Abstract
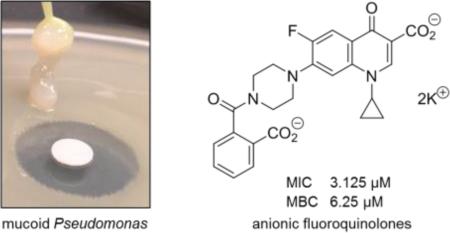
Pseudomonas aeruginosa is a common biofilm-forming bacterial pathogen implicated in diseases of the lungs. The extracellular polymeric substances (EPS) of respiratory Pseudomonas biofilms are largely comprised of anionic molecules such as rhamnolipids and alginate that promote a mucoid phenotype. In this paper, we examine the ability of negatively-charged fluoroquinolones to transverse the EPS and inhibit the growth of mucoid P. aeruginosa. Anionic fluoroquinolones were further compared with standard antibiotics via a novel microdiffusion assay to evaluate drug penetration through pseudomonal alginate and respiratory mucus from a patient with cystic fibrosis.
Keywords: fluoroquinolone, Pseudomonas, mucoid, biofilm, antibacterial
Graphical Abstract
Pseudomonas aeruginosa is an opportunistic bacterium that is frequently implicated in burn wound, bladder, and lower respiratory tract infections [1]. The Gram-negative aerobe is also the primary etiology of chronic pulmonary infections in cystic fibrosis (CF) due to its ability to evade host defenses within the accumulated mucus lining the airway of the lungs. When Pseudomonas colonizes the lower respiratory tract, neutrophils infiltrate the lungs and release reactive oxygen species (ROS) to suppress the infection. Exposure to ROS is thought to cause DNA-level mutations and activation of gene products that further protects P. aeruginosa from the immune system [2]. Among these are the enzymes and proteins involved in the biosynthesis and secretion of rhamnolipids [3] and alginate [4], two components of the extracellular polymeric substance (EPS) found in Pseudomonas biofilms of CF airways.
Like the sulfated and carboxylated components of mucus located in the lower respiratory tract, rhamnolipids and alginate possess an overall negative charged (Figure 1) [5]. In CF, the anionic environment of the lungs is amplified by DNA and F-actin released from necrotic neutrophils. The physiochemical properties of this dense complex mixture of negatively charged biomolecules consequentially serves as a barrier against cationic and zwitterionic antimicrobials [6]. It was rationalized from here that antibiotics carrying a net −1 or −2 charge would more readily transverse these barriers and reach the bacteria entrenched within due to the lack of ionic interactions with the EPS and mucus. On this premise, we set forth to evaluate anionic fluoroquinolones as growth inhibitors of biofilm-producing P. aeruginosa and their ability to penetrate the alginate component of Pseudomonas EPS and CF respiratory mucus.
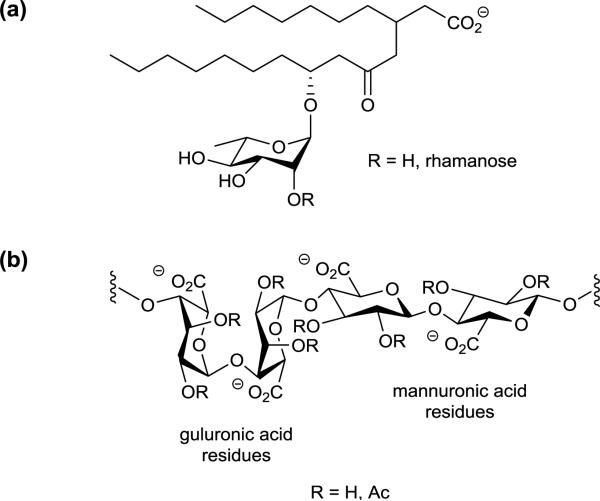
Figure 1.
Structures of (a) rhamnolipids and (b) alginate in biofilm-producing Pseudomonas aeruginosa.
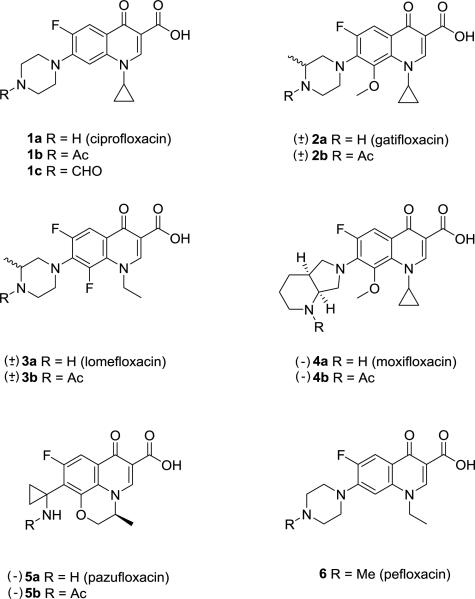
The initial series of anionic fluoroquinolones explored were derived from FDA-approved fluoroquinolones 1a-5a (Figure 2) including ciprofloxacin (1a), a first-line treatment for urinary tract infections due to P. aeruginosa. The initial design of the anionic fluoroquinolones was to acetylate the C-7 amino residue of the quinolone ring to preclude zwitterion formation at physiological pH. The reactions were performed in dichloromethane with acetic anhydride (1.5 equiv.), Et3N (1.3-2.6 equiv.), and DMAP (0.1 equiv.) to afford the N-acetyl fluoroquinolones 1b-5b. The derivatives and parent compounds were then evaluated for antipseudomonal activity against the reference non-mucoid P. aeruginosa PAO1 and its mucoid derivative, PAO581. The genomes of both bacterial strains have been sequenced [7,8] with the mutation causing the stable production of EPS alginate previously defined [9].
Figure 2.
N-Protio fluoroquinolones and N-substituted derivatives 1-6.
The N-acetyl fluoroquinolones 1b-5b were tested by the conventional disc diffusion (Kirby Bauer) assay on LB and PIA to compare the ability of each analog to inhibit bacterial viability on regular growth and mucoidy-promoting agar media, respectively (Table 1). The compounds were evaluated as the K+ salts using 10 mm cellulose discs impregnated with 10 μM of drug. Following overnight air incubation at 37 °C, the plates were examined for zones of growth inhibition and it was discovered that acetylation of the C-7 amino residue negated the inhibitory activity for most of the derivatives against both PAO variants. In contrast, the unmodified fluoroquinolones proved to be active displaying moderate to strong growth inhibitory effects with a zone diameter range of 10-38 mm.
Table 1.
Zones of growth inhibition (mm) against non-mucoid PAOl and mucoid PAO581 for fluoroquinolones 1-6.a
| strain | PAO1 | PAO1 | PAO581 | PAO581 |
|---|---|---|---|---|
| media | LB | PIA | LB | PIA |
| ciprofloxacin (1a) | 30 | 24 | 38 | 26 |
| gatifloxacin (2a) | 29 | 21 | 31 | 23 |
| lomefloxacin (3a) | 23 | 12 | 29 | 15 |
| moxifloxacin (4a) | 24 | 10 | 31 | 14 |
| pazufloxacin (5a) | 25 | 17 | 30 | 20 |
| pefloxacin (6) | 18 | 12 | 19 | 14 |
| 1b | 10 | 0 | 0 | 13 |
| 1c | 18 | (20)c | 21 | 9 |
| 2b | 0 | 0 | 0 | 0 |
| 3b | 0 | 0 | 0 | 0 |
| 4b | 0 | 0 | 0 | 0 |
| 5b | 0 | 0 | 0 | 0 |
Compounds were tested as K+ salts at 10 μM concentration in water.
b Pseudomonas isolation agar (PIA) is supplemented with 5% glycerol as a C source for mucoid production. Luria-Bertani (LB) agar lacks glycerol to promote the mucoid phenotype.
Partial inhibition.
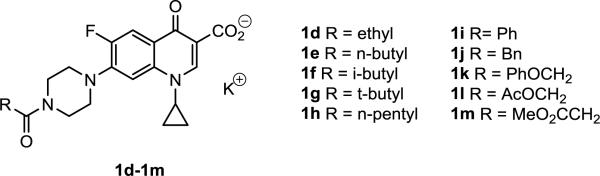
As expected, ciprofloxacin demonstrated the greatest antipseudomonal activity and its N-acetylated form 1b was the lone derivative in the series to inhibit growth. To further explore impact of lipophilicity on the activity, the N-formyl (1c, Figure 2) [10] and 10 additional N-acyl derivatives (1d-1m, Figure 3) of ciprofloxacin [11,12] were prepared for microbiological evaluation. The compounds were once again tested at 10 μM as K+ salts and with the exception of the N-formyl analog 1c, each was found to have no inhibitory effects on Pseudomonas growth. It was concluded from the data that the anionic character and lipophilicity (clogP 1.96 – 3.98) furnished to analogs 1b-1m of ciprofloxacin (clogP 1.63) had adversely affected antimicrobial activity. These findings further suggested that an ionizable group at the C-7 position of the quinolone ring may be a pre-requisite for transport and/or gyrase binding in Pseudomonas as noted for the reduced efficacy of pefloxacin (6, Table 1).
Figure 3.
N-Acyl fluoroquinolones 1d-1m.
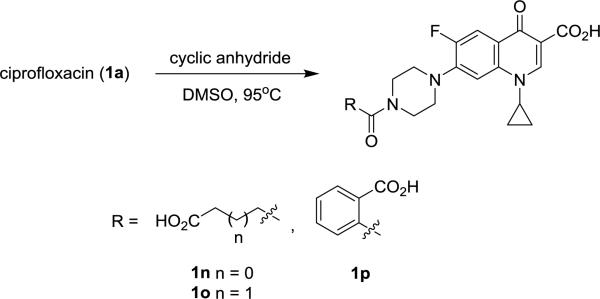
A second series of ciprofloxacin analogs modified with an ionizable carboxylate substituent on the C-7 piperazine ring were subsequently prepared (Scheme 1) and evaluated. Compounds 1n-1p were synthesized by heating ciprofloxacin (1 equiv.) with their respective cyclic anhydrides (1 equiv.) overnight in DMSO [13]. Activity comparisons were made with the polycationic aminoglycoside tobramycin and the carboxypenicillin ticarcillin, which like the analogs carries a net −2 charge.
Scheme 1.
Synthesis of carboxyfluoroquinlones 1n-1p.
In this series, the dianionic N-phthalyl derivative 1p was the only compound found to effectively inhibit the growth of Pseudomonas (Table 2). This was a surprising finding on comparison with the inactive N-benzoyl analog 1i. Moreover, a 53-58% reduction in zone size was observed for bacteria grown on the mucoidy-promoting PIA media in contrast to 20-32% for ciprofloxacin. When compared with tobramycin, the N-phthalyl analog exhibited similar activity while ticarcillin was found to be inactive suggesting that the PAO strains used in the study were harboring the genes for one or more penicillin-degrading β-lactamase (e.g., AmpC) [14].
Table 2.
Zones of growth inhibitions (mm) for fluoroquinolones 1n-1p.a
| strain | PAO1 | PAO1 | PAO581 | PAO581 |
|---|---|---|---|---|
| media | LB | PIA | LB | PIA |
| 1n | 0 | 0 | 0 | 0 |
| 1o | 0 | 0 | 0 | 0 |
| 1p | 23 | 11 | 31 | 13 |
| tobramycin | 18 | 13 | 20 | 17 |
| ticarcillin | 0 | 0 | 0 | 0 |
Compounds were tested as K+ salts at 10 μM concentrations in water.
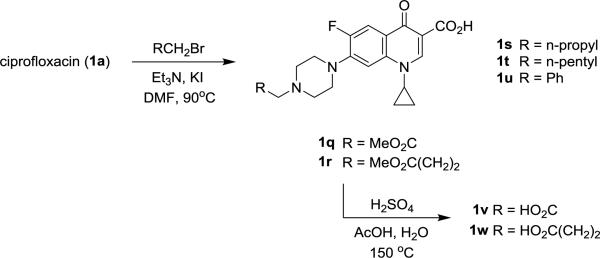
With these results, an additional series of carboxy-fluoroquinolones were evaluated. The acetic and butyric acid derivatives 1v and 1w were prepared by acid hydrolysis of their parent methyl esters 1q and 1r, respectively (Scheme 2) [15]. Additional N-alkyl derivatives 1s-1u were synthesized from their respective alkyl bromides for comparison with the analogs and the N-methyl fluoroquinolone, pefloxacin 6 (Figure 2). The antipseudomonal activity for this series was found to be mostly limited to N-alkylated analogs 1r and 1s (Table 3). On comparison with pefloxacin, the methyl ester derivative 1r displayed similar activity against mucoid PAO581.
Scheme 2.
Synthesis of N-alkyl fluoroquinolones 1q-1w.
Table 3.
Zones of growth inhibitions (mm) for fluoroquinolones 1q-1w.a
| strain | PAO1 | PAO1 | PAO581 | PAO581 |
|---|---|---|---|---|
| media | LB | PIA | LB | PIA |
| 1q | 0 | 0 | 0 | 0 |
| 1r | 16 | 6 | 17 | 9 |
| 1s | 23 | 0 | 13 | 0 |
| 1t | 0 | 0 | 0 | 0 |
| 1u | 0 | 0 | 13 | 0 |
| 1v | 0 | 0 | 0 | 0 |
| 1w | 0 | 0 | 0 | 0 |
| pefloxacin (6) | 18 | 12 | 19 | 14 |
Compounds were tested as K+ salts at 10 μM concentrations in water.
Minimum inhibitory and bactericidal concentrations were subsequently determined by broth microdilution for the two most active fluoroquinolones from the study, the N-formyl and N-phthalyl substituted ciprofloxacin analogs 1c and 1p, respectively (Table 4). The carboxy-fluoroquinolone 1p was found to be inferior to both ciprofloxacin and tobramycin in liquid culture. Likewise, the N-formyl analog 1c proved to be two fold less effective at inhibiting the pseudomonal growth compared to ciprofloxacin and tobramycin. Of note, the MIC results (i.e., ciprofloxacin ≈ tobramycin > 1c = 1p) did not correlate to the zone data (i.e., ciprofloxacin > 1p > tobramycin > 1c) performed on solid media. It is believed that the smaller zone sizes exhibited for the charged fluoroquinolones and tobramycin was a consequence of truncated diffusion through the uncharged agar media. These results prompted us to examine the influence of charge on the ability the antibiotics to penetrate through the alginate component of biofilm Pseudomonas EPS and CF respiratory mucus.
Table 4.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for select compounds.a
| strain | PAO1 | PAO1 | PAO581 | PAO581 |
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| 1c | 3.125 | 6.25 | 3.125 | 6.25 |
| 1p | 3.125 | 6.25 | 3.125 | 12.5 |
| ciprofloxacin | 1.56 | 6.25 | 1.56 | 6.25 |
| tobramycin | 3.125 | 1.56 | 0.78 | 6.25 |
Compounds were tested as K+ salts in Luria-Bertani broth. Values reported in μM.
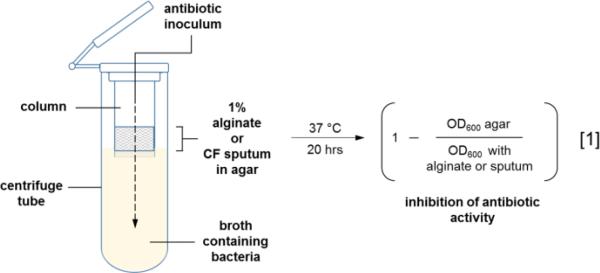
To probe the penetrating ability of charged antibiotics through solid media, a novel microdiffusion assay was developed using centrifuge filter columns containing 1% PAO581 alginate [16] or CF sputum in Noble agar. As depicted in Figure 4, the filter columns containing the semi-permeable barriers were inserted into a 1.5 mL centrifuge tube containing a 5 × 105 inoculum of P. aeruginosa and 50 μL of 25 μM antibiotic was applied to the agar surface. The tubes were sealed and incubated overnight at 37 °C with shaking. The penetrating ability was assessed by calculating the inhibition of antibiotic activity from the OD600 measurements and Equation 1 with Noble agar, an uncharged medium, representing 100% drug diffusion into the broth culture.
Figure 4.
Microdiffusion assay design.
The microdiffusion assay revealed that Pseudomonas alginate and the components in CF sputum likely modulate antibiotic penetration (Table 5). The data suggests that the negatively charged sugars in alginate (i.e., mannuronate, guluronate) reduces the EPS permeation of both charged and zwitterionic antibiotics through Pseudomonas biofilms. Based on the preliminary findings from this assay, the anionic fluoroquinolones 1c and 1p were less hindered by the alginate barrier compared to ciprofloxacin and tobramycin. Conversely, the constituents found in CF patient sputum did not appear to affect the penetration of these standard antibiotics. Follow up studies using sputum from different CF patient populations will be performed to further corroborate these findings.
Table 5.
Inhibition of antibiotic activity using PAO581 in the microdiffusion assay.
| strain | PAO581 alginate | CF sputum |
|---|---|---|
| 1c | 0.61 | 0.89 |
| 1p | 0.80 | 0.49 |
| ciprofloxacin | 0.97 | −0.33 |
| tobramycin | 0.98 | 0.0 |
n = 0 (drag bioavailability not affected)
n = 0 < to 1 (drug bioavailability decreased)
n < 0 (drug bioavailability increased)
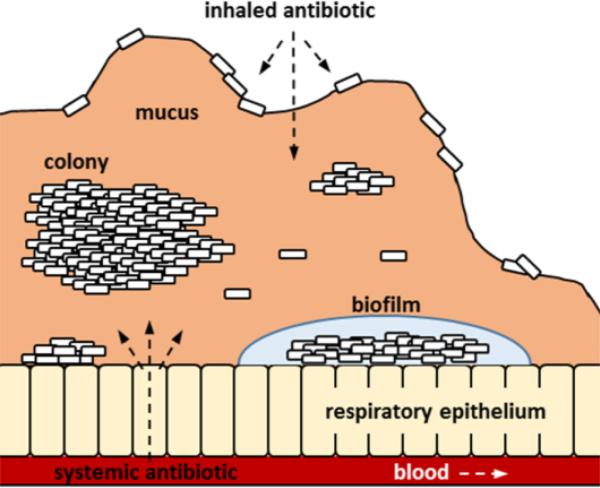
In summary, select anionic derivatives of the fluoroquinolone ciprofloxacin were found to possess antipseudomonal activity against biofilm-producing P. aeruginosa. The C-7 position was highly sensitive to chemical modification that may have affected DNA gyrase binding and/or cellular entry. It was further ascertained by the microdiffusion assay that the anionic fluoroquinolones appeared to have less chemical interactions with the alginate constituent from the EPS of PAO581 than ciprofloxacin and tobramycin. Conversely, the data suggests that the standard antibiotics penetrate CF respiratory mucus more effectively than the anionic fluoroquinolones. As a hallmark of the CF pathology, the hypersecretion of respiratory mucus facilitates persistent infection of the lower airways by Pseudomonas and adversely affects drug bioavailability (Figure 5). Treatment efficacy with inhaled and systemic antibiotics is not only predicated by the chemical constituents in the secreted mucus and Pseudomonas EPS but also the physiochemical properties of the drugs. This study established that ciprofloxacin can be chemically modified to modulate permeation in bacterial EPS and respiratory mucus as discerned by the microdiffusion assay. If developed further, this novel in vitro method to evaluate drug penetration against various biological barriers could become useful tool to help guide antimicrobial therapy in patients with chronic pulmonary disease.
Figure 5.
Respiratory mucus and EPS of Pseudomonas biofilms reduce the bioavailability of inhaled and systemic antibiotics.
Acknowledgements
This research was supported in part by the Marshall University School of Pharmacy FRS Grant Program and the Office of Experiential Learning for financial support. Special thanks is also given to NASA West Virginia EPSCoR grant program, the National Center for Research Resources, the National Center for Advancing Translational Sciences, and National Institutes of Health (NIH), through Grant UL1TR000117. HDY is supported by NIH P20GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence, and is the co-founder of Progenesis Technologies, LLC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Driscoll JA, Brody SL, Kollef MH. Drugs. 2007;67:351. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ciofu O, Riis B, Pressler T, Poulsen HE, Høiby N. Antimicrob. Agents. Chemother. 2005;49:2276. doi: 10.1128/AAC.49.6.2276-2282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinckx T, Wei Q, Matthijs S, Cornelis P. Microbiology. 2010;156:678. doi: 10.1099/mic.0.031971-0. [DOI] [PubMed] [Google Scholar]
- 4.Mathee K, Ciofu O, Sternberg C, Lindum PW, Campbell JI, Jensen P, Johnsen AH, Givskov M, Ohman DE, Molin S, Høiby N, Kharazmi A. Microbiology. 1999;145:1349. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 5.Mann EE, Wozniak DJ. FEMS Microbiol. Rev. 2012;36:893. doi: 10.1111/j.1574-6976.2011.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatch RA, Schiller NL. Antimicrob. Agents Chemother. 1998;42:974. doi: 10.1128/aac.42.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Nature. 2000;406:959. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 8.Yin Y, Withers TR, Govan JR, Johnson SL, Yu HD. Genome Announc. 2013;1:e00834–13. doi: 10.1128/genomeA.00834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu D, Eisinger VM, Head NE, Pier GB, Yu HD. Microbiology. 2008;154:2119. doi: 10.1099/mic.0.2008/017368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vavříková E, Polanc S, Kočevar M, Horváti K, Bosze S, Stolaříková J, Vávrová K, Vinšová J. Eur. J. Med. Chem. 2011;46:4937. doi: 10.1016/j.ejmech.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 11.Ji C, Miller PA, Miller MJ. ACS Med. Chem. Lett. 2015;6:707. doi: 10.1021/acsmedchemlett.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cormier R, Burda WN, Harrington L, Edlinger J, Kodigepalli KM, Thomas J, Kapolka R, Roma G, Anderson, Burda E, Turos E, Shaw LN. Bioorg. Med. Chem. Lett. 2012;22:6513. doi: 10.1016/j.bmcl.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noël S, Gasser V, Pesset B, Hoegy F, Rognan D, Schalk IJ, Mislin GL. Org. Biomol. Chem. 2011;9:8288. doi: 10.1039/c1ob06250f. [DOI] [PubMed] [Google Scholar]
- 14.Hengzhuang W, Ciofu O, Yang L, Wu H, Song Z, Oliver A, Høiby N. Antimicrob. Agents Chemother. 2013;57:196. doi: 10.1128/AAC.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fardeau S, Dassonville-Klimpt A, Audic N, Sasaki A, Pillon M, Baudrin E, Mullié C, Sonnet P. Bioorg. Med. Chem. 2014;22:4049. doi: 10.1016/j.bmc.2014.05.067. [DOI] [PubMed] [Google Scholar]
- 16.Damron FH, Yu HD. J. Bacteriol. 2011;193:286. doi: 10.1128/JB.01132-10. [DOI] [PMC free article] [PubMed] [Google Scholar]