Abstract
Background:
Pregnancy-associated stroke remains incompletely characterized because of the rarity of these potentially devastating events. We investigated whether mechanism and outcome of ischemic pathophysiology stroke differ between young pregnant and nonpregnant women.
Methods:
We identified 135 consecutive women ages 18–40 years admitted to our center from January 2008 through June 2014 with ischemic stroke, TIA, cerebral venous thrombosis, or nonaneurysmal subarachnoid hemorrhage due to reversible cerebral vasoconstriction syndrome (RCVS). We reviewed charts for pregnancy status, demographics, medical comorbidities, stroke severity, etiology, and discharge outcomes.
Results:
There were 33 women with pregnancy-associated stroke (PAS) and 102 with non–pregnancy-associated stroke (NPAS). Among women with PAS, 73% of strokes occurred postpartum. In the PAS group, the most common cause of cerebrovascular events was RCVS (n = 12), 11 postpartum and 4 in women with preeclampsia. There were no significant differences between the groups in demographics. Women with PAS were less likely to have vascular risk factors such as hyperlipidemia and history of thromboembolism but more likely to have cerebral venous thromboses (21% vs 7%, p = 0.02). Women with PAS were more likely to have RCVS as stroke mechanism (36% vs 1%, odds ratio 57.7, 95% confidence interval 7–468, p = 0.0001).
Conclusion:
Compared with nonpregnant women of the same age group, women with PAS had fewer vascular risk factors. Cerebral venous thrombosis and RCVS were more common in PAS, most of which occurred postpartum. These results provide further evidence for the unique pathophysiology of pregnancy-related stroke, raising important questions for future investigation.
With the release of its first-ever guidelines for stroke prevention in women, the American Heart Association/American Stroke Association (AHA/ASA) emphasized the need for dedicated research to identify and characterize stroke risk factors unique to women, highlighting pregnancy in particular as an area with insufficient evidence to make recommendations regarding screening or prevention.1 Stroke is a rare complication of the peripartum period, with low incidence (34.2 per 100,000 deliveries1,2) but potentially devastating consequences. Previous studies estimated that peripartum women are at 3-fold increased risk of stroke,2–6 with the postpartum period conferring the highest risk of both ischemic and hemorrhagic stroke.6,7 Of concern, the incidence of pregnancy-associated stroke (PAS) appears to be increasing.8
Risk factors for PAS identified in previous studies include preeclampsia/eclampsia, cesarean section, hypertension, chronic kidney disease, black race, pregnancy-related hematologic disorders, older age, migraine, gestational diabetes, primary hypercoagulable states, and smoking.2,7,9–11 However, our understanding of the complex processes underlying PAS remains incomplete. Most studies have included both hemorrhagic and ischemic strokes without further characterization of each subtype. Furthermore, previous studies relied on large administrative datasets without a systematic assessment of ischemic stroke subtype and rare associated conditions such as the reversible cerebral vasoconstriction syndrome (RCVS), cervical artery dissections, and cerebral venous thrombosis (CVT). Moreover, most studies have been conducted in northern European populations.1
With this retrospective cross-sectional study in an ethnically diverse group of young women, we aimed to closely characterize ischemic PAS and compare it with ischemic strokes in similarly aged nonpregnant women (non–pregnancy-associated stroke [NPAS]).
METHODS
Standard protocol approvals, registrations, and patient consents
Approval for the study was obtained from the institutional review board of Columbia University Medical Center, and the requirement for informed consent was waived because of the retrospective nature of the study and deidentification of data. Similarly, we were unable to obtain retrospective authorization from patients to disclose recognizable descriptions; therefore, any data that we felt could result in potential recognition of individual patients were omitted from the brief case descriptions in table 1.
Table 1.
Clinical characteristics of 33 women with pregnancy-associated stroke

Study population
Patients were identified using the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) and NIH StrokeNet database at Columbia University Medical Center (PI RSM) between January 2008 and June 2014. Patients at our center were included in this database if they were diagnosed with TIA, ischemic stroke, or hemorrhagic stroke either on or during admission. Vascular neurologists and coordinators meet daily to review all patients admitted or diagnosed during admission with any stroke or TIA, with the goal of complete capture of all cases.
Inclusion/exclusion criteria
All women aged 18–40 years at the time of the index event who were admitted or diagnosed during admission with TIA, ischemic stroke, CVT, or nonaneurysmal subarachnoid hemorrhage (SAH) due to RCVS were included. TIA was defined according to the current standard AHA/ASA definition: a transient episode of neurological dysfunction caused by focal brain, spinal cord or retinal ischemia, without acute infarction.12 We excluded women over 40 because pregnancy after age 40 remains rare; although rates of pregnancy after 40 are increasing, 93% of pregnancies still occur before the age of 40.13 We focused our study on ischemic pathophysiology, including hemorrhagic stroke only if it was due to venous thrombosis, hemorrhagic conversion of arterial ischemic stroke, or nonaneurysmal SAH due to RCVS. One patient with a ventricular assist device (VAD) was excluded to avoid skewing the data, because VADs are generally considered a contraindication to pregnancy. Out of a total of 5,986 patients included in the database during this time period, 135 met our inclusion criteria (figure 1).
Figure 1. CONSORT diagram.
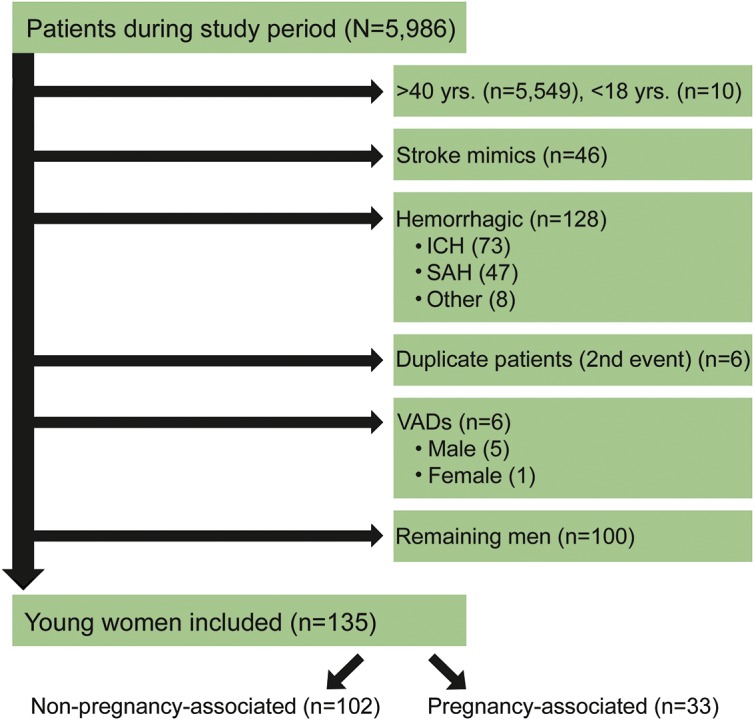
ICH = intracranial hemorrhage; SAH = subarachnoid hemorrhage; VAD = ventricular assist device.
Data acquisition
We reviewed all participants' charts for demographics, comorbidities, stroke severity, etiology, and outcomes. Multiple portions of the electronic medical record were reviewed, including administrative data, clinician notes, nursing documentation, and radiology reports. Participants were divided into 2 groups: women with PAS and women with NPAS. Women were considered to have PAS if the index event occurred during pregnancy or during the 12 weeks postpartum, including after elective or spontaneous termination of pregnancy. This time frame was chosen because of recent data showing that risk of thrombotic events remains elevated up to 12 weeks after delivery.9 We assessed vascular risk factors and prior vascular events by collecting data from the medical record regarding the following variables: body mass index, chronic hypertension (diagnosed prior to pregnancy), hyperlipidemia, diabetes (gestational or preexisting), heart disease, prior stroke, prior myocardial infarction, prior deep vein thrombosis or pulmonary embolism, active cancer, sickle cell disease, history of transplant, migraine, oral contraceptive use, active smoking, history of cocaine use, history of heavy alcohol use, underlying hypercoagulable states found on stroke evaluation, rheumatologic disease, patent foramen ovale, and family history of hypercoagulability. Infection at time of event, NIH Stroke Scale (NIHSS) on presentation, stroke location, stroke etiology according to TOAST criteria (cardioembolism, including thromboembolism, paradoxical embolism, and endocarditis; small vessel disease; large artery atherosclerosis; cryptogenic; or other/unspecified), and treatment (IV tissue plasminogen activator, neuroendovascular treatment, antiplatelet or anticoagulants) were also collected. We further classified the other/unspecified stroke subtype by associated rare causes such as CVT, RCVS, and cervicocephalic artery dissection. For the purposes of this study, patients were considered to have a diagnosis of RCVS if they fit the clinical syndrome (thunderclap headache with transient or persistent neurologic deficits) and had either radiographic/angiographic evidence of nonaneurysmal SAH or radiographic, angiographic, or sonographic evidence of vasoconstriction.14 All of the patients in our study considered to have RCVS were given this diagnosis by an attending vascular neurologist during the admission. In women with PAS, additional descriptive data related to pregnancy were collected, including parity, timing of stroke (first, second, or third trimester or postpartum), pregnancy complications including preeclampsia, assisted reproductive technology, multiple gestation, and breastfeeding status if postpartum.
The primary stroke outcome measure was good vs poor outcome; a good outcome was defined as a modified Rankin Scale (mRS) score ≤2 at discharge, signifying mild or no disability. Other outcomes assessed included intracranial bleeding after index event, stroke recurrence, and discharge disposition (home or acute rehabilitation vs nursing home or death).
Statistical analysis
Groups were compared using χ2 tests for categorical variables, with Fisher exact test where appropriate, and Wilcoxon rank sum test for continuous variables. Crude and adjusted logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) before and after adjusting for potential confounders. Missing data were not accounted for, as this was a hypothesis-generating study; however, the only exploratory variables with missing data were ethnicity (n = 18), body mass index (n = 6), patent foramen ovale (n = 47), and NIHSS score (n = 17).
RESULTS
We identified 135 young women with stroke, 33 of whom were pregnant or within 12 weeks postpartum. Clinical characteristics of the 33 women with PAS are detailed in table 1. There were no significant differences between the 2 groups with regard to age or demographics. Mean age of the entire group was 32 years (SD 6). A multiethnic cohort was represented (30% white, 13% black, 39% Hispanic, 4% other, 13% unknown). The pregnancy-associated group had fewer traditional vascular risk factors (hyperlipidemia, history of deep vein thrombosis or pulmonary embolism, active tobacco use). There were no significant differences between groups in proportion of history of migraine, underlying hypercoagulable state, sickle cell disease, history of prior stroke or myocardial infarction, concurrent infection, cocaine use, or family history of hypercoagulability (table 2).
Table 2.
Results of univariate analysis of PAS and NPAS
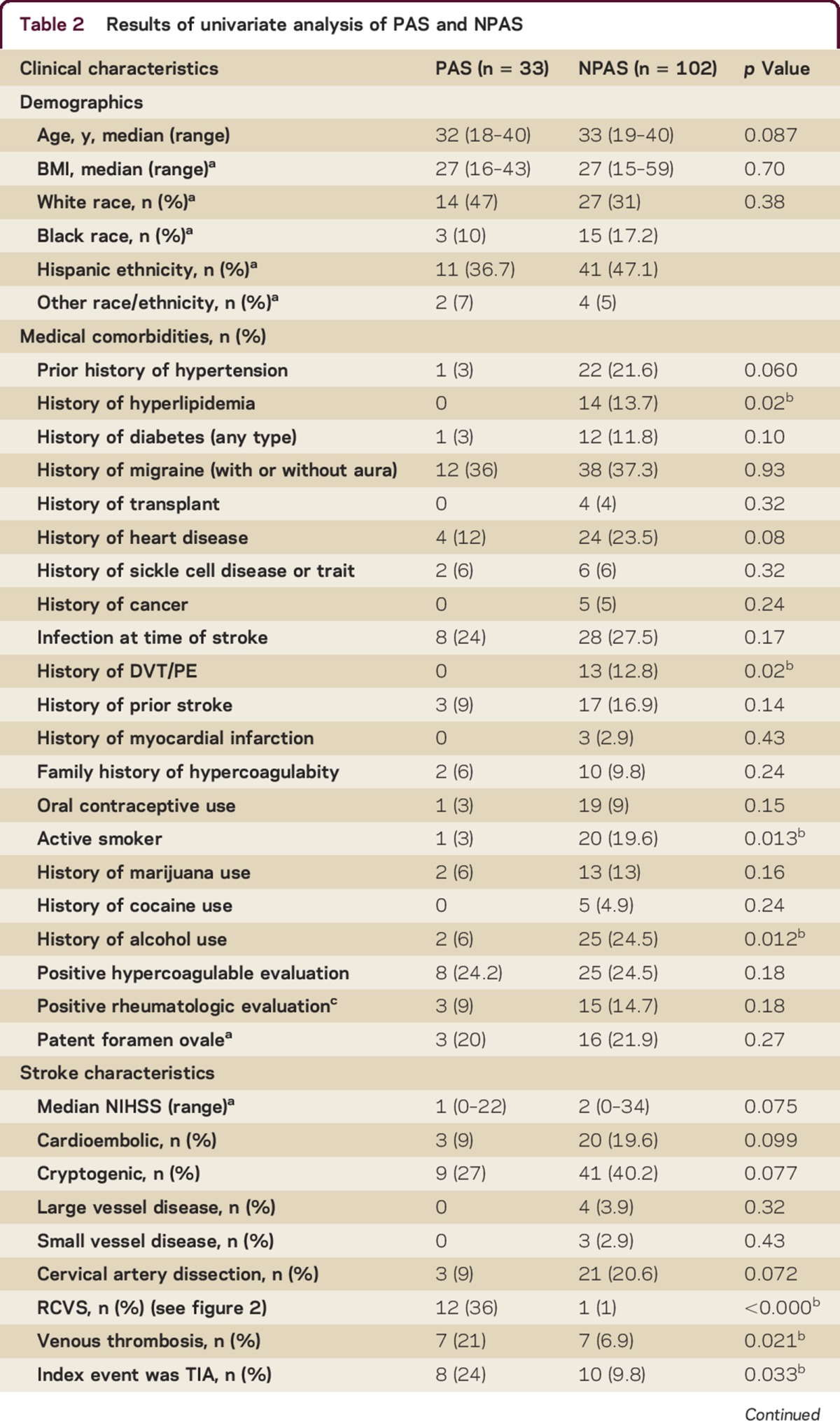
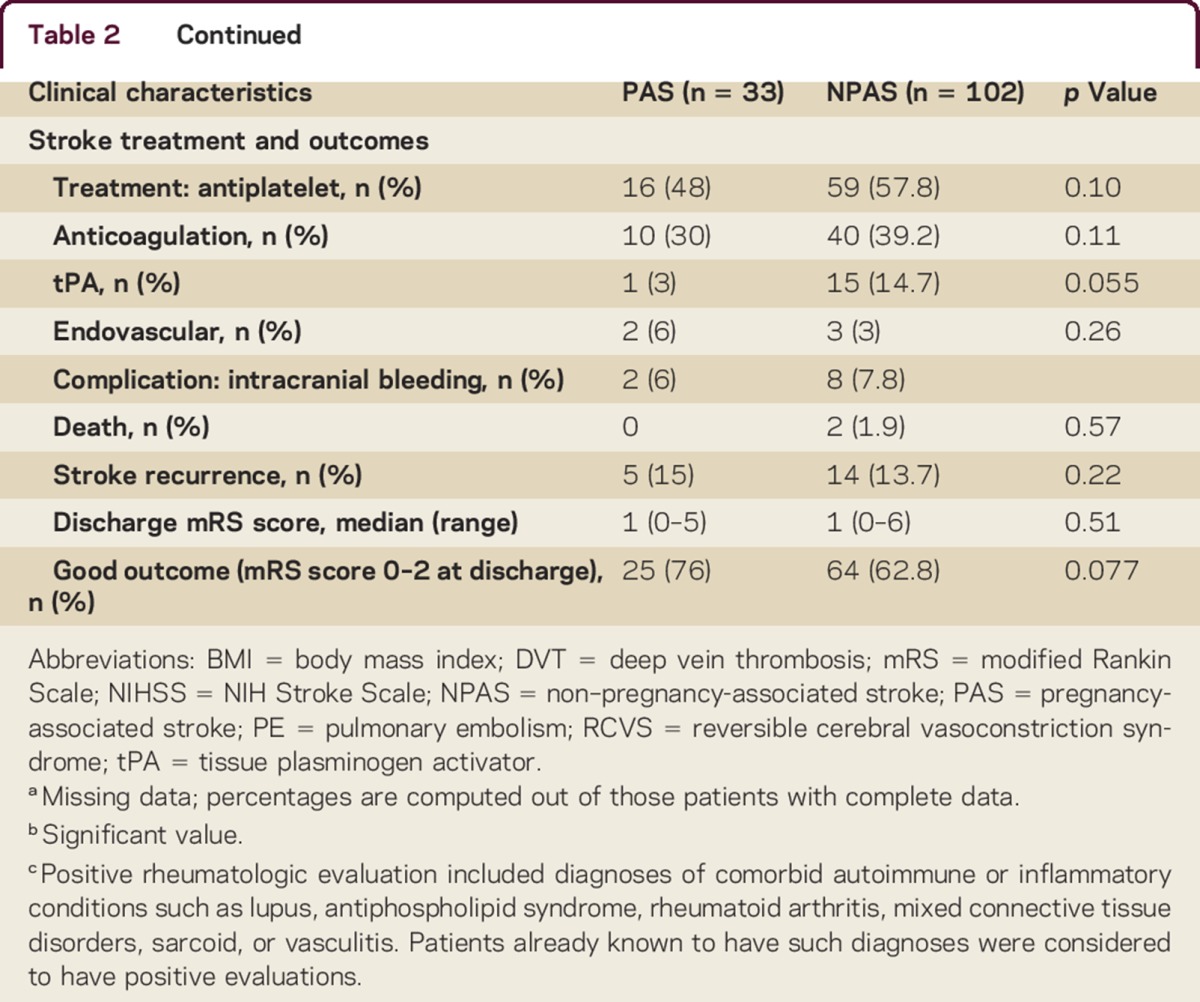
Among women with PAS, 73% of strokes (24 of 33) occurred postpartum, 4 (12%) occurred in the first trimester, 1 (3%) occurred in the second trimester, and 4 (12%) occurred in the third trimester. None of the strokes occurred during delivery. Of the 24 women with postpartum stroke, 21 (88%) had strokes after they had been discharged after delivery. The most common stroke mechanism for women with PAS was RCVS (12, 36%), followed by CVT (7, 21%), cardioembolism (3, 9%), and cervical artery dissection (3, 9%). No stroke mechanism was identified in 9 of the 33 women (27%). No strokes in the PAS group were attributed to large artery atherosclerosis or small vessel disease. Patients with NPAS and those with PAS had a statistically similar proportion of large artery atherosclerosis, small vessel, cardioembolic, and cryptogenic ischemic stroke subtypes; we also found no difference in the proportion of ischemic strokes due to cervical artery dissection.
Women with PAS had a higher proportion of CVT (21% vs 7% in NPAS, p = 0.02). Compared with women with NPAS, women with PAS had markedly greater odds of having RCVS as stroke etiology (36% vs 1%, unadjusted OR 57, 95% CI 7–468); 92% (11 of 12) of pregnancy-associated RCVS occurred postpartum.
Because of the dramatically increased odds of RCVS we found in the PAS group, we ran secondary analyses to assess for effect modifiers with regard to risk factors. Preeclampsia and migraine both appeared to play a role. Of the 12 women in the PAS group with RCVS, 4 (33%) had preeclampsia. In comparison, preeclampsia was present in 1 of the 8 women with cryptogenic stroke (13%) and in none of the women with cardioembolism or dissection as stroke mechanism. After adjusting for pregnancy, the odds of RCVS were increased for women in both groups with a history of migraine (OR 5.2, 95% CI 1.2–22.5). In the pregnancy group, women with a history of migraine had far higher odds of having RCVS as stroke mechanism (OR 75.4, 95% CI 8.64–659, p ≤ 0.0001). Thus there was evidence of effect modification between pregnancy and migraine.
DISCUSSION
In our small but detailed retrospective analysis, we found that the majority of PAS occurred postpartum, a finding that aligns with the results of prior studies.5,6,8 Stroke mechanisms in the PAS group appeared unrelated to traditional vascular risk factors. In contrast, women in the NPAS group were more likely to have stroke risk factors such as hyperlipidemia, history of thromboembolism, or active smoking. Stroke related to vascular risk factors in the young appears to be increasing,15 and the importance of primary prevention in this population cannot be overemphasized.
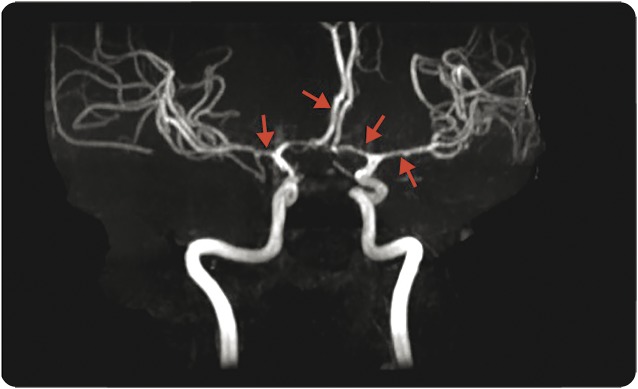
RCVS was the most common stroke mechanism in the PAS group, a finding that to our knowledge has been seldom reported in prior studies. This may reflect its relatively recent recognition as a cause of both ischemic stroke and nonaneurysmal SAH. Call and Fleming described the syndrome in 1988, terming it “reversible cerebral segmental vasoconstriction” and noting that previously described cases had often occurred in postpartum women.16 The authors postulated a hormonal influence and a connection with severe migraine syndromes. A representative magnetic resonance angiogram from our series is shown in figure 2. Although the term “reversible” implies a benign course, Call and Fleming noted that neurologic deficits could be permanent; indeed, a recent retrospective case series found that 20 of 59 patients with RCVS (34%) had worsening of neurologic deficits after diagnosis, including 4 patients who died.17 The majority of our RCVS cases had a favorable outcome, but some did not: 2 of our 11 postpartum RCVS cases had a discharge mRS score of 4 (moderately severe disability, unable to care for themselves or walk unassisted).
Figure 2. Reversible cerebral vasoconstriction syndrome.
Diffuse vasospasm in a 33-year-old woman with a history of migraine, postpartum day 8 after healthy pregnancy.
RCVS shares features with the posterior reversible encephalopathy syndrome (PRES); a case series documented coexisting PRES in 9% of patients with RCVS,18 and other authors suggest that the syndromes may coincide far more often.19 In the postpartum setting, RCVS, PRES, preeclampsia, and the HELLP syndrome (a severe form of preeclampsia with hemolysis, elevated liver enzymes, and low platelets) may all intersect under the umbrella term “postpartum cerebral angiopathy,”20,21 an incompletely understood disorder of systemic endothelial dysfunction and failure of cerebrovascular autoregulation.21 One study investigated the effects of pregnancy on the cerebral autoregulatory curve, noting that cerebral arteries from late-pregnant and postpartum animals constricted in reaction to high serotonin concentrations, whereas arteries from nonpregnant animals dilated.22 In a follow-up study, the authors noted that when plasma from women with HELLP syndrome was infused into the posterior cerebral arteries of rats it caused increased blood-brain barrier permeability and diminished vasodilation in response to calcium-activated potassium channels.23 In our group, 33% of the pregnancy-related RCVS cases had coexisting preeclampsia; none were documented as having HELLP syndrome.
The finding that a history of migraine appeared to increase the odds of pregnancy-associated RCVS is intriguing. Prior research has postulated a relationship between RCVS and migraine: a large case series documented prior migraine in 40% of patients with RCVS,24 significantly more than the approximate 15% prevalence of migraine in the general population.25 In a prospective cohort study, female sex and history of migraine were identified as independently associated risk factors for hemorrhage (subarachnoid, subdural, or intraparenchymal) in RCVS, and hemorrhagic RCVS, when compared with RCVS without hemorrhage, was highly associated with ischemic stroke and disability.26 Younger women with a history of migraine with aura and low Framingham cerebrovascular risk scores may paradoxically be at higher risk for ischemic stroke.27 A recent systematic review and meta-analysis found that migraine significantly increased the risk of both preeclampsia and ischemic stroke during pregnancy and proposed that hormonal and inflammatory changes associated with migraine might interact with pregnancy-related endothelial dysfunction to increase the risk of stroke.28 Our study raises the question of whether this increased stroke risk might be attributable to higher risk of RCVS. Further studies are needed, including both prospective clinical studies and basic research, to better characterize the complex interactions between pregnancy-related hormonal and inflammatory changes and cerebral autoregulation, and the factors that influence the development of RCVS.
Our study is limited by its retrospective nature and small numbers. The small sample size may have been underpowered to detect important but small differences between groups, increasing the likelihood of a type II error. Therefore, our results must be considered hypothesis-generating; ORs should be interpreted with caution because of wide CIs. Recall bias may have influenced the findings; most of our RCVS cases presented with headache, and women may have been more inclined to report a history of migraine in this setting (or clinicians may have been more inclined to ask about it). Because of the retrospective nature of the study, we were limited in our ability to assess patient outcomes after discharge. Disability at 90 days, ability of patients to care for their newborns, and outcomes of subsequent pregnancies will all be outcomes of interest in future planned prospective studies. Although our database screening was designed for 100% capture of strokes at our institution, some cases of PAS at our center may have been missed. Our results from a single urban institution may not apply to the general population, particularly given the high prevalence of Hispanics in our group. Finally, by excluding patients over 40 or under 18 and patients with aneurysmal SAH and primary intracranial hemorrhage, particularly hypertensive or spontaneous intracranial hemorrhage, we likely missed some instances of PAS; future studies are planned to include a wider age range and hemorrhagic strokes.
We found that in our cohort, women with PAS had fewer vascular risk factors than other young women with stroke. CVT and RCVS were more common in PAS, most of which occurred postpartum, and history of migraine appeared to increase the odds of postpartum RCVS. Additional studies are planned to further elucidate the relationship between migraine and postpartum RCVS and ideally to develop effective preventive strategies for stroke in this unique patient population.
AUTHOR CONTRIBUTIONS
Eliza Miller: data collection, drafting of manuscript. Shadi Yaghi: data analysis, critical revision of manuscript. Amelia Boehme: data analysis, critical revision of manuscript. Joshua Willey: critical revision of manuscript, supervision. Mitchell Elkind: critical revision of manuscript. Randolph Marshall: reviewing and revising the manuscript, supervision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
E. C. Miller reports no disclosures. S. Yaghi received funds from NINDS StrokeNet. A. K. Boehme is supported by NINDS NIH T32 NS007153-31. J. Z. Willey received funds from the NIH (NINDS K23 073104) and is a consultant for Heartware Incorporated. M. S. V. Elkind receives funding from NIH/NINDS, diaDexus, Inc, and BMS-Sanofi Pharmaceutical Partnership; serves as a consultant for BMS-Pfizer Partnership; serves on scientific advisory boards for Boehringer-Ingelheim, Inc., BMS-Pfizer Partnership, Daiichi-Sankyo, Janssen Pharmaceuticals, Biogen IDEC, Biotelemetry/Cardionet, and Sanofi-Regeneron Partnership; provides expert witness testimony for Merck/Organon related to Nuvaring and stroke and for BMS-Sanofi Partnership related to Plavix and stroke; serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; served as Resident and Fellow Section Editor for Neurology; and receives royalties from UpToDate for chapters related to cryptogenic stroke and hemicraniectomy. R. S. Marshall received funding from the NIH/NINDS, received publishing royalties for OnCall Neurology (Elsevier, 2003–present), and serves on the Editorial Boards of Neurology, Stroke, JAMA Neurology, and Annals of Neurology. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Bushnell C, McCullough LD, Awad IA, et al. ; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council for High Blood Pressure Research. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 2005;106:509–516. [DOI] [PubMed] [Google Scholar]
- 3.Petitti DB, Sidney S, Quesenberry CP, Bernstein A. Incidence of stroke and myocardial infarction in women of reproductive age. Stroke 1997;28:280–283. [DOI] [PubMed] [Google Scholar]
- 4.Grear KE, Bushnell CD. Stroke and pregnancy: clinical presentation, evaluation, treatment, and epidemiology. Clin Obstet Gynecol 2013;56:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med 1996;335:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaigobin C, Silver FL. Stroke and pregnancy. Stroke 2000;31:2948–2951. [DOI] [PubMed] [Google Scholar]
- 7.Hovsepian DA, Sriram N, Kamel H, Fink ME, Navi BB. Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke 2014;45:1947–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke 2011;42:2564–2570. [DOI] [PubMed] [Google Scholar]
- 9.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MSV. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014;370:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott CA, Bewley S, Rudd A, et al. Incidence, risk factors, management, and outcomes of stroke in pregnancy. Obstet Gynecol 2012;120:318–324. [DOI] [PubMed] [Google Scholar]
- 11.Lin SY, Hu CJ, Lin HC. Increased risk of stroke in patients who undergo cesarean section delivery: a nationwide population-based study. Am J Obstet Gynecol 2008;198:391.e1–391.e7. [DOI] [PubMed] [Google Scholar]
- 12.Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke 2009;40:2276–2293. [DOI] [PubMed] [Google Scholar]
- 13.Monte L, Ellis R. Fertility of Women in the United States: 2012. United States Census Bureau; 2014. Available at: http://www.census.gov/library/publications/2014/demo/p20-575.html. Accessed October 8, 2015. [Google Scholar]
- 14.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012;11:906–917. [DOI] [PubMed] [Google Scholar]
- 15.Kissela BM, Khoury JC, Alwell K, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 2012;79:1781–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke 1988;19:1159–1170. [DOI] [PubMed] [Google Scholar]
- 17.Katz BS, Fugate JE, Ameriso SF, et al. Clinical worsening in reversible cerebral vasoconstriction syndrome. JAMA Neurol 2014;71:68. [DOI] [PubMed] [Google Scholar]
- 18.Chen SP, Fuh JL, Wang SJ, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol 2010;67:648–656. [DOI] [PubMed] [Google Scholar]
- 19.Singhal AB. Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 2004;61:411–416. [DOI] [PubMed] [Google Scholar]
- 20.Feske SK, Singhal AB. Cerebrovascular disorders complicating pregnancy. Continuum (Minneap Minn) 2014;20(1 Neurology of Pregnancy):80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razmara A, Bakhadirov K, Batra A, Feske SK. Cerebrovascular complications of pregnancy and the postpartum period. Curr Cardiol Rep 2014;16:532. [DOI] [PubMed] [Google Scholar]
- 22.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension 2007;50:14–24. [DOI] [PubMed] [Google Scholar]
- 23.Wallace K, Tremble SM, Owens MY, Morris R, Cipolla MJ. Plasma from patients with HELLP syndrome increases blood-brain barrier permeability. Reprod Sci 2015;22:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhal AB, Hajj-Ali RA, Topcuoglu MA. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 2011;68:1005–1012. [DOI] [PubMed] [Google Scholar]
- 25.Loder S, Sheikh HU, Loder E. The prevalence, burden, and treatment of severe, frequent, and migraine headaches in US minority populations: statistics from National Survey studies. Headache 2015;55:214–228. [DOI] [PubMed] [Google Scholar]
- 26.Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke 2010;41:2505–2511. [DOI] [PubMed] [Google Scholar]
- 27.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ 2008;337:a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wabnitz A, Bushnell C. Migraine, cardiovascular disease, and stroke during pregnancy: systematic review of the literature. Cephalalgia 2015;35:132–139. [DOI] [PubMed] [Google Scholar]