Abstract
To assess surgical outcomes in synchronous multiple primary lung cancer (SMPLC) and correlations with clinicopathological features and prognostic/predictive factors.
We retrospectively reviewed patients diagnosed with early-stage nonsmall cell lung cancer (NSCLC) between January 2006 and June 2012. In total, 564 patients with resectable NSCLC underwent a preoperative positron emission tomography-computed tomography scan followed by anatomic resection. We reviewed the clinical features of 35 SMPLC patients. Surgical outcomes, prognosis, and tumor imaging features were evaluated (median follow-up = 44 months).
In total, 35 eligible SMPLC patients (6.21%) were identified (11 men [31%], 24 women [69%], mean age = 65 years]). The tumors were bilateral in 17 patients (49%) and in different lobes of the ipsilateral lung in 18 patients (51%). Most patients (26/35, 74%) had 2 primary tumors, and 26% (9/35) had more than 2 tumors (6 with 3 tumors; 3 with 4 tumors). The median size of the most advanced tumor was 3.0 cm (range 0.9–54). The median standard uptake value (SUV) of the largest tumor was 3.1 (range 1.0–13.3). The patients were treated as follows: 30 lobectomies, 2 sublobar resections, 2 sequential bilateral lobectomies, and 1 bi-lobectomy. Twenty-four patients (69%) received adjuvant therapy. The overall cumulative 5-year survival was 91.5% (median overall survival = 45.5 months). Patients with a reference tumor ≤ 3 cm and SUV ≤ 3.1 had an expected 5-year survival of 100%. Patients with a reference tumor > 3 cm and SUV > 3.1 had an expected 5-year survival rate of 53.3%.
SMPLC patients can benefit from aggressive surgery. The size and SUVmax of the reference tumor may predict postoperative outcomes.
INTRODUCTION
According to the most frequently cited definition, synchronous multiple primary lung cancers (SMPLCs) are diagnosed simultaneously with the index tumor; however, SMPLCs are physically separate from the main tumor and do not share its lymphatic drainage.1 With advancements in early diagnostic techniques such as multi-detector row computed tomography and positron emission tomography-computed tomography (PET-CT) scans, SMPLCs in 2 or more lobes are being diagnosed more frequently, leading to increased interest and controversy regarding the diagnosis and management of these lesions.2 However, SMPLCs are ambiguously assessed by the current tumor-node-metastasis (TNM) staging system, which classifies this condition as more advanced-stage disease.3 Without genetic or molecular analyses, it is often difficult to use clinical and histopathological findings to differentiate between SMPLCs and pulmonary metastases of the primary cancer. Furthermore, there are no standardized guidelines for the selection and management of such patients. Management is particularly difficult when the lung lesions have similar histology, as there are no rigorous criteria to distinguish SMPLCs from metastatic disease.4–6
Although several previous studies have reported successful resection in patients with multiple lung cancers,2,4–11 controversy remains regarding appropriate treatment strategies and postsurgical outcomes. Furthermore, there is little information regarding the risks of recurrence and factors influencing survival, especially for patients who would benefit from a surgical resection. Identifying the factors that affect tumor recurrence and overall survival (OS) could provide insight regarding the subgroups of patients who would most likely benefit from adjuvant treatment. Thus, the purpose of this study was to retrospectively review our experience with patients who underwent complete SMPLC resection and to assess factors that affected tumor recurrence and survival.
MATERIALS AND METHODS
Patients and Lesions
This study was approved by the Institutional Review Board of our hospital, and patient consent was waived (IRB 2-103-05-101). We retrospectively reviewed patients diagnosed with early-stage nonsmall cell lung cancer (NSCLC) between January 2006 and June 2012. In total, 564 patients with resectable NSCLC underwent a preoperative PET-CT scan followed by anatomic resection and 68 patients who had been diagnosed with multiple primary lung cancers were identified. Patients whose lesions all had the same histology of adenocarcinoma were required to have had a 18F-fluorodeoxyglucose (FDG) PET-CT study (as an adjunct to CT) within 4 weeks of lung resection, to have undergone complete surgical resection, and to have had at least 12 months of follow-up.
The following criteria, based on and modified from those of Martini and Melamed1 and Hanley and McNeil,12 were used for designation of SMPLCs: Presence of tumors with different histologies (eg, adenocarcinoma vs squamous cell carcinoma). Tumors of different histologic subtypes (eg, different estimated percentages of acinar vs papillary adenocarcinoma), regardless of nodal status. Tumors of similar histology in the absence of metastatic disease in intervening regional or mediastinal lymph node stations and the absence of extrathoracic disease. Tumors were defined as SMPLC when histology was different, when tumors were located in different lobes, or bilaterally, and in the absence of evidence of an extrathoracic disease. The current international staging system for lung cancer designates intralobar satellites as T3 disease. Tumors with satellite nodules (n = 7), defined as smaller tumors located close to the original cancer, in the same segment of same lobe had better survival and were not classified as SMPLC in our study (using strict criteria). Patients with bronchioloalveolar carcinoma (BAC; n = 9) were also excluded because of the pathological term of BAC was not used. In addition, carcinoid tumors (n = 2), metachronous cancer (n = 10), unilateral tumors of the same cell type and interlobar-lymph node involvement (n = 2), or who had received preoperative chemotherapy or radiotherapy (n = 3) were excluded from this study. As a result, 35 patients with SMPLC were included in this study. The medical case files of these patients were reviewed retrospectively. TNM staging of the patients was performed according to the criteria set forth in the 7th staging system.3 Age, sex, pulmonary function test results, tumor size, tumor location (ipsilateral different lobe or contralateral lung), tumor characteristics, histological type and grade, type of lung resection, tumor recurrence, patient survival, and the existence of adjuvant therapy were determined for each patient. All clinical, operative, imaging, and pathological findings were analyzed retrospectively by reviewing patient charts, pathological reports, and radiographic images, including PET-CT scans. Maximal standard uptake values (SUVmax) were measured prospectively in all patients. Follow-up information was acquired from clinic follow-up notes and the patient's primary care physician.
PET Acquisition
18F-FDG-PET-CT was performed using an integrated PET-CT scanner (München Germany). All patients fasted for 6 h prior to the examination, and the images were obtained 1 h after intravenous administration of 300 to 400 mBq 18-fluorodeoxyglucose (18F-FDG). Images were obtained from the head to the mid-thigh. Attenuation-corrected images were obtained and interpreted by an experienced nuclear medicine physician. Primary lesions were considered pathologically proven if a definite localized area of higher 18F-FDG uptake than the surrounding normal tissue existed (excluding physiological uptake).
PET Data Analysis
A nuclear medicine physician with 21 years of experience who was blinded to the recurrent status of the patients interpreted the FDG-PET images. The SUV for each patient was measured by creating regions of interest (ROIs) on the axial image of the tumor with the highest level of 18F-FDG uptake. 18F-FDG uptake in these ROIs was quantified by calculating the SUV in each pixel according to the following formula: SUV = activity concentration/(injected dose/body weight). To minimize partial volume effects, the maximum SUV within an ROI was used for further calculations. The maximum SUV within an ROI (SUVmax) was used as our reference measurement.
CT Technique
Chest CT scans (64-detector row scanner [Brilliance; Philips Medical Systems, Cleveland, OH]) from the lung apices through the adrenal glands were available for all patients.
Analysis of CT Image Features
CT findings were analyzed in the lung and mediastinal window setting (lung window: window level, −600 Hounsfield units [HU]; window width, 1500 HU; and mediastinal window: window level, 35 HU; window width, 400 HU) using a picture archiving and communication system (PACS) workstation (Brilliance; Philips Medical Systems, Cleveland, OH). All CT images were evaluated in consensus by 2 chest radiologists (H-HH and K-HK). The imaging features that were analyzed for each lesion included tumor size, location, border, margin, and density. Ground-glass opacity was defined as a hazy increase in lung density without obscuration of the underlying bronchial or vascular structures. A nodule was classified as part solid if it contained patches that completely obscured the lung parenchyma. The solid nodule was defined as a nodule that completely obscures the entire lung parenchyma within it.
Statistical Analysis
Statistical analyses were conducted using SPSS 14.0 (SPSS, Inc., Chicago, IL) software. Descriptive data are expressed as the mean ± standard deviation. Student t test was used to investigate continuous variables and the χ2 test was used to compare categorical variables between these groups. A receiver operating characteristic (ROC) curve of the largest tumor size and SUVmax was generated to obtain the cutoff value for the prediction of recurrence. The predictive value of the established model was assessed by calculating the area under the ROC curve (AUC). An AUC value of 0.71 to 0.80 indicates acceptable discrimination.12
Progression-free survival (PFS) was measured from the date of the first surgery until recurrence or the last follow-up date. OS was calculated from the date of the first surgery to either the date of death or the last follow-up date. Survival from the date of surgery was calculated using Kaplan–Meier survival analysis. Cox proportional hazards model was use to predict the risk factors for relapse. A P value < 0.05 was considered statistically significant.
RESULTS
During the study period, 564 consecutive patients with histologically proven NSCLC underwent complete surgical resection and a lymphadenectomy at our institution. A total of 68 eligible patients with SMPLC were identified, representing a frequency of 12.1% (68/564), and 35 patients were included in this study.
Patient and Tumor Characteristics
The patients ranged in age from 49 to 82 years (median 65 years) and consisted of 11 men (31%) and 24 women (69%). The tumors were bilateral in 17 patients (49%) and were located in different lobes of the same lung in 18 patients (51%). Most patients (26 of 35, 74%) had 2 primary lung cancers; however, 26% (9 of 35) had more than 2 tumors: 6 patients had 3 tumors and 3 patients had 4 tumors. The median size of the most advanced tumor was 3.1 cm (range 0.9–54.0); 51% of the most advanced tumors (18/35) were larger than 3 cm, and 49% of the lesions (17/35) were 3 cm or smaller. The median value of the SUVmax of the largest tumor was 3.1 (range 1.0–13.3). In all patients, a complete resection was performed while maximizing lung parenchymal preservation. The majority of patients (32 of 35, 91%), regardless of whether they underwent single or multiple operations, underwent either a single lobectomy (30 patients) and/or sublobar resections (2 patients). Two patients underwent sequential bilateral lobectomies, and 1 patient required a bilobectomy. None of these patients had mediastinal lymph node metastasis. No 30-day or in-hospital surgical mortality occurred. Twenty-four patients (69%) received adjuvant therapy (19 patients underwent chemotherapy, and 5 patients underwent radiotherapy), and the other 11 patients (31%) did not receive additional therapy after complete surgical resection. Chemotherapy therapy is indicated for the patients with high risk of recurrence (poor differentiation, visceral pleural invasion, lymph node involvement). Postoperative adjuvant radiotherapy is indicated for patients with closed margin (cutting end of bronchus). For other tumor (not index tumor), the imaging characteristics of CT may predict the patient's OS and PFS. Nonsolid type tumor had better OS and PFS.
The median follow-up time for the groups was 44 months (range, 16–86 months). At the last follow-up, 13 (37%) of the 35 patients had suffered a recurrence at a median time of 24 months after the first surgery; 7 of these patients died during follow-up at a median of 46 months after the first surgery. The 3-year recurrence-free survival (RFS) rate was 53.6%, with a 95% confidence interval (CI) of 37.1% to 70.1%. The overall cumulative 3- and 5-year survival rates were 91.5% (95% CI: 81.7–100.0) and 75.0% (95% CI: 60.7–89.3), respectively. The median OS was 45.5 months (95% CI: 36.4–51.3).
Association Among Tumor Size, PET, and Survival
The results of the univariate recurrence and survival analyses are shown in Table 1. The largest tumor size and SUVmax were significant predictors of recurrence (P = 0.018 and 0.003, respectively) and OS (P = 0.064 and 0.063, respectively). The following were not significant prognostic factors for recurrence or survival: age, sex, smoking status, lung function, preoperative serum carcinoembryonic antigen level, tumor location, histologic grade of differentiation, lymphovascular invasion, and treatment with adjuvant chemotherapy.
TABLE 1.
Univariate Analysis of Predictors Associated With PFS and OS in Synchronous Multiple Lung Cancers
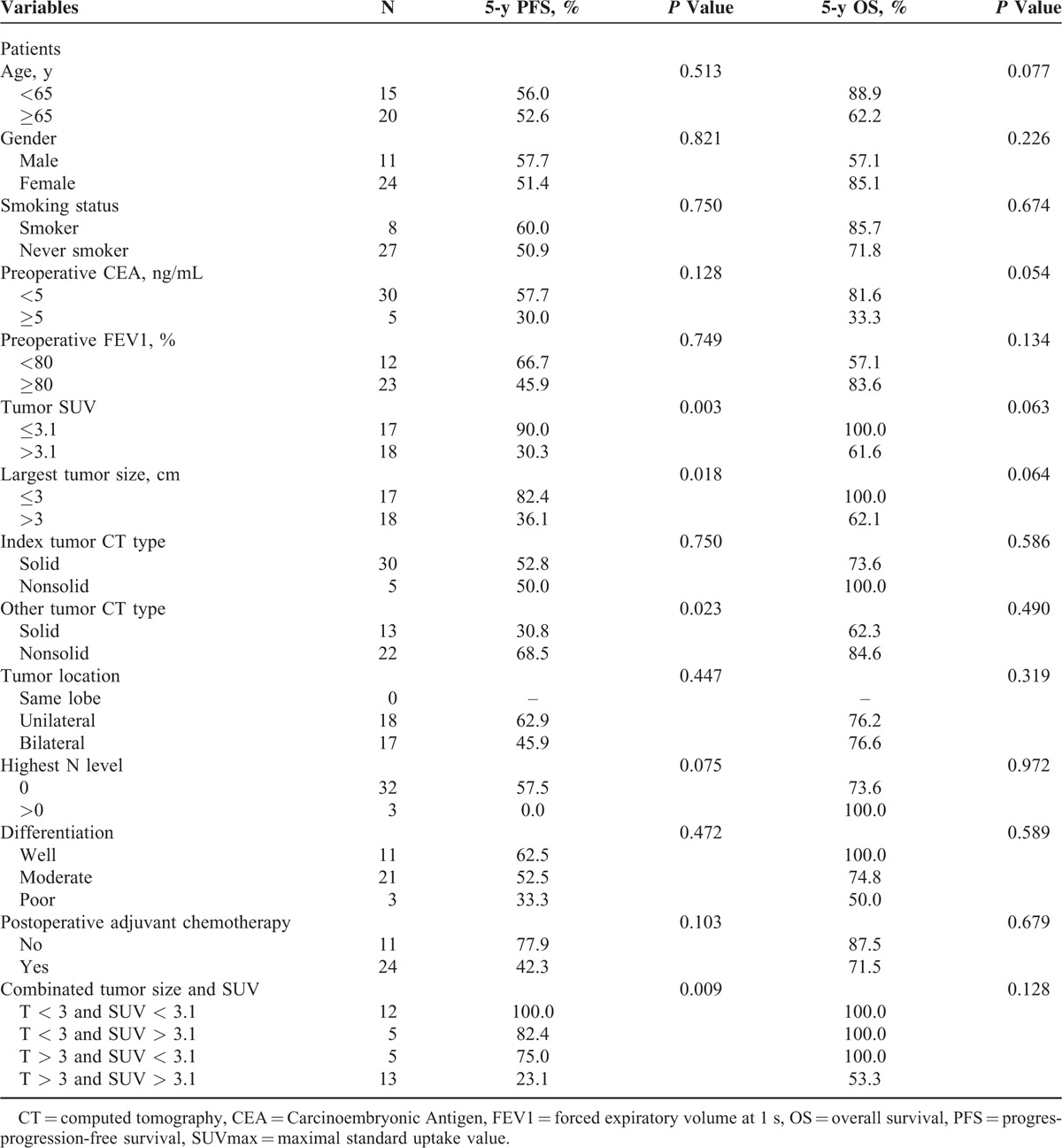
The ROC curves showed that the optimal cutoff value for predicting recurrence was 3 cm for tumor size and 3.1 for SUVmax. Using a cutoff value of 3 cm for tumor size yielded a sensitivity of 84.6% (95% CI: 72.6–96.6) and a specificity of 68.2% (95% CI: 52.8–83.6). The SUVmax value of 3.1 corresponded to a sensitivity of 92.3% (95% CI: 83.5–100.0) and a specificity of 72.7% (95% CI: 58.0–87.4). The AUC for predicting RFS was 0.71 for tumor size (95% CI: 0.53–0.88, P = 0.046) and 0.86 for SUVmax (95% CI: 0.74–0.99, P < 0.001; Figure 1).
FIGURE 1.
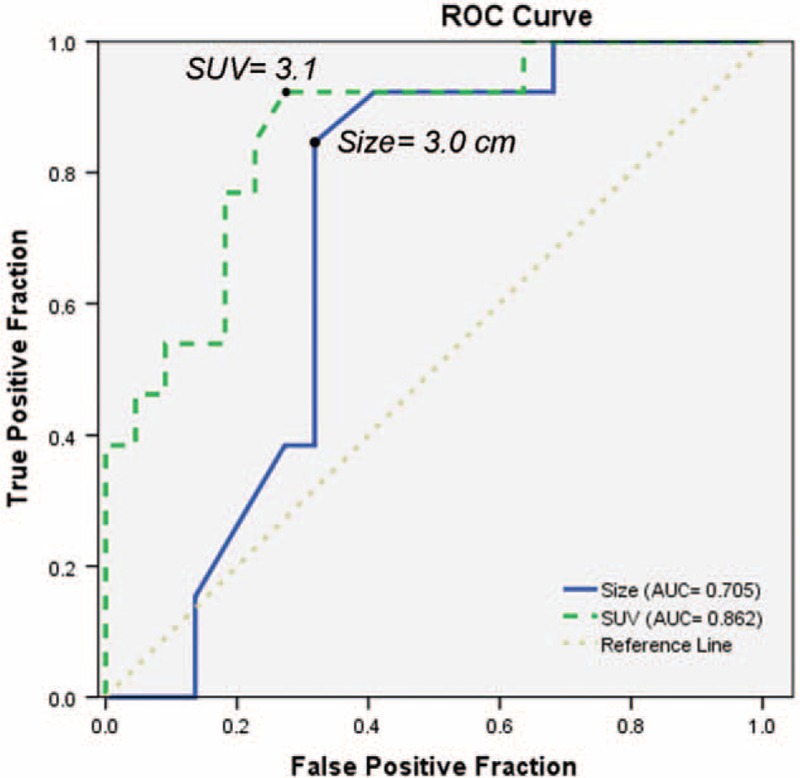
Receiver operating characteristic curves showed that the optimal cutoff value for predicting recurrence was 3 cm for tumor size and 3.1 for SUVmax.
In the multivariate analysis, large tumor size and a high SUVmax were independently associated with recurrence (P = 0.01 and <0.01, respectively) with hazard ratios (HRs) of 2.95 (95% CI: 0.63–13.73) and 8.24 (95% CI: 1.03–65.74), respectively. Large tumor size (>3 cm) and high SUVmax (>3.1) of referent tumor predicted the high probability of recurrences with HR of 6.79 (Table 2). The disease recurrence and survival rate, stratified for tumor size and SUVmax, are depicted in Figure 2A. Large tumor size (>3 cm) and high SUVmax (>3.1) of referent tumor predicted the poor OS (5-year survival 53.3%; Table 3). The OS stratified for tumor size and SUVmax, are depicted in Figure 2B.
TABLE 2.
Cox Regression Model for Progression-Tree Survival in SMPLC
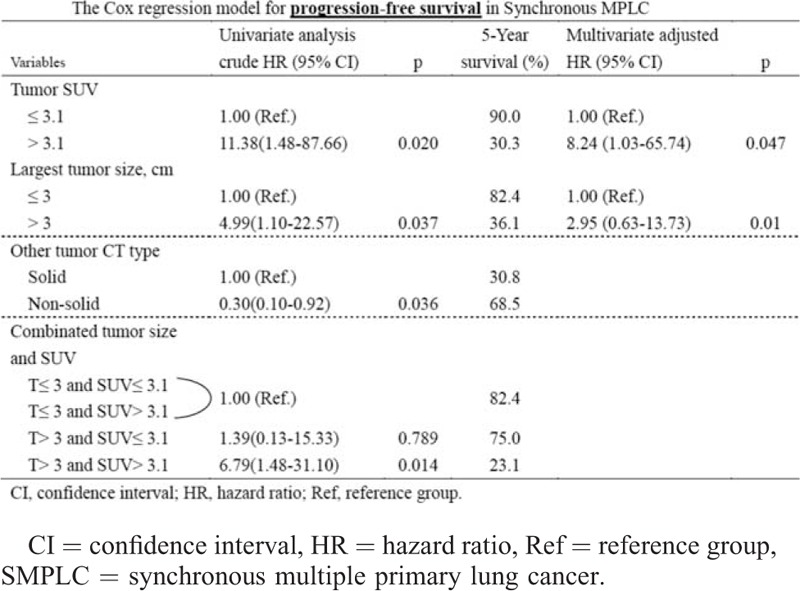
FIGURE 2.
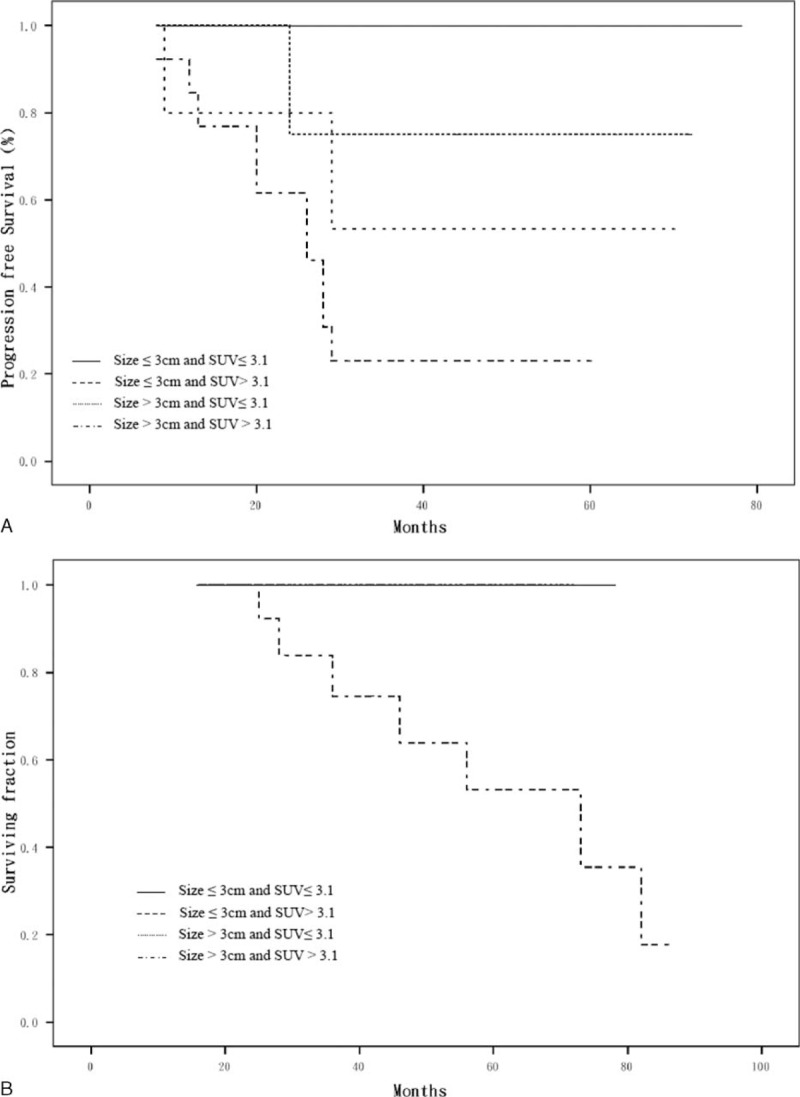
(A) Progression-free survival according to tumor size and SUVmax; (B) overall survival according to tumor size and SUVmax. Patients died only in T > 3 and SUVmax > 3.1 group.
TABLE 3.
Cox Regression Model for Overall Survival in SMPLC
DISCUSSION
It is known that patients with multiple primary pulmonary adenocarcinomas have a far better prognosis than those with other types of advanced disease or distant metastasis, if surgical resection is possible.5 The results of the present study as well as other large series have shown that the 5-year survival of patients with SMPLC without lymph node metastasis exceeds 50% despite similar histologic classification (despite similarities in histopathological features and similar histology) in most cases.2,4 The present results, despite the short follow-up period, indicated a 75% 5-year OS rate. However, very little is currently known regarding the risks of recurrence and factors that influence survival, especially in patients who could benefit from surgical resection. The prognostic implications of the presence of carcinomas in addition to the primary tumor remain controversial, and several previous series have presented contradictory results. Table 4 summarizes the most recent survival results and prognostic factors in surgically resected SMPLC patients.2,9–15 In the present study, we examined a relatively homogeneous series of consecutive patients with surgically treated SMPLCs to identify factors that affected postoperative recurrence and survival rates. The univariate analysis showed that SUVmax and tumor size (measured by CT), which can both be evaluated preoperatively and noninvasively, were significant independent postoperative prognostic factors for recurrence in patients with SMPLC. The combination of the highest tumor SUVmax and tumor size is useful for risk stratification of postoperative recurrence in these patients. The ability to predict recurrence has significant therapeutic and prognostic implications. If the prognosis can be determined preoperatively, it may be possible to identify candidates for intensive preoperative and postoperative treatment. Clearly, additional study is essential for further validation of the current results.
TABLE 4.
Survival and Prognostic Factors in Surgically Resected Synchronous Multiple Primary Lung Careers: Summary of the Literature Over the Last 10 y

The survival outcomes in the present study were satisfactory, regardless of the location of the additional primary tumors, and were better than expected under the current TNM staging system. The prognosis of the patients in this study was favorable compared to that other studies that have reported on surgical resection of SMPLC.14,15 One possible explanation for this observation is that the favorable survival in the present series of patients with SMPLC was because these patients were more likely to have had true SMPLCs. In the present study, an extensive preoperative workup (including PET imaging) was performed to rule out extrathoracic disease and identify fit candidates for complete surgical resection. The favorable survival observed in this series may also have been due to patient selection, as our patients were more highly selected than those with solitary cancers. Patients with nodal disease were excluded, thereby reducing the likelihood of including patients with true stage IIIB or metastatic disease.
The utility of FDG-PET in the differentiation of synchronous lung malignancy or metastases remains unclear. Recently, the SUV has been shown to be helpful in differentiating metastatic disease from second primary cancers in patients presenting with synchronous pulmonary lesions.16,17 As here we presented 1 patient with double primary lung cancer with different SUVmax of each tumor (Figure 3). We found no previous reports focusing on the prognostic significance of FDG uptake in surgically treated SMPLC patients. It has been reported that there is a significant relationship between 18F-FDG uptake and the degree of tumor cell proliferation, tumor growth rates, and tumor aggressiveness in pulmonary adenocarcinoma.18–20 FDG-PET imaging is currently extensively used clinically and is a significant prognostic factor for survival in patients with solitary lung cancers. New risk stratification factors and models are needed to preoperatively evaluate which patients with resected SMPLC are at highest risk for relapse and stand to benefit most from adjuvant therapy. Therefore, we performed disease-free survival and OS analyses to determine whether 18F-FDG uptake (SUV) can significantly predict for recurrence and survival in patients with SMPLC. In this study, multivariate analysis identified the SUV as the most significant independent factor for recurrence, and the most strongly significant cutoff point was found at an SUVmax of 3.1. Patients with the highest tumor SUVmax and the largest tumors appear to have the highest risk of recurrence; such patients may be appropriate candidates for trials of intensive preoperative and postoperative treatment, including radiotherapy and chemotherapy. If a group of high-risk patients who are likely to have microscopic metastases at presentation could be identified, improved outcomes may be possible. This information may help guide treatment strategies. However, a larger, multi-institutional prospective study is needed for further validation of the current results.
FIGURE 3.
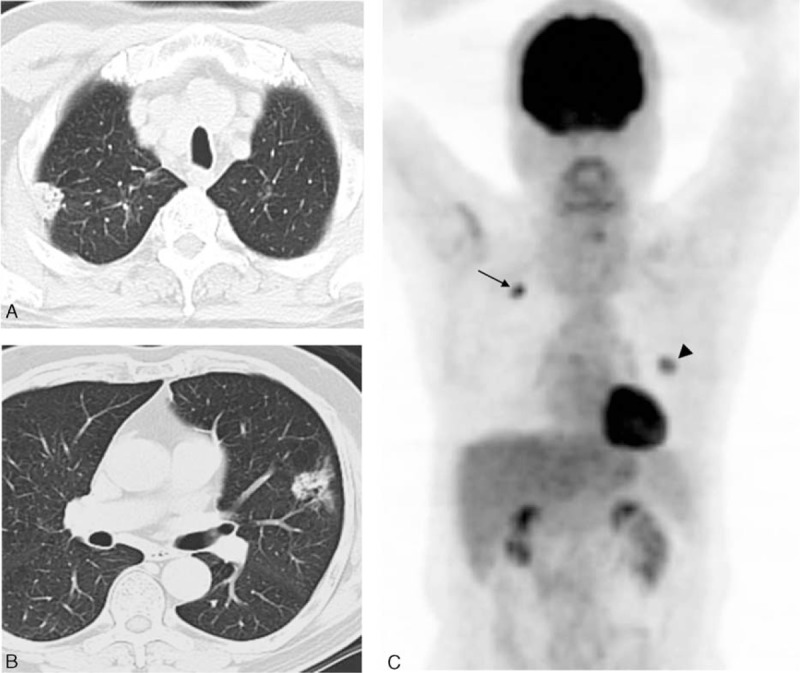
Representative CT and 18F-FDG PET images of a 62-y-old man with 2 synchronous primary lung tumors. (A, B) Axial CT scans in lung window setting showing a 2.0-cm solid nodule in the right upper lobe (RUL) of lung and another 3.1-cm lesion mixed with solid and ground glass components in the left upper lobe (LUL). (C) Whole-body maximum-intensity projection of FDG PET demonstrating a moderately differentiated adenocarcinoma in the RUL with an SUVmax of 9.4 (arrows) and a second primary with similar histology but a different colonel origin in the LUL with an SUVmax of 4.3 (arrowheads). CT = computed tomography, 18F-FDG = 18F-fluorodeoxyglucose, PET = positron emission tomography.
Tumor size itself may have a strong influence on potential local progression or metastasis. Because tumor size may roughly reflect the number of tumor cells and is more readily known to clinicians than histology or genetic analysis, this information may be important when deciding whether to recommend an aggressive surgical approach with a curative objective. Generally, tumor size is an important prognostic factor for early-stage lung cancer.21 However, little is known about the prognostic significance of tumor size among patients with SMPLC.11 In this study, a prognostic difference was seen according to tumor size of the reference tumor >3 cm and 2 variables of SUVmax and tumor size were found to provide excellent independent prognostic discrimination. The combination of SUVmax and tumor size together identified a subgroup of patients at highest risk for a result of recurrent disease after surgical resection.
This study has some limitations. First, it was retrospective, and selection bias was introduced by limiting entry to patients with adenocarcinoma who had undergone CT and PET examinations and surgery; therefore, our data are subject to the so-called verification or work-up bias22 and may not represent the general population with SMPLC. Second, some variables, such as tumor colony (subclassification of adenocarcinoma), lung function, and comorbidities, were not included in our analysis. Therefore, the results regarding patient prognoses may not be generalizable to the entire patient population. Nonetheless, we believe that the prognostic factors derived from this study may be useful for clinical decision making. Further analysis in a larger series is needed to more clearly address these concerns. In addition, it is unclear whether other treatment approaches, such as stereotactic body radiation or targeted therapy, would yield better results than the surgical approach.23
In conclusion, the management of multiple lung cancers is challenging, and patient prognoses vary. Patients with SMPLC can benefit from aggressive surgery. The size and SUVmax of the reference tumor may predict postoperative outcomes.
Acknowledgment
The authors thank the Cancer Registry Group of Tri-Service General Hospital for the support of the data.
Footnotes
Abbreviations: AUC = area under the ROC curve, BAC = bronchioloalveolar carcinoma, CI = confidence interval, CT = computed tomography, FDG = fluorodeoxyglucose, GGO = ground-glass opacity, HU = Hounsfield units, MDCT = multi-detector row computed tomography, NSCLC = nonsmall cell lung cancer, OS = overall survival, PACS = picture archiving and communication system, PET = positron emission tomography, RFS = recurrence-free survival, ROC = receiver operating characteristic, SMPLC = synchronous multiple primary lung cancer, SUV = maxmaximal standard uptake value, TNM = tumor-node metastasis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70:606–612. [PubMed] [Google Scholar]
- 2.Trousse D, Barlesi F, Loundou A, et al. Synchronous multiple primary lung cancer: an increasing clinical occurrence requiring multidisciplinary management. J Thorac Cardiovasc Surg 2007; 133:1193–1200. [DOI] [PubMed] [Google Scholar]
- 3.American Joint Committee on Cancer. Cancer Staging Manual—Lung. 7th ed.Chicago: American Joint Committee on Cancer; 2010. [Google Scholar]
- 4.Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg 2007; 134:630–637. [DOI] [PubMed] [Google Scholar]
- 5.Tung YW, Hsu CP, Shai SE, et al. Surgical feasibility of ipsilateral multifocal non-small cell lung cancer in different lobes: excellent survival in node-negative subgroup. Eur J Cardiothorac Surg 2003; 24:1008–1012. [DOI] [PubMed] [Google Scholar]
- 6.Van Rens MT, Zanen P, Brutel de La Rivière A, et al. Survival in synchronous vs. single lung cancer: upstaging better reflects prognosis. Chest 2000; 118:952–958. [DOI] [PubMed] [Google Scholar]
- 7.Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004; 78:1194–1199. [DOI] [PubMed] [Google Scholar]
- 8.Tsunezuka Y, Matsumoto I, Tamura M, et al. The results of therapy for bilateral multiple primary lung cancers: 30 years experience in a single centre. Eur J Surg Oncol 2004; 30:781–785. [DOI] [PubMed] [Google Scholar]
- 9.Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010; 5:197–205. [DOI] [PubMed] [Google Scholar]
- 10.Yu YC, Hsu PK, Yeh YC, et al. Surgical results of synchronous multiple primary lung cancers: similar to the stage-matched solitary primary lung cancers? Ann Thorac Surg 2013; 96:1966–1974. [DOI] [PubMed] [Google Scholar]
- 11.Tanvetyanon T, Robinson L, Sommers KE, et al. Relationship between tumor size and survival among patients with resection of multiple synchronous lung cancers. J Thorac Oncol 2010; 5:1018–1024. [DOI] [PubMed] [Google Scholar]
- 12.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143:29–36. [DOI] [PubMed] [Google Scholar]
- 13.Rostad H, Strand TE, Naalsund A, et al. Resected synchronous primary malignant lung tumors: a population-based study. Ann Thorac Surg 2008; 851:204–209. [DOI] [PubMed] [Google Scholar]
- 14.Voltolini L, Rapicetta C, Luzzi L, et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. Eur J Cardiothorac Surg 2010; 37:1198–1204. [DOI] [PubMed] [Google Scholar]
- 15.Kocaturk CI, Gunluoglu MZ, Cansever L, et al. Prognosis in patients with non-small cell lung cancer and satellite tumors. Thorac Cardiovasc Surg 2011; 596:360–363. [DOI] [PubMed] [Google Scholar]
- 16.Dijkman BG, Schuurbiers OC, Vriens D, et al. The role of (18)F-FDG PET in the differentiation between lung metastases and synchronous second primary lung tumours. Eur J Nucl Med Mol Imaging 2010; 37:2037–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obando JA, Samii JM, Yasrebi M. A case of two synchronous primary lung tumors demonstrated by FDG positron emission tomography. Clin Nucl Med 2008; 33:775–777. [DOI] [PubMed] [Google Scholar]
- 18.Vesselle H, Salskov A, Turcotte E, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol 2008; 3:971–978. [DOI] [PubMed] [Google Scholar]
- 19.Higashi K, Ueda Y, Ayabe K, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun 2000; 21:707–714. [DOI] [PubMed] [Google Scholar]
- 20.de Geus-Oei LF, van Krieken JH, Aliredjo RP, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer 2007; 55:79–87. [DOI] [PubMed] [Google Scholar]
- 21.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2007; 2:694–705. [DOI] [PubMed] [Google Scholar]
- 22.Sica GT. Bias in research studies. Radiology 2006; 238:780–789. [DOI] [PubMed] [Google Scholar]
- 23.Tanvetyanon T, Finley DJ, Fabian T, et al. Prognostic factors for survival after complete resections of synchronous lung cancers in multiple lobes: pooled analysis based on individual patient data. Ann Oncol 2013; 24:889–894. [DOI] [PubMed] [Google Scholar]


