Abstract
Both periodontitis and osteoporosis have similar sign of bone resorption in nature. However, the relationship of the severity between these 2 bone-loss diseases is still uncertain.
The aim of this study was to investigate the association between the severity of osteoporosis and periodontitis regarding the impact of oral hygiene maintenance. In total, 35,127 osteoporosis patients and 50,498 comparisons were derived from the Longitudinal Health Insurance Database of Taiwan between 2000 and 2010. The population was subdivided into groups according to the different level oral hygiene maintenance and the severity of periodontitis and osteoporosis. The association between osteoporosis and periodontitis was estimated by multinomial logistic regression and rank correlation by Kendall rank correlation test, presented by odds ratio (OR), and 5% confidence intervals (CIs).
After controlling the age, sex, and comorbidities, variables in the good oral hygiene maintenance population, we found that periodontitis raised 1.29-fold risk of osteoporosis (95% CI = 1.12–1.49); the risk of osteoporosis was increased with the elevated severity of periodontitis from 1.27 (95% CI = 1.08–1.48) to 1.38 (95% CI = 1.01–1.89). There is a positive correlation between the severity of periodontitis and osteoporosis occurrence in this population (OR = 1.27–1.46; Kendall rank correlation test P = 0.0003). In the poor oral hygiene maintenance population, periodontitis patients had 6.02-fold risk of osteoporosis than those who without periodontitis (95% CI = 4.65–7.81); the risk of osteoporosis was increased with periodontitis severity from 5.96 (95% CI = 4.48–7.92) to 6.37 (95% CI = 3.36–12.1).
This result indicated the periodontitis and osteoporosis are conjunctive. The sudden periodontal breakdown of those who with good oral hygiene maintenance might be an indicator for the risk of osteoporosis; if those who were diagnosed as osteoporosis must pay more attention to their periodontal health. Good oral hygiene maintenance might be a crucial factor for preventing the deterioration of osteoporosis progressing; the oral hygiene maintenance plays a significant influence on the association between periodontitis and osteoporosis.
INTRODUCTION
The similar clinical sign of osteoporosis and periodontitis is bone resorption in nature. These 2 diseases have similar risk factors. Osteoporosis is the most common skeletal disorder in elderly population. This entity is characterized by the imbalance between bone resorption and bone formation due to the deucedly activated osteoclast.1,2 It is popular to define osteoporosis as low bone mineral density (BMD) detected by dual-energy x-ray absorptiometry (DXA) scan. The osteoporotic condition is usually represented as T-score after DXA examination3,4 and the impaired bone quality causes the compromised bone strength then increases the risk of fracture.5 Periodontitis is an infected periodontal disease because of specific pathogenic bacteria flora in the subgingival area. It usually induces the gradual alveolar bone resorption and loss of the soft tissue attachment around the teeth due to chronic inflammation. The bony destruction mechanism of periodontitis is a common cause of the tooth loss in adults.6 Both osteoporosis and periodontitis are multifactorial etiologic factors in nature, although the mediator factors or mechanism may be different. How to prevent and treat these 2 bone-loss diseases is always an important issue in public health. Recently, the relationship between osteoporosis and periodontitis is valued gradually.7
Since 1960 the association between these 2 bone-loss diseases had been discussed. Although there is only incipient evidence about the relationship between osteoporosis and periodontitis, the positive association between the two diseases had been elaborated in the previous research. In 2007, Gomes-Filho et al8 indicated the close correlation between low skeletal BMD and interproximal alveolar bone loss among a large population of postmenopausal women. Other researches also mentioned that postmenopausal women with osteoporosis had higher risk of periodontitis than those who without osteoporosis.9,10 Osteoporosis is regarded as a synergistic factor for the progression of alveolar bone loss in postmenopausal women with pre-existed periodontitis during 2 or 3-year follow-up. Although some researches indicated osteoporosis increased the risk of the alveolar bone and tooth loss, osteoporosis and periodontitis were closely related,9–14 other studies indicated that there was no significant association between BMD and the severity of periodontal disease.15,16 Until now the association between osteoporosis and periodontitis remains a controversial issue, this correlation and potential mechanisms linking of these 2 bone-loss diseases are still uncertain.
Most of the previous researches usually aimed at the osteoporosis impacted on the periodontal condition and less information was discussed whether severe periodontal breakdown might increase the possibility of osteoporosis occurrence. Furthermore, in the past studies they never subdivided the different severity of these 2 bone-loss diseases and there was limited literature to assess the causality between osteoporosis and periodontitis. The issue about the association with periodontitis severity and osteoporosis severity needs to be explored in depth. In this study, we tried to investigate not only the association between periodontitis and osteoporosis based on different severity of these 2 bone-loss diseases, but also considered the impact of oral hygiene maintenance on the osteoporosis patients.
METHODS
A Longitudinal Health Insurance Database that is a part of National Health Insurance Research Database (NHIRD) was retrieved for this study. The population derived from the National Health Insurance (NHI) system of Taiwan between 2000 and 2010. NHIRD was set up by Taiwan Bureau of National Health Insurance (TBNHI) on March 1, 1995. All population in Taiwan joined this NHI program under obligation of insurance law. NHIRD included insurant information, medical visits, and medical treatment from the start of 1996 to the end of 2011. The Longitudinal Health Insurance Database contained 1 million insurant randomly selected people who joined the NHI program under the 2000 year. Codes of the International Classification of Diseases, Ninth Revision and Clinical Modification (ICD-9-CM) were used to identify diseases in the database of NHIRD. Insurant identification was recoded by TBNHI before being sent to researcher. This study was approved by Institutional Review Board at China Medical University Hospital.
The study population was divided into 2 groups according to different levels of oral hygiene maintenance. The patients received dental scaling at least 3 times within 5 years were defined as good oral hygiene maintenance population; those patients who never received dental scaling within 5 years were attributed to poor oral hygiene maintenance population. Moreover, the osteoporosis population was selected in those patients with new osteoporosis diagnosis (ICD-9-CM 733.0, V13.51, and V82.81) between 2000 and 2010. The comparison group was selected from those who had no osteoporosis history in corresponding population. They were also frequency matched with the age stratum (5 years, eg, 0–4, 5–9, 10–14, and so on) and sex as osteoporosis group at 4:1 and 1:1 rates in the good and poor oral hygiene maintenance population group respectively (Figure 1).
FIGURE 1.
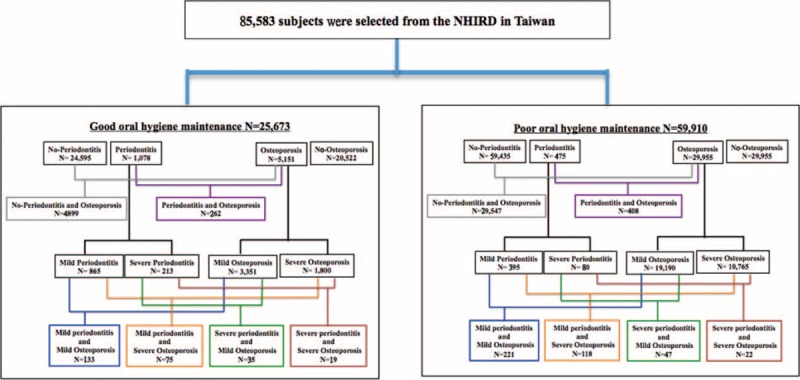
Flow chart of subjects selected from the National Health Insurance Research Database (NHIRD).
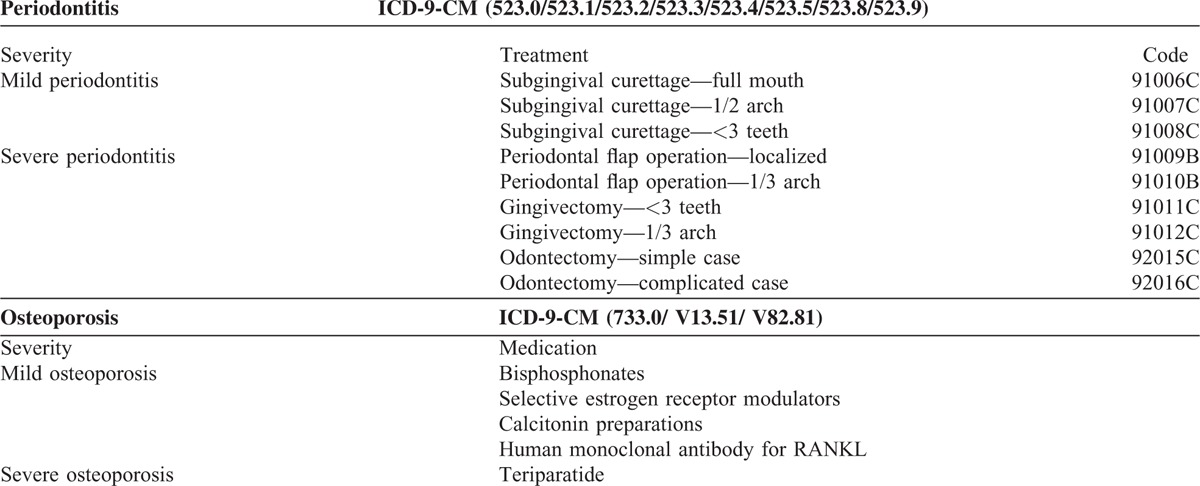
Based on a T-score a clinician makes therapeutic decisions to manage osteoporosis and fit the guideline from TBNHI. Specific pharmacologic interventions such as bisphosphonates, selective estrogen receptor modulators, calcitonin preparations, or human monoclonal antibody for RANKL are given on those patients whose T-scores of DXA are between −2.5 and −3.0; those who are treated with teriparatide when T-score is less than −3.0. In this study, the severity of osteoporosis was grouped into mild and severe groups based on different medicine used. Osteoporosis patients received teriparatide, were defined as severe osteoporosis group and the other was defined as mild one. Additionally, the severity of periodontitis was also grouped into mild and severe groups based on the different dental treatments. The periodontitis patients received flap operation, gingivectomy or dental extraction (odontectomy) was defined as severe periodontitis population; those who only received subgingival curettage were defined as mild group (Table 1). In order to clarify the association between osteoporosis and periodontitis, we investigated the occurrence of periodontitis within 1 year before or after osteoporosis was diagnosed.
TABLE 1.
Define Different Severity of Periodontitis and Osteoporosis
The difference of age group (<50, 50–64, 65–79, and >80 years old), sex, and comorbidities, including diabetes (ICD-9-CM 250), hypertension (ICD-9-CM 401-405), hyperlipidemia (ICD-9-CM 272), coronary artery disease (CAD, ICD-9-CM 410-414), and obesity (ICD-9-CM 278), were distributed in the study population and the demographics difference between osteoporosis patients and comparison groups was analyzed by χ2 test. All comorbidities were defined before the index date. The risk of osteoporosis in periodontitis patients compared with the population without periodontitis regarding the oral hygiene maintenance was estimated by multivariable logistic regression after the confounding factors such as age, sex, and comorbidities were adjusted. The association between the osteoporosis severity and periodontitis severity in the good and poor oral hygiene maintenance population was estimated by multinomial logistic regression and measured of rank correlation by Kendall rank correlation test after controlling age, sex, and comorbidities. All results were presented by odds ratio (OR) and 95% confidence intervals (CIs). A 2-sided P < 0.05 was considered statistically significant. All statistical analyses were performed by SAS statistical software (version 9.4 for Windows; SAS Institute Inc, Cary, NC).
RESULTS
In the Good Oral Hygiene Maintenance Population
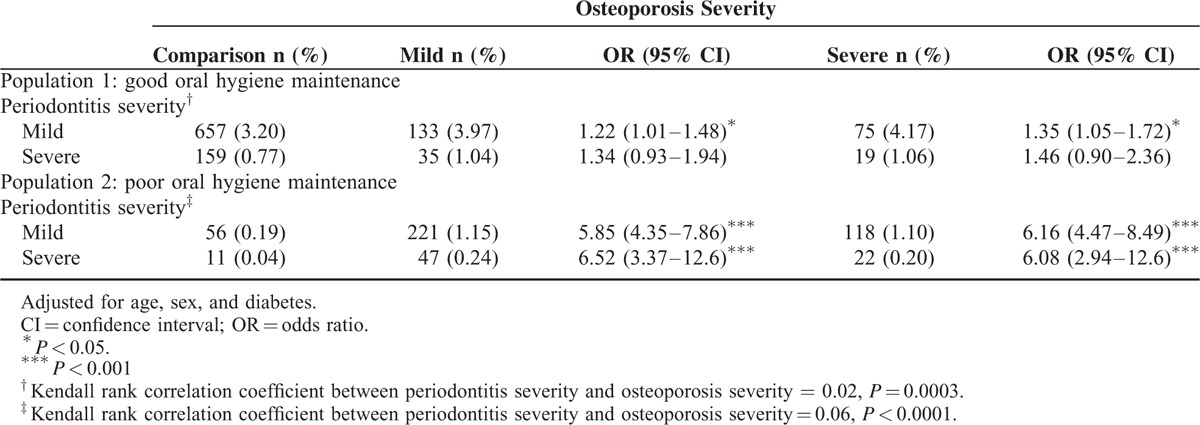
In total, 5151 osteoporosis patients and 20,522 age- and sex-matched comparisons were enrolled into the study (Table 2). In osteoporosis population, women were more than men (74.3% vs. 25.7%) and the mean age was 61.7-year old (standard deviation = 12.4). More than half of osteoporosis patients occurred between 50 and 79-year olds. Osteoporosis patients suffered from higher prevalence of diabetes (18.5% vs. 16.4%, P = 0.0004), hypertension (47.6% vs. 45.0%, P = 0.0006), hyperlipidemia (36.2% vs. 30.1%, P < 0.0001), and CAD (29.2% vs. 23.6%, P < 0.0001) than comparisons. Compared with patients without periodontitis, those patients who suffered from periodontitis had a 1.29-fold risk of osteoporosis after age, sex, and comorbidity had been adjusted (95% CI = 1.12–1.49, Table 3). The risk of osteoporosis was increased with the elevated severity of periodontitis from 1.27 (95% CI = 1.08–1.48) to 1.38 (95% CI = 1.01–1.89) compared with those who without periodontitis. This result revealed the positive correlation between osteoporosis severity and periodontitis severity (Table 4). The odds ratio was increased from 1.22 (in mild periodontitis to mild osteoporosis) to 1.46 (in severe periodontitis to severe osteoporosis) as compared with patients without periodontitis (Kendall rank correlation test P = 0.0003).
TABLE 2.
Demographics Between Osteoporosis and Comparison Group
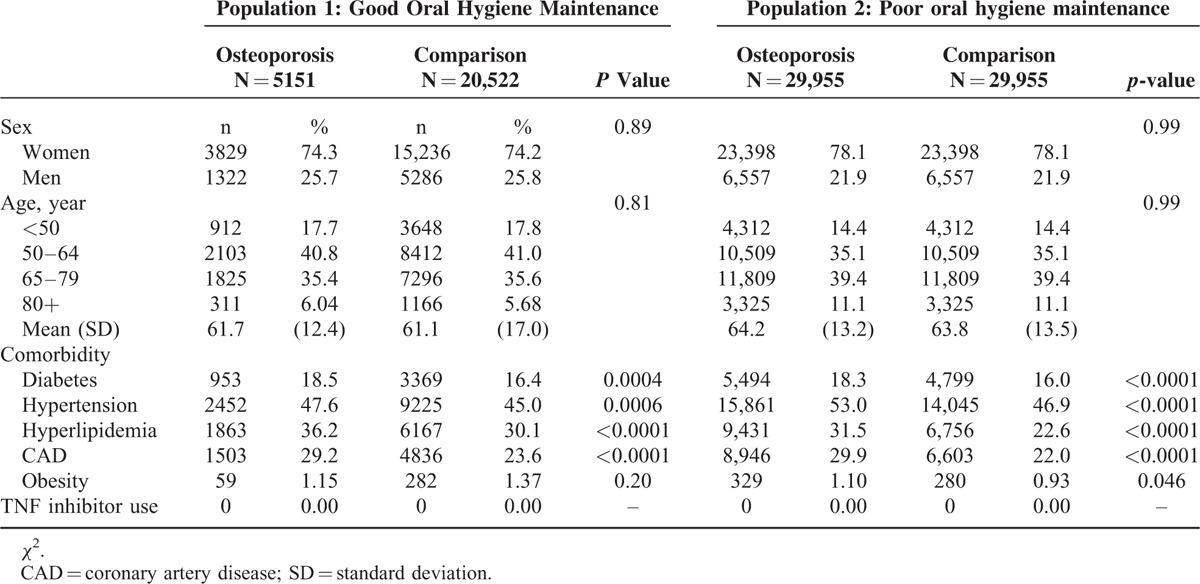
TABLE 3.
Risk of Osteoporosis Among Different Periodontitis Severity in Age- and Sex-Adjusted Logistic Regression
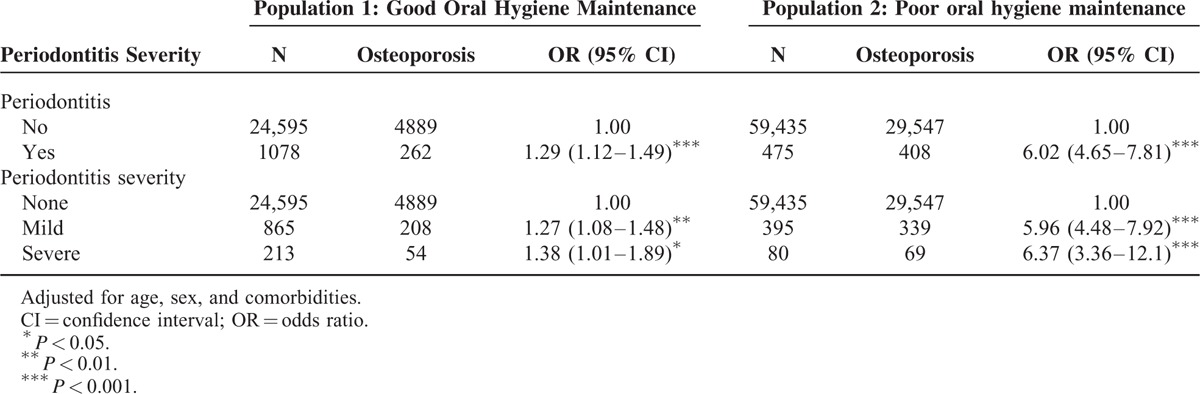
TABLE 4.
The Association Between Osteoporosis Severity and Periodontitis Severity in Age, Sex, and Comorbidity-Adjusted Logistic Regression
In the Poor Oral Hygiene Maintenance Population
In total, 29,976 osteoporosis patients and 29,976 age- and sex-matched comparisons were selected as study subjects from those who never received scaling treatment within 5 years (Table 2). In osteoporosis patients, women were the major proportion and the mean age was 64.2-year old (standard deviation = 13.2). Osteoporosis group had higher prevalence of diabetes, hypertension, hyperlipidemia, CAD, and obesity than comparison group (P < 0.05). Periodontitis patients had 6.02-fold risk of osteoporosis than those who without periodontitis (85.9% vs. 49.7%, 95% CI = 4.65–7.81) after age, sex, and comorbidity had been adjusted (Table 3). The risk of osteoporosis increased with periodontitis severity from 5.96 (95% CI = 4.48–7.92) to 6.37 (95% CI = 3.36–12.1). Regardless of the severity of periodontitis, the risk for osteoporosis severity was over 5.85-fold compared with patients without periodontitis (Table 4).
DISCUSSION
In this study, there were 35,127 osteoporosis patients including 27,227 women and 7889 men from different ranges of age; it was not limited to the postmenopausal women as previous studies (Table 2).8,9,11,13,16 This demographic analysis indicated that diabetes, hypertension, hyperlipidemia, and CAD increased the risk of osteoporosis significantly. This result was coincident to the past researches that those comorbidities have high relevance to the osteoporosis occurrence. Moreover, many literatures had also expressed that diabetes, hypertension, hyperlipidemia, and CAD are related to periodontitis development. To investigate the association between osteoporosis and periodontitis more precisely, we had adjusted for age, sex, and comorbidities in this study. Previous researches almost focused on the correlation between osteoporosis and periodontitis regardless of the severity of the 2 bone-loss diseases. In order to accurately determine whether the severity of osteoporosis and periodontitis is positive correlated, we subdivided the osteoporosis patients into mild and severe groups according to the usage of bisphosphonate. In Taiwan osteoporosis patients receive bisphosphonate therapy under TBNHI support when their T score of DXA is less than -2.5.17 For this reason the bisphosphonate used or not can represent the severity of osteoporosis, so we could distinguish the different osteoporosis severity based on the criteria in this study. Gingival inflammation is represented as the increasing periodontal pocket and probing depth (PD).18 Assessing PD is the most common periodontal examination to evaluate the topography of the gingiva and related structures in general dental practice.19 According to the guideline offered by the American Academy of Periodontology for periodontal therapy, comprehensive subgingival curettage is recommended to treat irregular root surface and alter caused by periodontal pathogen if PD is over 5 mm. If the PD is not reduced based on the post-treatment evaluation, various mucogingival flap procedures are performed to eliminate the periodontal pocket.20 In Taiwan, periodontitis is diagnosed on the basis of dental examination including a periodontal examination, probing depth checked, and radiographs at semiannual checkups which are covered by NHI. The periodontal treatments include scaling, subgingival curettage, and periodontal surgery depending on the periodontal pocket depth around tooth and the degree of alveolar bone destruction. According to periodontal surgery performance such as flap operation, gingivectomy, or odontectomy or not, we differentiate the severity of periodontitis into mild and severe ones. However, oral hygiene care greatly impacts the chronic periodontitis occurrence, it also might contribute to some systemic diseases including CAD, diabetes, hypertension, and so on. So we included the criteria of oral hygiene care to discuss the impact on the osteoporosis occurrence. In this study, those who received regular scaling annually were regarded as the group with good oral hygiene maintenance. On the contrary, the others who never received scaling were grouped into the poor oral hygiene maintenance population.
The consensus published by Chang et al21 indicated the patients with osteoporosis may have higher risk of periodontitis as compared with those individuals without osteoporosis during 5-year follow-up according to the nationwide Taiwanese population-based database. There is some correlation between these 2 bone-loss diseases although the osteoporosis is a condition of reducing bone mass and periodontitis is a bony destruction disease due to chronic inflammation. Most researches in the past to explore the relationship between periodontitis and osteoporosis usually focused on periodontal breakdown occurred after osteoporosis.2,6,7,22 There is less information about whether periodontitis raise the risk of osteoporosis. In this result, the periodontitis occurrence plays significant influence on osteoporosis development in all population (P < 0.001). This showed the risk of osteoporosis and periodontitis occurrence had positive correlation based on the result and represented consensus. Furthermore, if the poor oral hygiene maintenance population suffered from periodontitis, they had higher risk of osteoporosis than those who with good oral hygiene maintenance (OR, 6.20; 95% CI, 4.78–8.03). The data also indicated the increased severity of periodontitis would raise the morbidity of osteoporosis in all population; we can infer there is high relevance of the periodontitis severity and the risk of osteoporosis (Table 3). The result was coincident to the proposed viewpoints that had indicated various local and systemic conditions as “risk factors” and that is directly related to periodontal destruction accelerating the bacterial invasion and affects the onset and progression of periodontal infection.13,23,24 Good oral hygiene maintenance had been recognized as the better way to prevent periodontitis and control the progression of periodontal breakdown. The literature indicated good oral hygiene maintenance decreases the possibility of other systemic problems associated with periodontal disease.25 According to this result we speculated the oral hygiene maintenance could affect the development of osteoporosis; if the oral hygiene care is good it might reduce the risk of osteoporosis. Maintenance of oral hygiene might contribute to prevention of not only periodontitis but also osteoporosis.
Another significant finding in this study was a linear relationship between the severity of periodontitis and osteoporosis in the good oral hygiene maintenance population (Kendall rank correlation coefficient is 0.02, P = 0.0003) (Table 4). This is a major discovery for dentists in clinic to detect the possibility of osteoporosis occurrence based on the periodontal breakdown among those who with good oral hygiene maintenance. Until now, there is little relevant literature in the past to make a similar point of the prospective issue. Those periodontitis patients with good oral hygiene would be able to rule out the stimulation of the pathogenic microorganism; we are able to tentatively speculate the sudden periodontal breakdown may be related to the osteoporosis occurrence. Osteoporosis as a silent disease is difficult to be perceived in the early stage because there is no distinct symptom until bone fractured in the progressing disease. Most of the women usually are aware of the osteoporosis crisis after menopause, but osteoporosis may also occur in patients with certain metabolic disorders from different ranges of age. According to this result, the periodontal status might be used for osteoporosis-detecting sign if those patients have good oral hygiene maintenance. On the other hand, in the good oral hygiene maintenance population in the event that the patient was diagnosed as osteoporosis, dentists must pay more attention to the periodontal status through intensive dental care. However, in the poor oral hygiene population there is no such trend in the association with periodontal severity and osteoporosis severity (Kendall rank correlation coefficient is 0.06, P < 0.0001), the osteoporosis occurrence is unpredictable according to the periodontal status in dental clinic. Because there are many confounding factors to interfere the relationship between periodontitis and osteoporotic conditions, the periodontal destruction cannot be used as the indicator for osteoporosis occurrence in dental clinic. Consequently, the oral hygiene maintenance might play a crucial role in preventing the deterioration of osteoporosis progression.
In the previous studies the association of osteoporosis and periodontitis had been supported, but the reports were usually criticized for the small sample sizes and inadequate controlled confounding factors.26–28 Moreover, the extent of the relationship between these 2 bone-loss diseases is still uncertain now. There is less information to discuss whether severe periodontal breakdown might increase the risk of osteoporosis. This study provided the preliminary information about the interrelationship of the osteoporosis and periodontitis according to different severity of these 2 diseases regarding the impact of the oral hygiene maintenance. The subjects surveyed from large and nationwide population of osteoporosis patients could increase the validity and realism. Large sample size may also contribute the sufficient magnitude to confirm the contribution of systemic bone loss to periodontal destruction and the long-term follow-up (from 2000 to 2010) is another strength of this study. The nationwide population combined long-term follow-up period might compensate some shortcomings such as unavoidable error or flaws of the healthcare declaration. Although the results provided a realistic perspective for the extent relationship of these 2 bone-loss diseases, there were still some limitations. NHIRDs of Taiwan include both dental and medical records but lack some required information such as dental images, periodontal examination (eg, probing depth, plaque index) and lifestyle behavior (eg, smoking, betel-nut chewing, alcohol). It caused some difficulties to perceive the oral hygiene status regarding the plaque accumulation and detect the real periodontal condition precisely before osteoporotic condition in this database. Ideally, the extent of oral hygiene maintenance should be distinguished according to the plaque accumulation in dental examination. But it was difficult to trace the data of plaque index in the study; we only could define the status of oral hygiene maintenance based on the annual frequency of dental scaling which, maybe, can represent the positive degree of self-care oral hygiene. Furthermore, the smoking and betel-nut chewing habit are related to periodontitis development; these confounding factors of personal habits could not be included into discussion. We hope these problems can be overcome in the subsequent research. Moreover, tooth loss is a typical symptom for severe periodontitis because of attachment loss. There is another limitation in this study if tooth lost before dental treatment might be difficult to be traced on the NHIRD. However, Taiwan's NHI is a national healthcare program covering >99.5% of the population,29 periodontal treatment programs are widely covered by NHI and popularized in Taiwan. To the point that most of the severe periodontitis patients suffered from teeth lost suddenly usually seek for further dental treatment. When patients received full--mouth periodontal treatment under TBNHI support, we could distinguish the severity of periodontitis based on different treatment modalities. We recognized that extremely few edentulous patients suffered from severe periodontitis who hesitate periodontal treatment might be missed in this study; this shortcoming could be overcome because of the large-scaled population base. In physical condition the activity of bone resorption and formation are coupled, the pathological process causes the imbalance of bone remodeling to result in bone loss. Periodontitis is an inflammatory disease, the inflammatory reaction stimulates immune cells to secrete a variety of cytokines to mediate the activity of osteoclast. Impaired function of the osteoclast and osteoblast leads to bone resorbing activity outstrips normal bone cell regeneration, osteoporosis may occur. Periodontitis and osteoporosis have similar sign of bone resorption in nature and this result indicated the closed correlation of the 2 bone-loss diseases, even their bone-resorbing mechanisms are not exactly the same. In this study, we infer the good oral hygiene care not only reduces the periodontitis according to this result but also may be able to decrease the risk of osteoporosis. It is required for well-controlled prospective studies or basic research in the correlation between the atrophic form, the state of hygiene, and the incidence of osteoporosis.
CONCLUSION
The periodontitis and osteoporosis are conjunctive and there is positive correlation between the severity of periodontitis and osteoporosis occurrence. In the good oral hygiene care population the sudden onset of periodontal breakdown might be an indicator for the risk of osteoporosis progression; if those who diagnosed as osteoporosis must pay more attention to their periodontal health. This result demonstrated the good oral hygiene maintenance for preventing the deterioration of osteoporosis progression is very important; the oral hygiene maintenance plays a significant influence on the association between periodontitis and osteoporosis.
Footnotes
Abbreviations: BMD = bone mineral density, CAD = coronary artery disease, CI = confidence interval, DXA = dual-energy x-ray absorptiometry, ICD-9-CM = International Classification of Diseases, Ninth Revision and Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, OR = odds ratio, PD = probing depth, TBNHI = Taiwan Bureau of National Health Insurance.
All authors substantially contributed to: conception (C-ZW, Y-FH) and design (C-ZW, Y-FH, C-TC, S-PL), or analysis (C-HM) and interpretation of data (all authors); drafting the manuscript (Y-FH) or revising it critically for important intellectual content (all authors); and final approval of the submitted manuscript (all authors). Y-FH had full access to all the data in the study and takes responsibility for the accuracy and integrity of the data analysis.
This study was supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Chang Gung Memorial Hospital (CMRPG3E1341), Lo-Hsu Foundation Inc, Lotung Poh-Ai Hospital Research Grant (104-E126), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, Katsuzo and Kiyo Aoshima Memorial Funds, Japan, and China Medical University Hospital (1MS1).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling: principles and implications for diagnosis and therapy. J Periodontol 2002; 73:1377–1391. [DOI] [PubMed] [Google Scholar]
- 2.Guiglia R, Di Fede O, Lo Russo L, et al. Osteoporosis, jawbones and periodontal disease. Med Oral Patol Oral Cir Bucal 2013; 18:e93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis KJ, Shypailo RJ. Bone mineral and body composition measurements: cross-calibration of pencil-beam and fan-beam dual-energy X-ray absorptiometers. J Bone Miner Res 1998; 13:1613–1618. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen TV, Sambrook PN, Eisman JA. Sources of variability in bone mineral density measurements: implications for study design and analysis of bone loss. J Bone Miner Res 1997; 12:124–135. [DOI] [PubMed] [Google Scholar]
- 5.Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol 1996; 67 (10 Suppl):1076–1084. [DOI] [PubMed] [Google Scholar]
- 6.Lai YL. Osteoporosis and periodontal disease. J Chin Med Assoc 2004; 67:387–388. [PubMed] [Google Scholar]
- 7.Reddy MS. Osteoporosis and periodontitis: discussion, conclusions, and recommendations. Ann Periodontol 2001; 6:214–217. [DOI] [PubMed] [Google Scholar]
- 8.Gomes-Filho IS, Passos Jde S, Cruz SS, et al. The association between postmenopausal osteoporosis and periodontal disease. J Periodontol 2007; 78:1731–1740. [DOI] [PubMed] [Google Scholar]
- 9.Payne JB, Reinhardt RA, Nummikoski PV, et al. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 1999; 10:34–40. [DOI] [PubMed] [Google Scholar]
- 10.Geurs NC, Lewis CE, Jeffcoat MK. Osteoporosis and periodontal disease progression. Periodontology 2000 2003; 32:105–110. [DOI] [PubMed] [Google Scholar]
- 11.Tezal M, Wactawski-Wende J, Grossi SG, et al. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol 2000; 71:1492–1498. [DOI] [PubMed] [Google Scholar]
- 12.Nicopoulou-Karayianni K, Tzoutzoukos P, Mitsea A, et al. Tooth loss and osteoporosis: the OSTEODENT Study. J Clin Periodontol 2009; 36:190–197. [DOI] [PubMed] [Google Scholar]
- 13.Sultan N, Rao J. Association between periodontal disease and bone mineral density in postmenopausal women: a cross sectional study. Med Oral Patol Oral Cir Bucal 2011; 16:e440–447. [DOI] [PubMed] [Google Scholar]
- 14.Lin TH, Lung CC, Su HP, et al. Association between periodontal disease and osteoporosis by gender: a nationwide population-based cohort study. Medicine 2015; 94:e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 1990; 63:218–222. [DOI] [PubMed] [Google Scholar]
- 16.Elders PJ, Habets LL, Netelenbos JC, et al. The relation between periodontitis and systemic bone mass in women between 46 and 55 years of age. J Clin Periodontol 1992; 19:492–496. [DOI] [PubMed] [Google Scholar]
- 17.Black DM, Bauer DC, Schwartz AV, et al. Continuing bisphosphonate treatment for osteoporosis: for whom and for how long? N Engl J Med 2012; 366:2051–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johannsen A, Rydmark I, Soder B, et al. Gingival inflammation, increased periodontal pocket depth and elevated interleukin-6 in gingival crevicular fluid of depressed women on long-term sick leave. J Periodontal Res 2007; 42:546–552. [DOI] [PubMed] [Google Scholar]
- 19.Listgarten MA. Periodontal probing: what does it mean? J Clin Periodontol 1980; 7:165–176. [DOI] [PubMed] [Google Scholar]
- 20.Parameter on periodontal maintenance. American Academy of Periodontology. J Periodontol 2000; 71 (5 Suppl):849–850. [DOI] [PubMed] [Google Scholar]
- 21.Chang WP, Chang WC, Wu MS, et al. Population-based 5-year follow-up study in Taiwan of osteoporosis and risk of periodontitis. J Periodontol 2014; 85:e24–30. [DOI] [PubMed] [Google Scholar]
- 22.Esfahanian V, Shamami MS, Shamami MS. Relationship between osteoporosis and periodontal disease: review of the literature. J Dent (Tehran) 2012; 9:256–264. [PMC free article] [PubMed] [Google Scholar]
- 23.Beck JD. Methods of assessing risk for periodontitis and developing multifactorial models. J Periodontol 1994; 65 (5 Suppl):468–478. [DOI] [PubMed] [Google Scholar]
- 24.Grossi SG, Genco RJ, Machtei EE, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol 1995; 66:23–29. [DOI] [PubMed] [Google Scholar]
- 25.Park YD, Patton LL, Kim HY. Clustering of oral and general health risk behaviors in Korean adolescents: a national representative sample. J Adolesc Health 2010; 47:277–281. [DOI] [PubMed] [Google Scholar]
- 26.Krook L, Whalen JP, Lesser GV, et al. Human periodontal disease and osteoporosis. Cornell Vet 1972; 62:371–391. [PubMed] [Google Scholar]
- 27.Kribbs PJ, Smith DE, Chesnut CH., 3rd Oral findings in osteoporosis. Part II: relationship between residual ridge and alveolar bone resorption and generalized skeletal osteopenia. J Prosthet Dent 1983; 50:719–724. [DOI] [PubMed] [Google Scholar]
- 28.Kribbs PJ, Chesnut CH, 3rd, Ott SM, et al. Relationships between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent 1989; 62:703–707. [DOI] [PubMed] [Google Scholar]
- 29.Universal Health Coverage in Taiwan. Available at: http://www.nhi.gov.tw/Resource/webdata/21717_1_20120808UniversalHealthCoverage.pdf Accessed May 2012 [Google Scholar]


