Abstract
Transepithelial photorefractive keratectomy (tPRK), where both the epithelium and stroma are removed in a single-step, is a relatively new procedure of laser refractive error correction. This study compares the 3-month results of myopia and compound myopic astigmatism correction by tPRK or conventional alcohol-assisted PRK (aaPRK).
This prospective, nonrandomized, case–control study recruited 148 consecutive patients; 93 underwent tPRK (173 eyes) and 55 aaPRK (103 eyes). Refractive results, predictability, safety, and efficacy were evaluated during the 3-month follow-up. The main outcome measures were uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), and mean refractive spherical equivalent (MRSE).
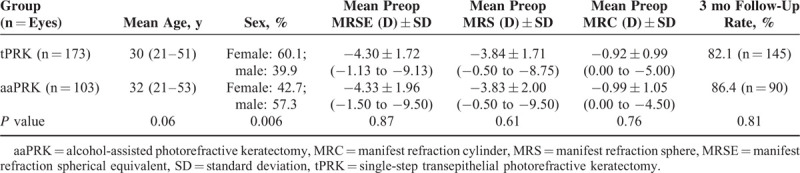
Mean preoperative MRSE was −4.30 ± 1.72 D and −4.33 ± 1.96 D, respectively (P = 0.87). The 3-month follow-up rate was 82.1% in the tPRK group (n = 145) and 86.4% in aaPRK group (n = 90), P = 0.81. Postoperative UDVA was 20/20 or better in 97% and 94% of eyes, respectively (P = 0.45). In the tPRK and aaPRK groups, respectively, 13% and 21% of eyes lost 1 line of CDVA, and 30% and 31% gained 1 or 2 lines (P = 0.48). Mean postoperative MRSE was −0.14 ± 0.26 D in the tPRK group and −0.12 ± 0.20 D in the aaPRK group (P = 0.9). The correlation between attempted versus achieved MRSE was equally high in both groups.
Single-step transepithelial PRK and conventional PRK provide very similar results 3 months postoperatively. These procedures are predictable, effective, and safe for correction of myopia and compound myopic astigmatism.
INTRODUCTION
The original method to remove the epithelium before the excimer laser ablation was manual mechanical scraping, which was later enhanced by using an alcohol solution or brush.1 In 2003, Camellin2 proposed a new alcohol-assisted technique called laser-assisted subepithelial keratectomy (LASEK) that enabled the epithelium to be preserved as a flap and reapplying it to the ocular surface after treatment. Epithelial laser in situ keratomileusis (Epi-LASIK) is another method that uses an epithelial flap, but is performed with a microkeratome with a blunt oscillating blade.3 In the late 1990s, transepithelial photorefractive keratectomy (PRK) was introduced where removal of the epithelium is carried out with laser phototherapeutic ablation followed by a laser refractive ablation of the stroma. This 2-step technique was not widely used due to the prolonged surgery time with the older generation of lasers, increased pain, and a lack of adjusted nomograms.4–7
Newer generation of faster lasers and improved ablation algorithms and nomograms have over the years, allowed development of a new (tPRK) variant of transepithelial PRK. Single-step transepithelial PRK allows removing the epithelium and stroma in a single step with 1 ablation profile. This profile is calculated taking into account data from the literature estimating the central epithelial thickness of a normal cornea to be 55 and 65 μm at 4 mm from the center,8 superimposed on the corneal wavefront guided aspheric ablation profiles. Several studies have compared 2-step transepithelial PRK to standard PRK performed with alcohol or mechanical epithelial removal with variable results.4–7 tPRK is a relatively new procedure and a limited number of publications are currently available. Only 3 direct comparisons between a single-step tPRK and conventional PRK are published so far.9–11 Meanwhile, the procedure has undergone several minor modifications and nomogram adjustments.12–14 Thus, there is a need for updated comparative evaluations based on a larger number of eyes. The aim of this study is to compare 3-month refractive results, predictability, safety, and efficacy of single-step transepithelial PRK with alcohol-assisted PRK (aaPRK) when used to correct myopia and compound myopic astigmatism.
METHODS
This prospective, consecutive, nonrandomized case–control study comprised eyes that underwent either single-step transepithelial PRK (tPRK) or aaPRK between August 2012 and April 2014, at the Oftalmika Eye Hospital, Bydgoszcz, Poland.
Before the procedure, each patient was adequately informed about the study as well as the risks and benefits of the surgery, and provided signed informed consent in accordance with the Declaration of Helsinki. The study was approved by the local ethical board committee.
Inclusion criteria were as follows: age over 21 years, primary myopia or compound myopic astigmatism, preoperative manifest refraction spherical equivalent (MRSE) within the range of −1.0 to −9.5 D, a stable refractive error for at least 12 months before the surgery, contact lens discontinuation for at least 3 weeks, estimated corneal stromal bed thickness of more than 300 μm at the thinnest point. Exclusion criteria were previous ocular surgery, any diagnosed ocular disease, a history of ocular trauma, irregular astigmatism on corneal topography, systemic disease that could affect corneal wound healing, and pregnancy.
A total of 277 eyes of 148 consecutive patients were included. One patient developed retrobulbar neuritis of the left optic nerve 6 weeks after the surgery and this eye was excluded from the analysis. One hundred seventy-three eyes underwent tPRK and 103 eyes underwent aaPRK. The choice of the procedure was based on patients’ preferences as tPRK is a more expansive procedure. Patient demographics and preoperative variables are given in Table 1. There are no significant differences in preoperative variables of patients in the tPRK and aaPRK groups, except the gender. The percentage of females is 60.1 in tPRK group and 42.7 in aaPRK group. Patients who attended all visits, thus without any missing data, were included into analysis.
TABLE 1.
Demographics and Preoperative Variables of Patients in the tPRK and aaPRK Groups
Preoperative Examination
Preoperative information on general and ocular medical history, contact lens wear, and medication use was obtained from each patient. The examination included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest and cycloplegic refraction, slit lamp biomicroscopy, tonometry, specular microscopy (EM-3000, Tomey, Erlangen, Germany), pupillometry, Scheimpflug camera tomography (Sirius, SCHWIND eye-tech-solutions GmbH, Kleinostheim, Germany), ocular aberrometry (IRX-3, Imagine Eyes, Paris, France), and fundus examination. All ocular aberrations were measured for pupil diameter of 4 mm.
Surgical Technique
All surgeries were performed with 6th-generation Amaris excimer laser, version 750 S (Schwind eye-tech-solutions). Ablations were based on aberration-free algorithms calculated using ORK-CAM software. Treatments were performed by 2 surgeons using an identical surgical protocol. The treatments were mostly aimed at emmetropia baring a few eyes with a target refraction of −0.25 D or −0.5 D (nondominant eye in case of older patients). However, in statistical analysis and standardized graphs presented as results in this study, only eyes targeted plano (plano target) were taken it into account wherever necessary.
Before the surgery, proparacaine hydrochloride 0.5% drops (Alcaine, Alcon, Fort Worth, TX) were instilled 3 times within a 5-min interval. The eyelids were opened using a wire lid speculum. In the aaPRK group, the cornea was exposed to a 20% ethyl alcohol solution for 30 s with the use of a well. Subsequently, a superficial cut of the epithelium was made with either an 8.5- or 9.5-mm diameter trephine. The epithelium was mechanically debrided with a spatula. In the tPRK group, where aspheric aberration-free TransPRK ablation algorithm was used (Schwind eye-tech-solutions), the epithelium was removed during laser ablation only from the area of the total ablation zone, which is the sum of the optical and transition zones. In both groups in all cases, 0.02% mitomycin C (MMC) was applied for 2 min based on the standard protocol.15 MMC application was followed by generous irrigation of the eye with room temperature balanced solution. Intraoperative complications were not noted. After the surgery, a bandage contact lens was applied (Acuvue Oasis, J&J, New Brunswick, TX) for 7 days. The postoperative regimen included 0.3% tobramycin drops (Tobrexan, Alcon, New Brunswick, TX) for 1 month, 0.1% diclofenac drops (Dicloabak, Laboratoires THEA, Clermont-Ferrand, France) for 1 month, 0.15% hyaluronic acid drops (Biolan, Penta Arzneimittel, Stulln, Germany) for 3 months, and 0.1% dexamethasone drops (Dexafree, Laboratoires THEA, Stulln, Germany) 3 times daily for the first month, twice daily for the second month, and once daily for the third month.
Postoperative Examinations
Patients were instructed to visit the clinic for postoperative examinations after 1 day, 1 week, 1 month, and 3 months. Examinations at 1 day, 1 week, and 1 month included UDVA, CDVA, manifest refraction, tonometry, and slit lamp biomicroscopy. Corneal haze was evaluated as proposed by Fantes at al16 (0 = no haze; 0.5 = trace haze on oblique illumination; 1 = corneal cloudiness not interfering with the visibility of fine iris details; 2 = mild effacement of fine iris details; 3 and 4 = details of the lens and iris not discernible). At the last visit, Scheimpflug camera tomography and ocular aberrometry were also performed. All ocular aberrations were measured for pupil diameter of 4 mm. Moreover, immediately after the surgery and 1 week after we used a discrete, 10-category verbal rating scale (VRS, 1—no pain and 10—the worst possible pain) to evaluate pain level. Three months after the surgery patients were asked about overall satisfaction with the surgery and (high, moderate, low, not satisfied), and whether they would decide to have a surgery again (yes, no).
Statistical Analysis
Data were analyzed using Datagraph-med version 4.20 d (Ingenieurbüro Pieger GmbH, Wendelstein, Germany), which is a relational database designed for refractive data analysis. Statistical analysis was performed using Statistica 10 software (Statsoft Inc., Tulsa, OK). The normality of the data was verified with Kolmogorov–Smirnov test. When numeric data were compared the z-test was used (for distribution of Mean preop MRSE, Mean preop MRS, Mean preop MRC, and respective postoperative values). For the comparison of nominal data the chi-squared test (for sex, follow-up rate, majority of complications, and satisfaction) or Fisher exact test (for haze) were used. For all tests, P < 0.05 was considered statistically significant.
RESULTS
The mean ablation time was 44.35 ± 13.68 s in the tPRK group and 16.85 ± 9.58 s in the aaPRK group (P < 0.001), whereas mean time of the whole procedure, from introduction to removal of the lid speculum, was 165 ± 24.62 s and 254 ± 32.14 s, respectively (P < 0.001). In the tPRK and aaPRK group, respectively, the mean diameter of the optical zone was 6.96 ± 0.45 and 7.11 ± 0.43 mm (P = 0.47), and the transition zone was 1.39 ± 0.50 and 1.29 ± 0.63 mm (P = 0.55). The minimal estimated stromal residual thickness was 306 μm among all the analyzed eyes (309 μm in tPRK group and 306 μm in aaPRK group).
The 3-month follow-up rate was 82.1% in the tPRK group (n = 145) and 86.4% in the aaPRK group (n = 90), because some of the patients were not able or not willing to attend all of the required visits. Mean postoperative MRSE was −0.14 ± 0.26 D in the tPRK group and −0.12 ± 0.20 D in the aaPRK group (P = 0.9). In the tPRK group, the postoperative refractive spherical equivalent in 63% of eyes were within ±0.13 D, 12% within +0.14 to +0.5 D, 24% within −0.14 to −0.5 D, and 1% within −0.5 and −1.0 D; the respective values in the aaPRK group were 70%, 4%, 26%, and 0%. The differences were not statistically significant.
Refractive results, predictability, safety, and efficacy 3 months after the surgery are shown in Figures 1 and 2 as standard graphs recommended for reporting refractive surgery outcomes.17 UDVA was 20/20 or better in 97% of eyes in the tPRK group and 94% in the aaPRK group (P = 0.45). In the tPRK group, 13% of eyes lost 1 line of CDVA and 30% gained a line or 2. In the aaPRK group, 21% of eyes lost 1 line of CDVA and 31% gained a line or 2 (P = 0.48).
FIGURE 1.
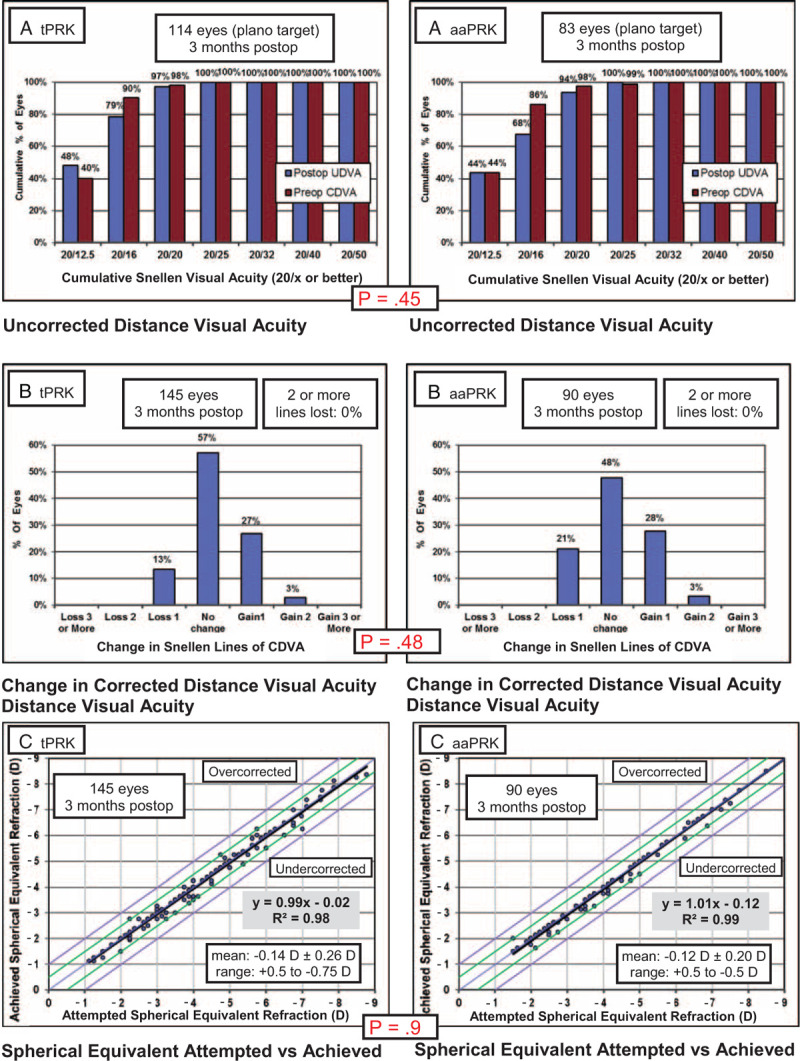
Comparison of uncorrected distance visual acuity (A), change in corrected distance visual acuity (B), and attempted vs achieved spherical equivalent (C) in single-step transepithelial photorefractive keratectomy (tPRK; left panels) and alcohol-assisted photorefractive keratectomy (aaPRK; right panels) groups.
FIGURE 2.
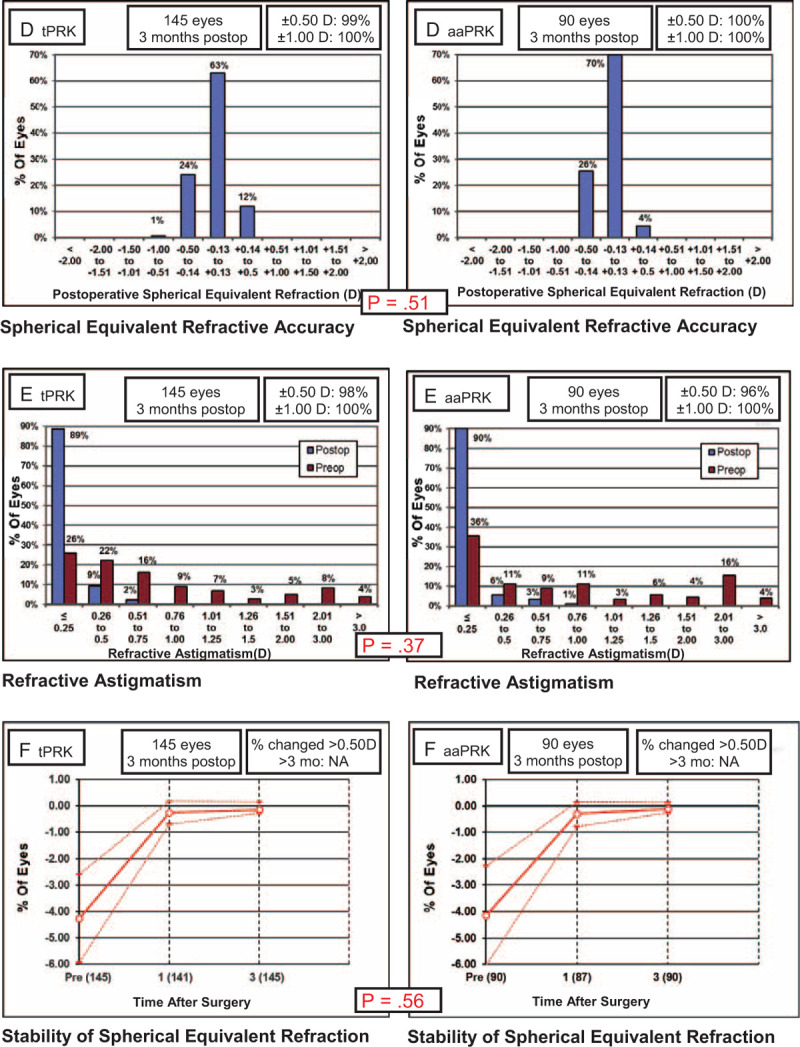
Comparison of the spherical equivalent refractive accuracy (D), refractive astigmatism (E), and stability of spherical equivalent refraction (F) in single-step transepithelial photorefractive keratectomy (tPRK; left panels) and alcohol-assisted photorefractive keratectomy (aaPRK; right panels) groups.
Mean preoperative higher order RMS for 4 mm pupil was 0.15 ± 0.15 μm in the tPRK group and 0.17 ± 0.21 μm in the aaPRK group (P = 0.21), and the postoperative values were 0.21 ± 0.23 and 0.19 ± 0.12 μm (P = 0.37), respectively. The differences between the preoperative and postoperative higher-order RMS were not significant for both groups (P = 0.13 for tPRK group and P = 0.27 for the PRK group).
The mean pain scores after the surgery were 4.78 ± 2.65 in the tPRK group and 4.59 ± 2.85 in the aaPRK group (P = 0.85). There were also no differences in pain intensity during first days after the surgery (mean scores of 4.46 ± 2.54 and 4.51 ± 2.36 in the tPRK and aaPRK groups, respectively; P = 0.86). After tPRK, 86.25% of patients declared high satisfaction with the surgery compared to 88.24% patients after aaPRK (P = 0.46). The ratio for moderate satisfaction was 13.75% for tPRK and 11.76% for aaPRK, respectively (P = 0.54). All patients would consider having the surgery again.
The total postoperative complication rate was 19.31% in the tPRK group and 14.44% in the aaPRK group (P = 0.13). A slightly higher incidence of haze was detected with the slit lamp for the tPRK group (13.79%), compared to the aaPRK group (8.89%); however, the results of the Fisher exact test suggest that the proportions of patients falling in each subcategory within each group did not differ significantly (P = 0.09). The intensity of haze was also not statistically significantly different between groups and was at the 0.5 level in all but 2 eyes after tPRK and 1 eye after aaPRK in which the incidence of haze was evaluated at level 1. During the follow up, corneal haze intensity had a tendency to decrease.
There was no statistically significant difference in the incidence of other postoperative complications, which included elevated IOP in 1.38% of eyes after tPRK and 2.22% after aaPRK; decreased visual acuity at night: 1.38% of eyes after tPRK and 1.11% after aaPRK; more intensive dry eye symptoms in 2.76% of eyes after tPRK and 2.22% after aaPRK. No postoperative complications, such as epitheliopathy, delayed re-epithelialization or recurrent corneal erosion, were not reported to a level of clinical significance in our cohort.
DISCUSSION
Aspheric aberration-free ablation profile of single-step transepithelial PRK (TransPRK) used in the study, has many implications over the standard aaPRK procedures. The ablation profile is calculated estimating that the central epithelial thickness of a normal cornea is 55 and 65 μm at 4 mm from the center.8 Therefore, the epithelial thickness profile resembles a slight hyperopic treatment (<0.75 D) and proper compensation helps to avoid hyperopic shift. The laser system is tuned to compensate for a difference in photoablative rates of the stroma and the epithelial tissue, which is approximately 20% higher in the epithelium. Since 1 epithelial ablation algorithm is used for all eyes in tPRK, regardless of the actual epithelial topometry, more stroma might be ablated than necessary in eyes with a thin epithelium, whereas in eyes with a thick epithelium the refractive part of the ablation might begin where there is still some epithelium left on the surface. In corneas with high toxicity, the epithelial thickness profile along the steepest meridian may differ from the thickness profile along the flattest meridian. Moreover, in light of the studies by Reinstein et al18 and Kanellopoulos and Asimellis19 the assumption that the epithelium thickness map is rotationally symmetrical may not be appropriate.
By means of very high-frequency digital ultrasonography, Reinstein et al18 found that the mean epithelial thickness at the corneal vertex was 53.4 ± 4.6 μm, and the average epithelial thickness map showed that the corneal epithelium was thicker inferiorly than superiorly (5.9 μm at the 3-mm radius, P < 0.001) and thicker nasally than temporally (1.3 μm at the 3-mm radius, P < 0.001). The location of the thinnest epithelium was temporally displaced on average 0.33 mm, and 0.90 mm superiorly with reference to the corneal vertex. Quite similar results were published recently by Kanellopoulos and Asimellis.19 They used spectral-domain anterior-segment optical coherence tomography and measured a mean epithelial thickness at the pupil center of 53.28 ± 3.34 μm, superiorly 51.86 ± 3.78 μm, and inferiorly 53.81 ± 3.44 μm. Both papers show high inter-individual variability of the central epithelial thickness and 3-dimensional epithelial maps. In theory, the above-mentioned findings may deteriorate the refractive results, predictability, safety, and efficacy of TransPRK ablations in comparison to the standard aaPRK procedures.
However, our clinical results analyzing largest material published so far, show the opposite. In our study in which the latest version of the tPRK algorithm was used, we did not observe statistically significant differences between the tPRK group and the aaPRK group in terms of UDVA, CDVA, and MRSE, 3 months after the surgery. The correlation between attempted versus achieved MRSE was very high in both groups with no statistically significant difference between the 2 groups. Shortly after the procedure was introduced, Luger et al,9 Aslanides et al,10 and Fadlallah et al11 evaluated relatively small cohorts and came to the similar conclusions; in myopia and compound myopic astigmatism correction, the refractive and visual outcomes of single-step transepithelial PRK are not different from those of conventional PRK. However, Luger et al9 reported that the postoperative mean spherical equivalent in tPRK was slightly hyperopic at +0.07 ± 0.23 D. Moreover, our results in both the tPRK and aaPRK groups were among the best achieved with surface ablation in terms of postoperative UDVA, postoperative refractive spherical equivalent, and refractive astigmatism.20
Unequal preoperative epithelial thickness might potentially also be a source of higher-order aberrations, but we did not find statistically significant differences in the preop and postop higher-order RMS between the groups. Thus, the natural interindividual variability of epithelial thickness maps did not deteriorate the clinical results in the studied population in a noticeable way. One reason for that may be the changes in epithelial thickness after the surgery. One may expect that the epithelial map would have some similar features before and after the surgery, for example, the epithelium may be thicker inferiorly than superiorly and thicker nasally than temporally. Reinstein et al21 and Kanellopoulos and Asimellis22 confirmed thickening of the epithelium centrally and progressively less thickening centrifugally across the central 6 mm after myopic correction. In both studies, the epithelial thickness was averaged within annular bands centered on the corneal vertex; thus, these studies do not verify the above-mentioned hypothesis.
Another potential disadvantage of transepithelial PRK is the higher total excimer laser energy load. In our study, mean ablation time was 163% longer in the TransPRK group; however, the majority of the laser energy was delivered to the epithelium. Excimer laser energy, among other effects, causes increase of the temperature of the stromal tissue, which is the main risk factor for haze formation, an inherent complication of excimer laser refractive surgery. After surface ablation, up to 52% of eyes develop some degree of corneal haze in the first months, with values most often reported are within the range of 5% to 20%.20 In our study population up to 3 months after the surgery, haze was more often detected in the tPRK group; however, the difference did not reach statistical significance (13.79% vs 8.89%, P = 0.09). In all eyes, MMC was applied for 2 min, followed by generous irrigation of the eye. The intensity of haze was very low, in almost all cases at the 0.5 level, and the difference in haze intensity between groups was not statistically significant. The nonsignificant differences in haze intensity in the 2 groups could be attributed to the use of MMC. It must be accounted here that the use of MMC does not allow us to evaluate haze formation for the 2 techniques in an unbiased way.
A weak point of our study, except lack of randomization, is that the haze was evaluated subjectively with the slit lamp by an unmasked examiner. In our opinion, the intensity of haze after transepithelial PRK and the impact of MMC on haze formation need to be further studied, preferably with masked examiners and with tools that provide objective measurements, like optical densitometry. Another week point of the paper is the difference in sex proportions between groups. There are statistical more females in tPRK group, which may be a source of bias especially in pain evaluation, as pain perception may be positively influenced by female gender.23
High-resolution spectral optical coherence tomography (OCT) with speckle contrast reduction also failed to detect differences in the corneal healing processes after tPRK and aaPRK, except for the shorter time to cover the stroma with epithelium in the tPRK group.12 The main reason explaining this observation is that the diameter of epithelial removal matches the total ablation zone in transepithelial PRK treatments, decreasing the wound surface, and shortening the epithelial closure time.10,12
Another advantage of tPRK is reduced surgery time. In our study, the total surgery time was reduced by 35% in comparison to aaPRK. Surgery itself is less stressful for the patient and very comfortable for the surgeon. Aslanides et al10 and Fadlallah et al11 reported decreased postoperative pain after the single-step transepithelial PRK; however, our results failed to confirm these findings.
Transepithelial approaches allow maximum correspondence between the corneal topography and the ablation profile, which may be especially useful in customized treatments of irregular corneal astigmatism. The topographic map corresponds more closely with the epithelial surface than with the stromal surface since the epithelium acts as a natural mask, thinning over stromal protrusions and thickening over excavations.24 Several papers confirmed the value of customized, topography-guided transepithelial ablation in correcting irregular corneal astigmatism.25 However, in current transepithelial customized ablation profiles, a difference in photoablative rates of the stroma and the epithelial tissue cannot be compensated precisely as long as an epithelial thickness map is not taken into account. In the future, the advent of a high-resolution OCT technique could allow proper representation of the epithelial layer, potentially leading to improved customized epithelial ablations.
In conclusion, single-step transepithelial PRK and conventional PRK performed on regular corneas produce very similar results 3 months after the surgery. These procedures are predictable, effective, and safe for correction of myopia and compound myopic astigmatism.
Correction
Dr. Samuel Arba Mosquera's name has been corrected from Samuel A. Mosquera.
Footnotes
Abbreviations: aaPRK = alcohol-assisted photorefractive keratectomy, CDVA = corrected distance visual acuity, Epi-LASIK = epithelial laser in situ keratomileusis, LASEK = laser-assisted subepithelial keratectomy, MMC = mitomycin C, MRSE = mean refractive spherical equivalent, OCT = optical coherence tomography, tPRK = single-step transepithelial photorefractive keratectomy, TransPRK = single-step transepithelial PRK by Schwind eye-tech-solutions Germany, UDVA = uncorrected distance visual acuity, VRS = verbal rating scale.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Abad JC, An B, Power WJ, et al. A prospective evaluation of alcohol-assisted versus mechanical epithelial removal before photorefractive keratectomy. Ophthalmology 1997; 104:1566–1574. [DOI] [PubMed] [Google Scholar]
- 2.Camellin M. Laser epithelial keratomileusis for myopia. J Refract Surg 2003; 19:666–670. [DOI] [PubMed] [Google Scholar]
- 3.Pallikaris IG, Katsanevaki VJ, Kalyvianaki MI, et al. Advances in subepithelial excimer refractive surgery techniques: Epi-LASIK. Curr Opin Ophthalmol 2003; 14:207–212. [DOI] [PubMed] [Google Scholar]
- 4.Carr JD, Patel R, Hersh PS. Management of late corneal haze following photorefractive keratectomy. J Refract Surg 1995; 11:S309–S313. [DOI] [PubMed] [Google Scholar]
- 5.Kanitkar KD, Camp J, Humble H, et al. Pain after epithelial removal by ethanol-assisted mechanical versus transepithelial excimer laser debridement. J Refract Surg 2000; 16:519–522. [DOI] [PubMed] [Google Scholar]
- 6.Clinch TE, Moshirfar M, Weis JR, et al. Comparison of mechanical and transepithelial debridement during photorefractive keratectomy. Ophthalmology 1999; 106:483–489. [DOI] [PubMed] [Google Scholar]
- 7.Lee HK, Lee KS, Kim JK, et al. Epithelial healing and clinical outcomes in excimer laser photorefractive surgery following three epithelial removal techniques: mechanical, alcohol, and excimer laser. Am J Ophthalmol 2005; 139:56–63. [DOI] [PubMed] [Google Scholar]
- 8.Sin S, Simpson TL. The repeatability of corneal and corneal epithelial thickness measurements using optical coherence tomography. Optom Vis Sci 2006; 83:360–365. [DOI] [PubMed] [Google Scholar]
- 9.Luger MH, Ewering T, Arba-Mosquera S. Consecutive myopia correction with transepithelial versus alcohol-assisted photorefractive keratectomy in contralateral eyes: one-year results. J Cataract Refract Surg 2012; 38:1414–1423. [DOI] [PubMed] [Google Scholar]
- 10.Aslanides IM, Padroni S, Arba Mosquera S, et al. Comparison of single-step reverse transepithelial all-surface laser ablation (ASLA) to alcohol-assisted photorefractive keratectomy. Clin Ophthalmol 2012; 6:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadlallah A, Fahed D, Khalil K, et al. Transepithelial photorefractive keratectomy: clinical results. J Cataract Refract Surg 2011; 37:1852–1857. [DOI] [PubMed] [Google Scholar]
- 12.Kaluzny BJ, Szkulmowski M, Bukowska DM, et al. Spectral OCT with speckle contrast reduction for evaluation of the healing process after PRK and transepithelial PRK. Biomed Opt Express 2014; 5:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adib-Moghaddam S, Arba-Mosquera S, Salmanian B, et al. On-line pachymetry outcome of ablation in aberration free mode TransPRK. Eur J Ophthalmol 2014; 24:483–489. [DOI] [PubMed] [Google Scholar]
- 14.Arba Mosquera S, Awwad ST. Theoretical analyses of the refractive implications of transepithelial PRK ablations. Br J Ophthalmol 2013; 97:905–911. [DOI] [PubMed] [Google Scholar]
- 15.Raviv T, Majmudar PA, Dennis RF, et al. Mytomycin-C for post-PRK corneal haze. J Cataract Refract Surg 2000; 26:1105–1106. [DOI] [PubMed] [Google Scholar]
- 16.Fantes FE, Hanna KD, Waring GO, III, et al. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol 1990; 108:665–675. [DOI] [PubMed] [Google Scholar]
- 17.Dupps WJ, Jr, Kohnen T, Mamalis N, et al. Standardized graphs and terms for refractive surgery results. Cataract Refract Surg 2011; 37:1–3. [DOI] [PubMed] [Google Scholar]
- 18.Reinstein DZ, Archer TJ, Gobbe M, et al. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg 2008; 24:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanellopoulos AJ, Asimellis G. In vivo three-dimensional corneal epithelium imaging in normal eyes by anterior-segment optical coherence tomography: a clinical reference study. Cornea 2013; 32:1493–1498. [DOI] [PubMed] [Google Scholar]
- 20.Taneri S, Weisberg M, Azar DT. Surface ablation techniques. J Cataract Refract Surg 2011; 37:392–408. [DOI] [PubMed] [Google Scholar]
- 21.Reinstein DZ, Archer TJ, Gobbe M. Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg 2012; 28:195–201. [DOI] [PubMed] [Google Scholar]
- 22.Kanellopoulos AJ, Asimellis G. Longitudinal postoperative Lasik epithelial thickness profile changes in correlation with degree of myopia correction. J Refract Surg 2014; 30:166–171. [DOI] [PubMed] [Google Scholar]
- 23.Omulecki W, Laudanska-Olszewska I, Synder A. Factors affecting patient cooperation and level of pain perception during phacoemulsification in topical and intracameral anesthesia. Eur J Ophthalmol 2009; 19:977–983. [DOI] [PubMed] [Google Scholar]
- 24.Allan BD, Hassan H. Topography-guided transepithelial photorefractive keratectomy for irregular astigmatism using a 213 nm solid-state laser. J Cataract Refract Surg 2013; 39:97–104. [DOI] [PubMed] [Google Scholar]
- 25.Camellin M, Arba Mosquera S. Simultaneous aspheric wavefront-guided transepithelial photorefractive keratectomy and phototherapeutic keratectomy to correct aberrations and refractive errors after corneal surgery. J Cataract Refract Surg 2010; 36:1173–1180. [DOI] [PubMed] [Google Scholar]


