptibility of MDR isolates.
| Clinical isolates | Number of isolates | MIC |
|---|---|---|
| S. aureus (MRSA) | 1 | 1 : 4096 |
| 3 | 1 : 8192 | |
| 5 | 1 : 16384 | |
| 1 | 1 : 65536 | |
| E. faecium (VRE) | 2 | 1 : 1024 |
| 8 | 1 : 2048 | |
| P. aeruginosa (XDR) | 7 | 1 : 512 |
| 3 | 1 : 1024 | |
| K. pneumoniae (XDR) | 7 | 1 : 512 |
| 2 | 1 : 1024 | |
| 1 | 1 : 4096 | |
| E.coli (XDR) | 1 | 1 : 2048 |
| 9 | 1 : 4096 | |
| A. baumannii (XDR) | 1 | 1 : 512 |
| 6 | 1 : 1024 | |
| 2 | 1 : 2048 | |
| A. baumannii (MDR) | 1 | 1 : 1024 |
Discussion
Hospital-acquired infections are leading causes of preventable morbidity and mortality [2, 20]. Skin decolonization with CHG-impregnated cloths has been shown to reduce the risk of some types of these infections [11, 9, 12, 13, 16, 21]. This method was shown to be an effective and cost saving way to reduce the risk of transmission of MDROs such as MRSA and VRE in the hospital, a setting where rapid emergence and spread of MDROs is well known [9, 12, 15, 10, 1]. The in vitro time kill studies confirmed that all of the German clinical isolates tested, including MDR and XDR gram-negative bacteria, MRSA, and vancomycin-resistant E. faecium, were highly susceptible to all concentrations of the proprietary CHG formulation used. This was true at the earliest time point tested (15 seconds). These results corroborate and quantify the effectiveness of the proprietary CHG formulation against clinical isolates of MDR and XDR gram-negative bacteria in vitro.
MIC data were more variable than time kill data. We observed relatively low MICs for MRSA, E. coli, A. baumannii and vancomycin-resistant E. faecium, but higher MICs for P. aeruginosa and K. pneumoniae. Lack of a consistent relationship between the CHG MIC and increased CHG killing has been described previously with MRSA [22]. The authors noted that the MIC did not appear to affect in vitro rate of kill or in vivo skin test results, and did not represent CHG resistance. The discordance between MIC and kill rate did not affect the utility of CHG decolonization in that study, and the same may well be true for the pathogens we studied. For example, even the MICs we observed against P. aeruginosa (e.g., 19.53 μg/mL or 1 : 512) would be at least 10-fold lower than the lowest concentrations of CHG deposited on skin when the 2% proprietary CHG formulation was used [11]. We conducted our in vitro evaluations using the proprietary CHG formulation specifically because the CHG impregnated cloths have been shown to provide consistently high concentrations of antiseptic coverage when applied to skin [11].
Conclusions
The present study provides in vitro evidence that the proprietary CHG formulation is effective against MDR gram-negative organisms, MRSA, and VRE. The solution we studied is available only in CHG-impregnated cloths which are known to provide effective protection against colonization and infection by drug resistant pathogens. Of course, the 60 clinical isolates tested would not be representative of all strains a patient might encounter in a German hospital. In addition, higher concentrations of the CHG product than were tested in vitro may also be more representative of the amount deposited by cloths in real-life use. Future research should evaluate the potential of the cloths to prevent MDROs from colonizing the skin and leading to hospital-acquired infections.
ACKNOWLEDGMENTS
Writing assistance was provided by Wendy Horn, Insight Communications Group LLC, and funded by Sage Products LLC. This research was funded by Sage Products LLC, Cary, Illinois, USA.
Partial results were presented at the 3rd International Conference on Prevention & Infection Control (ICPIC), 2015."
References
- 1.Luyt C-E, Bréchot N, Trouillet J-L, et al. Antibiotic stewardship in the intensive care unit. Critical Care. 2014;18:480–491. doi: 10.1186/s13054-014-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 3.Mutters NT, Mersch-Sundermann V, Mutters R, et al. Control of the spread of vancomycin-resistant Enterococci in hospitals: epidemiology and clinical relevance. Dtsch Arztebl Int. 2013;110:725–731. doi: 10.3238/arztebl.2013.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson MU, Bizzarro MJ, Dembry LM, et al. One size does not fit all: Why universal decolonization strategies to prevent methicillin-resistant Staphylococcus aureus colonization and infection in adult intensive care units may be inappropriate for neonatal intensive care units. J Perinatol. 2014;34:653–655. doi: 10.1038/jp.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwab F, Meyer E, Geffers C, et al. Understaffing, overcrowding, inappropriate nurse: ventilated patient ratio and nosocomial infections: which parameter is the best reflection of deficits? J Hosp Infect. 2012;80:133–139. doi: 10.1016/j.jhin.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Cheah AL, Spelman T, Liew D, et al. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin Microbiol Infect. 2013;19:E181–E189. doi: 10.1111/1469-0691.12132. doi:10.1111/1469-0691.12132. [DOI] [PubMed] [Google Scholar]
- 7.Lambert ML, Suetens C, Savey A, et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: a cohort study. Lancet Infect Dis. 2011;11:30–38. doi: 10.1016/S1473-3099(10)70258-9. [DOI] [PubMed] [Google Scholar]
- 8.Mattner F, Bange F-C, Meyer E, et al. Preventing the spread of multidrug-resistant gram-negative pathogens. Dtsch Arztebl Int. 2012;108:39–45. doi: 10.3238/arztebl.2012.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368:533–542. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang SS, Septimus E, Avery TR, et al. Cost savings of universal decolonization to prevent intensive care unit infection: Implications of the REDUCE MRSA Trial. Infect Control Hosp Epidemiol. 2014;35:S23–S31. doi: 10.1086/677819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmiston CE, Jr, Krepel CJ, Seabrook GR, et al. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg. 2008;207:233–239. doi: 10.1016/j.jamcollsurg.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet Infect Dis. 2013;381:1099–1106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin- resistant enterococci. Arch Intern Med. 2006;166:306–312. doi: 10.1001/archinte.166.3.306. [DOI] [PubMed] [Google Scholar]
- 15.Derde LPG, Dautzenberg MJD, Bonten MJM. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med. 2012;38:931–939. doi: 10.1007/s00134-012-2542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassir N, Papazian L, Fournier PE, et al. Insights into bacterial colonization of intensive care patients' skin: the effect of chlorhexidine daily bathing. Eur J Clin Microbiol Infect Dis. 2015;34:999–1004. doi: 10.1007/s10096-015-2316-y. [DOI] [PubMed] [Google Scholar]
- 17.Cassir N, Thomas G, Hraiech S, et al. Chlorhexidine daily bathing: Impact on health care-associated infections caused by gram-negative bacteria. Am J Infect Control. 2015 doi: 10.1016/j.ajic.2015.02.010. doi: 101016/ jajic201502010 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 19.Gebel J, Werner HP, Kirsch-Altena A, et al. Standardmethoden der DGHM zur Prüfung chemischer Desinfektionsverfahren. Berlin: MHP Verlag; 2001. [Google Scholar]
- 20.Umscheid CA, Mitchell MD, Doshi JA, et al. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32:101–114. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 21.Quach C, Milstone AM, Perpête C, et al. Chlorhexidine bathing in a tertiary care neonatal intensive care unit: impact on central line-associated bloodstream infections. Infect Control Hosp Epidemiol. 2014;35:158–163. doi: 10.1086/674862. [DOI] [PubMed] [Google Scholar]
- 22.Cookson BD, Bolton MC, Platt JH. Chlorhexidine resistance in methicillin-resistant Staphylococcus aureus or just an elevated MIC? An in vitro and in vivo assessment. Antimicrobial Agents Chemother. 1991;35:1997–2002. doi: 10.1128/aac.35.10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


