Abstract
This study was designed and performed to establish the relationship between plasma and synovial fluid (SF) levels of thioredoxin reductase (TrxR) and disease activity in Chinese patients with rheumatoid arthritis (RA).
This study consisted of a total of 224 patients diagnosed with RA, 224 age and sex-matched healthy controls, and 156 patient controls. The disease activity of RA patients was calculated as diseases activity score that include 28-joint counts (DAS 28), which was divided into low-diseases activity (LDA) and high-diseases activity (HDA) groups.
Increased plasma TrxR was detected in patients with RA than healthy controls (P < 0.0001). With an area under the curve (AUC) of 0.874, plasma TrxR showed a evidently greater discriminatory ability than C-reactive protein (CRP; AUC, 0.815), antistreptolysin-O (ASO; AUC, 0.631), rheumatoid factor (RF, AUC, 0.793), and erythrocyte sedimentation rate (ESR, AUC, 0.789) in diagnosing RA. RA patients with HDA had significantly elevated TrxR levels in plasma and SF than did those with LDA (P < 0.0001). With an AUC of 0.874, plasma TrxR levels as an indicator for screening of HDA showed a significantly greater discriminatory ability than CRP (AUC, 0.690), ASO (AUC, 0.597), RF (AUC, 0.657), and ESR (AUC, 0.603). Similarly, SF TrxR levels as an indicator for screening of HDA also showed a significantly greater discriminatory ability as compared with above biomarkers.
TrxR levels in plasma and SF were positively correlated with the severity of RA. TrxR levels may therefore serve as a new biomarker in addition of the traditional biomarkers for assessing the risk and severity of RA. Further analysis of TrxR release machinery may give us a new understanding of pathogenesis of RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a common long-lasting autoimmune disorder that primarily affects joints resulted from the inflammation of the synovial membrane of multiple joints and further causing damage to the cartilage and bones.1 The cause of RA is unknown, and the prognosis is guarded, where half of the risk for RA is believed to be genetic2. Furthermore, smoking is suggested to be the most significant nongenetic risk factor; besides, vitamin D deficiency is more common in people with RA than in the general population. Importantly, cytokines are directly implicated in the pathogenesis of RA as part of a complex regulatory network related to specific immunological processes that promote autoimmunity, chronic inflammation, and tissue destruction.3
In addition, oxidative stress is a feature of much pathology of human diseases, which has been proposed to be closely correlated with the pathogenesis of RA.4,5 The clear relationship between cellular redox homeostasis imbalance and increased oxidative damage has been proposed, possible reason might be that there was a concomitant association of increased expression of pro-inflammatory factors at sites of inflammation, including cytokines, prostaglandins, reactive nitrogen species (RNS), and reactive oxygen species (ROS).6 These reactive species function significantly in the following 3 aspects: leading to the destructive, proliferative synovitis evident in RA; working as a key modulators of the immune responses (synovial T lymphocytes) that take place in the inflamed synovium of RA patients; and playing a critical role in regulating events of cell-signaling.7 Coincidently, there was previous evidence proved this supposition, for example, in the study performed by Rall et al,8 observably higher levels of oxidative stress was found in subjects with RA when compared to those in the healthy individuals. Besides, there was research mentioned that via the application of antioxidants, a suppressing of its serum levels was documented to predispose for the development of RA,9 emphasizing the effect of elevated ROS production in the disease clinically. Furthermore, multiple products generated by ROS have been identified in clinical samples from the joints in patients with RA.10
According to the previous evidence on the exploration of the relationship between oxidative stress and RA, we speculated that redox imbalance might play a role in the pathology of RA associated with previous studies.11 Thioredoxin system included thioredoxin reductase (TrxR) and thioredoxin (TRX) is 1 of the important regulators of the redox state of proteins.12 The mammalian TrxRs are consisting of a conserved-Cys-Val-Asn-Val-Gly-Cys-redox catalytic site, to glutathione reductases.13 Importantly, TrxRs can contribute to the catalysis of the NADPH-dependent reduction of the redox protein TRX. In addition, TRX gene was found to have a novel cis-regulatory element responsible for oxidative stress in its promoter region, and can be strongly induced by oxidative stress.14
Increased production of TRX in synovial tissue and blood of RA patients were recently reported consistent with the hypothesis that TRX/TrxR activities may contribute to disease activity in RA.15,16 For instance, Jikimoto et al17 showed that plasma TRX could be regarded as a useful biomarker for RA disease activity and as an index of oxidative activity and may therefore reflect higher oxidative stress levels in RA, which was also proved by another paper indicated that increased expression of TRX presented a positive association with the disease activity in RA.1 In sharp contrast, few studies investigate the relationship of the TrxR in plasma and synovial fluid (SF) with the screening of RA and the confirmation of disease activity of RA. Herein, this study was designed to establish the association between plasma and SF levels of TrxR and disease activity via the detection of TrxR in the plasma and SF in Chinese patients with RA.
PATIENTS AND METHODS
Ethnic Statement
The ethics committee of the Zhejiang Chinese Medical University approved this study before the performance of the operation. All participants (or their relatives) were notified of the study protocol and signed informed consent approved by the institutional ethics committee.
Patients
In 2014, a total of 224 patients with RA who fulfilled the 201018 American College of Rheumatology criteria for RA were recruited from the Outpatient unit of Rheumatology Department at the Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou and enrolled in this study for the following experiment. The included eligible patients should have paired plasma and SF samples. Exclusion criteria: patients who have previous history of smoking history over 5 years; patients had alcohol intake during the past 1 years; patients with narcotic drugs intake; patients were diagnosed with hypertension, diabetes mellitus, hypothyroidism/hyperthyroidism, and any other form of arthritis except RA; and patients who received a previous treatment of steroids ([10 mg/d, including parenteral administration) and have a previous history of biologic therapy.
Two hundred eighteen age and sex-matched subjects who were enrolled for health physical examination during the same hospital with no clinical and radiological evidence of RA served as the control group. In addition, 156 age and sex-matched patients who diagnosed as ankylosing spondylitis, Reiter's syndrome, or infectious arthritis were designated as the patient control group. These patients came from our orthopedic clinic. The exclusion criteria for RA patients also apply to the control cases.
Clinical Variable
Standard demographic details and biomarkers were collected including age, sex, disease duration, white blood cells (WBC), C-reactive protein (CRP), rheumatoid factor (RF), antistreptolysin-O (ASO), anticitrullinated protein/peptide antibodies (ACPA), and erythrocyte sedimentation rate (ESR). Disease activity was assessed using a variety of variables including ESR and CRP, swollen joint count (SJC), tender joint count (TJC), and diseases activity score 28 (DAS 28) according to the method of Prevoo et al.19 Consisting of 28 joint swelling and 28 joint tender, values DAS 28 ≤3.2 meant low-diseases activity [LDA], and values DAS 28 > 3.2 meant moderate and high-diseases activity [HDA]); together with ESR and visual analog score (VAS) (“no pain” [left end 0 mm] and “excruciating pain” [right end 100 mm]).
Laboratory Test
Morning fasting blood samples were collected in 5 mL BD Vacutainer Rapid Serum Tube, Mississauga, Ontario, Canada (NJ) from each participant, were quickly centrifuged to separate the plasma from the cells, and were then immediately frozen at −80°C until analysis. Before any treatment, SF was obtained from RA patients. Control subjects gave only plasma samples. All samples and indicators examination were detected and analyzed in duplicates. Investigators who were recruited to measure the concentration of the included biomarkers were blinded to the clinical outcome. Levels of TrxR were measured by enzyme-linked immunosorbent assay (ELISA). The coefficients of variation (CVs) of inter-assay and intra-assay were 5.8% to 8.9% and 6.8% to 9.9%, respectively. ACPA levels in plasma and SF in RA patients were tested by ELISA method. Other biomarkers, such as WBC, Hs-CRP, ASO, RF, and ESR, were also tested. In addition, undetectable levels were preset to equal to the lower limit in the experiment.
Statistical Analysis
In this experiment, continuous variables were expressed as means (standard deviation, SD) and medians (interquartile ranges, IQR). The difference between groups was estimated via the application of the Mann–Whitney U test and chi-squared test. Spearman rank correlation test analyzed the possible correlations among laboratory parameters. The diagnostic accuracy of TrxR in RA and its disease severity were assessed by receiver operating characteristic (ROC) curve. Area under the curve (AUC) was used to assess cut-point choices to ensure the accuracy of the test. In addition, with multivariate adjustment for possible confounders (age, gender, body mass index [BMI], disease duration, levels of WBC, CRP, ASO, RF, and ESR), ordered logistic regression models were applied for the investigation of the association between the levels of TrxR and disease severity. The association between TrxR and disease severity of RA were expressed as adjusted odds ratios (ORs) and 95% confidence interval (CI). Statistical calculation was tested by SPSS (version 19.0, SPSS, Inc., Chicago, IL) and the version1.0-2ROCR package (http://cran.r-project.org/). Differences were considered significant at P < 0.05.
RESULTS
Patient Demographics
In our study, there was a sum of 224 patients recruited who were diagnosed with RA that had paired plasma and SF samples. In the study population, 80 (36.7%) were male and the median age was 51 years (IQR, 37–58). In those patients, 188 (83.9%) patients had ACPA-positive results. The TrxR levels in plasma and SF were no significantly difference in those RA patients who had ACPA-positive as compared with those who had ACPA-negative (All P > 0.05). Out of the 224 patients, 109 (48.7%) had HDA disease (DAS-28 > 3.2). Detailed information with respect to the baseline characteristics between groups were shown in Table 1.
TABLE 1.
Baseline Characteristics of Patients With RA and Normal Cases
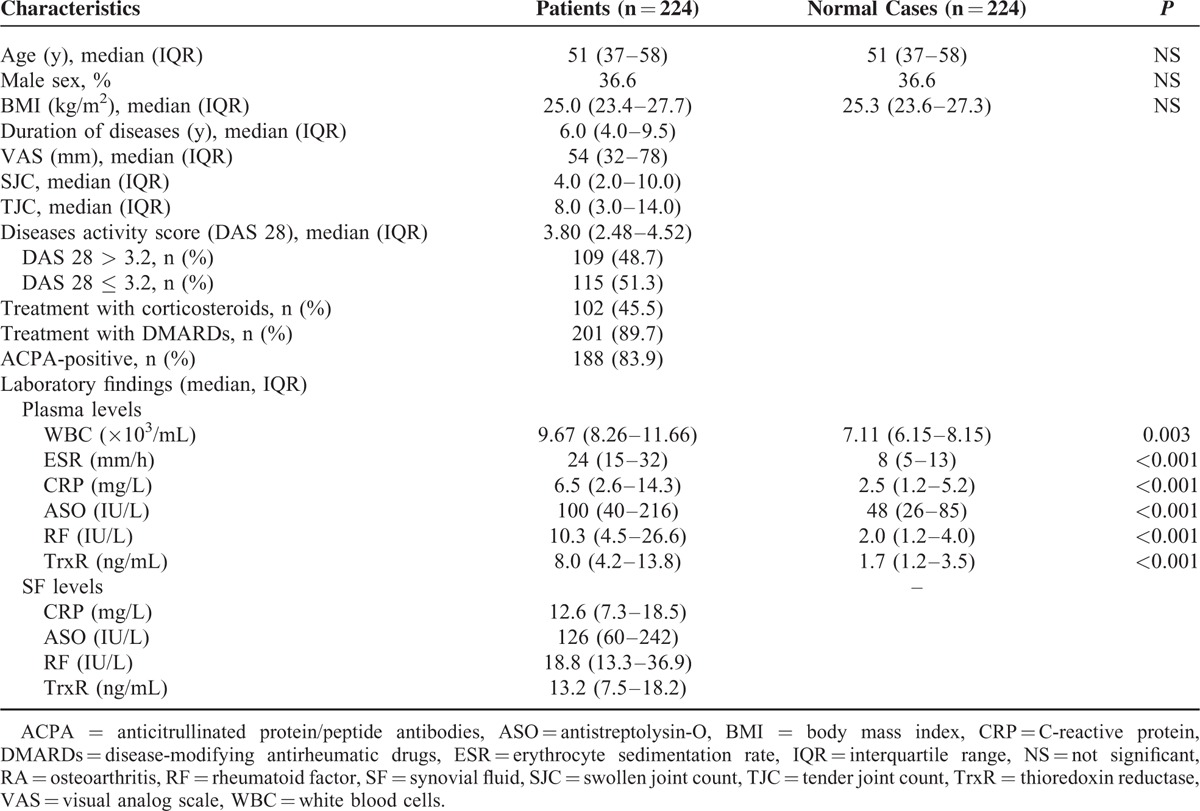
TrxR Is Increased in Patients With RA Compared With Controls
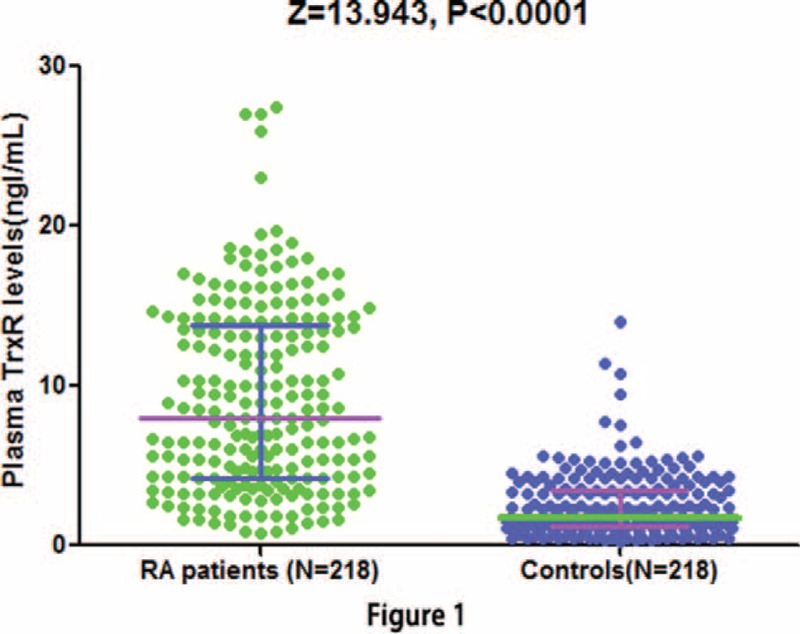
As shown in Figure 1, when compared to healthy controls, plasma TrxR was much higher in patients with RA (8.0 [IQR, 4.2–13.8] ng/mL vs 1.7 [IQR, 1.2–3.5] ng/mL; Z = 13.943, P < 0.0001). Besides, the cutoff value of plasma TrxR levels was 3.5 ng/mL for screening RA (sensitivity: 84.6 %, specificity: 72.5%), and AUC at 0.874 (95% CI, 0.843–0.906; P < 0.0001; Figure 2). With an AUC of 0.874, TrxR has greater discriminatory ability than CRP (AUC, 0.815; 95% CI, 0.774–0.857; P < 0.01), ASO (AUC, 0.631; 95% CI, 0.577–0.684; P < 0.0001), RF (AUC, 0.793; 95% CI, 0.750–0.836; P < 0.001), and ESR (AUC, 0.789; 95% CI, 0.748–0.831; P < 0.001). On the other hand, the combination model (TrxR/CRP/ASO/RF/ESR) was indicated to have a relatively higher discriminatory accuracy (AUC, 0.965; 95% CI, 0.925–0.979) than those markers alone.
FIGURE 1.
Plasma levels of TrxR in patients with RA and controls. All data are medians and interquartile ranges (IQR). Mann–Whitney U test. RA = rheumatoid arthritis, TrxR = thioredoxin reductase.
FIGURE 2.
Receiver operator characteristic (ROC) curve demonstrating sensitivity as a function of 1-specificity for diagnosing RA based on the combined model incorporating (TrxR/RF/CRP/ASO/ESR) and the relative contribution of each plasma biomarker alone (initial cohort). This combined model had an area under the ROC curve of (AUC, 0.965; 95% CI, 0.925–0.979). ASO = antistreptolysin-O, AUC = area under the curve, CI = confidence interval, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, RA = rheumatoid arthritis, RF = rheumatoid factor, TrxR = thioredoxin reductase.
Similarly, there was also a same tendency with regard to the association of the increased plasma TrxR level and the screening of RA (8.0 [IQR, 4.2–13.8] ng/mL vs 2.5 [IQR, 1.7–4.1] ng/mL; Z = 8.326, P < 0.0001). Furthermore, the optimal cutoff value of plasma TrxR levels was 3.9 ng/mL (sensitivity: 78.6 %, specificity: 70.7%, AUC, 0.848, 95% CI, 0.792–0.891; P < 0.0001). With an AUC of 0.848, TrxR showed greater ability of discrimination as compared with CRP (AUC, 0.778; 95% CI, 0.715–0.826; P < 0.01), ASO (AUC, 0.604; 95% CI, 0.538–0.655; P < 0.001), RF (AUC, 0.758; 95% CI, 0.702–0.801; P < 0.01), and ESR (AUC, 0.676; 95% CI, 0.622–0.724; P < 0.001). Interestingly, higher discriminatory accuracy was detected in the combined model (TrxR/CRP/ASO/RF/ESR) (AUC, 0.889; 95% CI, 0.827–0.926) when compared to those markers alone. In addition, the combined model (TrxR/CRP/ASO/RF/ESR) compared with a model without TrxR showed a greater discriminatory ability (AUC, 0.818; 95% CI, 0.775–0.867; P < 0.01).
Relationship Between Disease Activity and TrxR Levels
Accordingly, there was an evidently positive correlation between increased plasma TrxR levels and increasing severity of RA (DAS-28) (r = 0.448, P < 0.0001). RA patients with HDA had significantly elevated TrxR levels in plasma than did those with LDA (12.0 [IQR, 5.6–15.2] ng/mL vs 6.0 [IQR, 3.5–10.0] ng/mL; P < 0.0001; Figure 3A). Similarly, RA patients with HDA had significantly elevated TrxR levels in SF than did those with LDA (17.3 [IQR, 11.9–24.3] ng/mL vs 10.4 [IQR, 5.3–14.5] ng/mL; Figure 3B). In addition, there was a significantly correlation between levels of TrxR in plasma and in SF (r = 0.409, P < 0.0001), similar tendency was also found with respect to the relationship between plasma levels of TrxR and CRP (r = 0.306, P < 0.001), as well as SF levels of TrxR levels and CRP (r = 0.328, P < 0.001). In addition, a correlation between TrxR and WBC in SF was found (r = 0.198, P = 0.012). On the other hand, there was no influence of age, sex, BMI, ASO, RF, WBC, and ESR on TrxR in RA patients, with no statistical difference (all P > 0.05).
FIGURE 3.
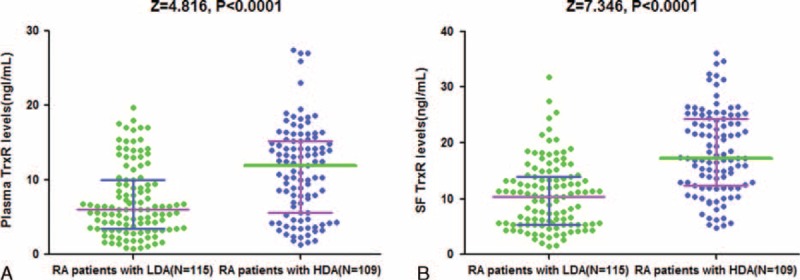
Levels of TrxR in different groups. (A) Plasma levels of TrxR in RA patients with HAD and LDA; (B) SF levels of TrxR in RA patients with HAD and LDA. All data are medians and interquartile ranges (IQR). HAD = high-diseases activity, LDA = low-diseases activity, RA = rheumatoid arthritis, SF = Synovial fluid, TrxR = thioredoxin reductase.
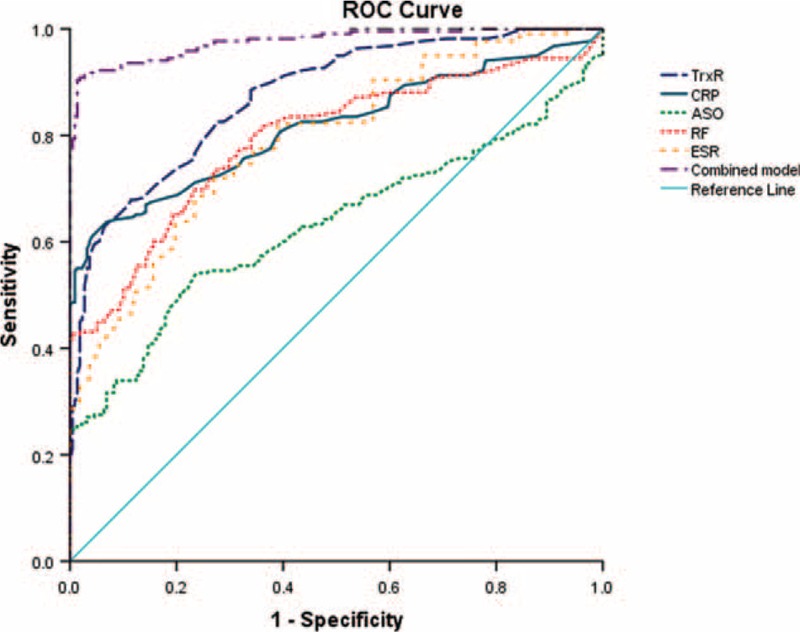
Considering plasma TrxR levels as an indicator for screening of HDA, the optimal cutoff value of plasma TrxR levels was 8.0 ng/mL (sensitivity: 79.6%, specificity: 69.8%; AUC, 0.769, 95% CI, 0.705–0.833; P < 0.0001; Figure 4A). With an AUC of 0.874, TrxR showed a significantly greater discriminatory ability as compared with CRP (AUC, 0.690; 95% CI, 0.620–0.770; P < 0.001), ASO (AUC, 0.597; 95% CI, 0.521–0.673; P < 0.0001), RF (AUC, 0.657; 95% CI, 0.584–0.730; P < 0.001), and ESR (AUC, 0.603; 95% CI, 0.528–0.678; P < 0.0001). And the combination model (TrxR/CRP/ASO/RF/ESR) shown a greater discriminatory accuracy (AUC, 0.833; 95% CI, 0.780–0.885) than those markers alone. Further, increased risk of HDA was positively associated with plasma TrxR levels ≥8.0 ng/mL (unadjusted OR, 10.45; 95% CI, 4.13–24.08). Furthermore, after adjusting for sex, age, duration of diseases, and other biomarkers, increased risk of HDA also presented a positive association when plasma TrxR levels ≥8.0 ng/mL (OR, 5.99; 95% CI, 3.28–11.44; P < 0.0001).
FIGURE 4.
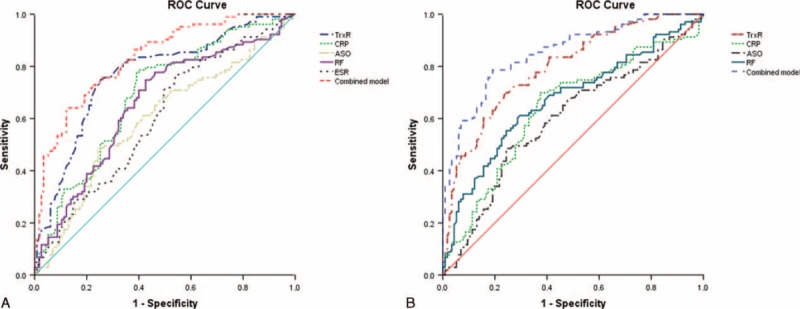
(A) Receiver operator characteristic (ROC) curve demonstrating sensitivity as a function of 1-specificity for diagnosing HDA based on the combined model incorporating (TrxR/RF/CRP/ASO/ESR) and the relative contribution of each plasma biomarker alone (initial cohort). This combined model had an area under the ROC curve of (AUC, 0.833; 95% CI, 0.780–0.885). (B) ROC curve demonstrating sensitivity as a function of 1-specificity for diagnosing HDA based on the combined model incorporating (TrxR/RF/CRP/ASO) and the relative contribution of each SF biomarker alone (initial cohort). This combined model had an area under the ROC curve of (AUC, 0.851; 95% CI, 0.801–0.901). ASO = antistreptolysin-O, AUC = area under the curve, CI = confidence interval, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, HDA = high-diseases activity, RA = rheumatoid arthritis, RF = rheumatoid factor, SF = Synovial fluid, TrxR = thioredoxin reductase.
In addition, the cutoff value of SF TrxR levels for screening of HDA was 11.5 ng/mL (sensitivity: 81.5%, specificity: 68.4%; AUC, 0.795, 95% CI, 0.736–0.553; P < 0.0001; Figure 4B). With an AUC of 0.795, CXCL12 showed greater discriminatory ability than CRP (AUC, 0.638; 95% CI, 0.564–0.713; P < 0.01), ASO (AUC, 0.602; 95% CI, 0.529–0.691; P < 0.001), and RF (AUC, 0.671; 95% CI, 0.698–0.743; P < 0.01). Also, the combination model (TrxR/CRP/ASO/RF) shown a significantly higher accuracy of discrimination (AUC, 0.851; 95% CI, 0.801–0.901) than those markers alone. In multivariate analysis, there was an increased risk of HDA associated with SF TrxR levels ≥11.5 ng/mL (OR, 5.13; 95% CI, 3.11–10.75; P < 0.0001) after adjusting for above possible confounders.
DISCUSSION
There was previous data suggested that oxidative stress measurement could help to monitor disease severity in RA,20 where DNA oxidation, lipid peroxidation, ROS,6 protein carbonylation,21 and genetic polymorphisms22 were used as biomarkers evaluating oxidative stress in RA. In this study, we have proposed that there was a strong correlation established between TrxR both in peripheral blood or SF and DAS 28 score, emphasizing that measurement of TrxR, irrespective of the source contribute a lot to predict RA development as well as to elucidate the mechanisms of RA pathogenesis. Similarly, another study suggested that oxidative/nitrosative stress markers may be important for the prediction of the development of RA.23 Our study also showed significant correlation between the HDA and higher levels TrxR, which was also proved by previous evidence.4,6 However, there were other authors insisted that there might be no observably association of this topic in patients with RA.24
Do the elevated TrxR levels in plasma of RA patients merely reflect the local TrxR production in inflamed joints? Interestingly, the positive correlation between plasma and SF level of TrxR and SF highlighted that the important role of the measurement of TrxR in peripheral blood might contribute to the indirect measure of the oxidant status at the sites of tissue damage. These findings suggest that plasma TrxR might be originated from the SF in RA patients. TrxR produced in synovial cavity can move to peripheral circulation due to the cavity pressure is raised in inflamed joints and exceeded the capillary perfusion pressure.25
What kind of cells produces TrxR in SF of RA patients? To be specific, the production of TRX might be stimulated by local environment in the rheumatic joint and there was a positive association between TRX levels and the leukocyte number in the SF,1 this result was also examined in our study. Thus, leukocytes could be seen as a candidate of TRX producers in the RA synovial tissue.26 Previous study reported that SF of RA patients contained large amount of cytokines such as tumor necrosis factor (TNF) and interleukin (IL)-1.17 Thus, the TrxR may be produced by synovial cells, because H2O2 and TNF were reported to induce TRX/TrxR in synovial fibroblasts.1
This study was also performed to confirm whether elevated TrxR levels in plasma and SF have physiological or pathological roles in RA patients. In this study, no link between ACPA and TrxR had been found. Some other possible mechanisms should be taken into account. One study provided strong evidence that oxidative stress is significantly involved in cartilage degradation in experimental arthritis.27 TrxR might contribute to the disease activity in RA associated with its multiple effects in immunologic and inflammatory responses. First, local and systemic oxidative stress was indicated to be deeply involved in the development of chronic and destructive inflammation.28 Redox status can be disturbed by excessive production of ROS, in which ROS production was suggested to modulate the expression of inflammatory chemokines and affecting tissue damage in RA.29 However, in multivariate analysis, there was an increased risk of HDA associated with elevated plasma TrxR levels, showing a different mechanism of TRX production and consumption from the reactive protein. Second, comparisons between mediators suggest a unique correlation of TRX with macrophage migration inhibitory factor (MIF).30 Previous study suggested that MIF is an important member of the cytokine hierarchy in RA.31 In addition, Radstake et al32 found in their established mice model that MIF may be responsible for the development of collagen type II-induced arthritis. Third, pivotal role of TNF has been mentioned in the pathogenesis of RA.33 TNF could result in the induction of increased TRX expression by fibroblast-like synoviocytes.1 In this regard, the positive feedback regulation of both mediators could be part of a self-perpetuating inflammatory response. Fourth, oxidative stress at site of chronic inflammation can cause permanent genetic change.34 Genetic polymorphism of the p53 has been reported to be detected in synovial cells in RA and may contribute to the transformed-appearing phenotype.35 Lastly, by exerting the role of antiapoptosis and growth promoting capacities, TrxR may therefore contribute to both survival of inflammatory cells and hypertrophy of synoviocytes in RA.36 Kabuyama et al37 demonstrated that TrxR1 suppresses hydrogen peroxide and inhibits apoptosis of RA synovial cells. Meanwhile, TRX can trans activate activator protein 1 and nuclear factor kB, their activation may influence in the synovial macrophages and fibroblast-like synoviocytes.1 This study has several potential limitations. First, the sample size was not large enough to reach definitive conclusions. Further studies with great numbers are warranted. Second, this cross-sectional design study must be confirmed by future longitudinal studies. Third, with respect to the adoption of a “gold standard,” ELISA, for the detection of TrxR, hemoglobin interference can frequently cause a false positive signal. Fourth, only TrxR was measured, and in this regard we failed to determine the relationship of other antioxidant defense parameters with TrxR levels and RA. Last, we did not assess the differences of TrxR levels in SF levels between RA patients and control subjects because of ethical concerns.
CONCLUSIONS
TrxR levels in plasma and SF were positively correlated with the severity of RA. TrxR levels in plasma may serve as a new biomarker in addition of the traditional methods for assessing the risk and severity of RA. Further analysis of TrxR release machinery may give us a new understanding of pathogenesis of RA, leading a chance of exogenous TrxR as a new tool for the treatment of RA.
Acknowledgments
Authors also acknowledge the contribution of Derbali Fatma (Medicine faculty of Sousse) and other reviewers who have helped us to improve the manuscript. All authors have read the journal's authorship agreement.
Footnotes
Abbreviations: ACPA = anti-citrullinated protein/peptide antibodies, ASOa = ntistreptolysin-O, AUC = area under the curve, CIc = onfidence interval, CRP = C-reactive protein, CVsc = oefficients of variation, DASd = iseases activity score, ELISAe = nzyme-linked immunosorbent assay, ESRe = rythrocyte sedimentation rate, HDAh = igh-diseases activity, IQRi = nterquartile ranges, LDAl = ow-diseases activity, ORo = dds ratio, RAr = heumatoid arthritis, RFr = heumatoid factor, RNSr = eactive nitrogen species, ROCr = eceiver operating characteristic, ROSr = eactive oxygen species, SF = synovial fluid, SJCs = wollen joint count, TJCt = ender joint count, TNFt = umor necrosis factor, TRX = thioredoxin, TrxR = thioredoxin reductase, WBCw = hite blood cells.
ZX and JS have contributed equally to this study.
This study was supported by National Natural Science Foundation of China (No. 81273680 and 81373633).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Maurice MM, Nakamura H, Gringhuis S, et al. Expression of the thioredoxin-thioredoxin reductase system in the inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum 1999; 42:2430–2439. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 365:2205–2219. [DOI] [PubMed] [Google Scholar]
- 3.da Rocha LF, Duarte ÂL, Dantas AT, et al. Increased serum interleukin 22 in patients with rheumatoid arthritis and correlation with disease activity. J Rheumatol 2012; 39:1320–1325. [DOI] [PubMed] [Google Scholar]
- 4.Hassan SZ, Gheita TA, Kenawy SA, et al. Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: relationship to disease manifestations and activity. Int J Rheum Dis 2011; 14:325–331. [DOI] [PubMed] [Google Scholar]
- 5.Kundu S, Bala A, Ghosh P, et al. Attenuation of oxidative stress by allylpyrocatechol in synovial cellular infiltrate of patients with rheumatoid arthritis. Free Radical Res 2011; 45:518–526. [DOI] [PubMed] [Google Scholar]
- 6.Kundu S, Ghosh P, Datta S, et al. Oxidative stress as a potential biomarker for determining disease activity in patients with rheumatoid arthritis. Free Radic Res 2012; 46:1482-1289. [DOI] [PubMed] [Google Scholar]
- 7.Gringhuis SI, Leow A, Papendrecht-Van Der Voort EA, et al. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol 2000; 164:2170–2179. [DOI] [PubMed] [Google Scholar]
- 8.Rall LC, Roubenoff R, Meydani SN, et al. Urinary 8-hydroxy-2-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: effect of progressive resistance training. J Nutr Biochem 2000; 11:581–584. [DOI] [PubMed] [Google Scholar]
- 9.Heliövaara M, Knekt P, Aho K, et al. Serum antioxidants and risk of rheumatoid arthritis. Ann Rheum Dis 1994; 53:51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winyard PG, Ryan B, Eggleton P, et al. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem Soc Trans 2011; 39:1226–1232. [DOI] [PubMed] [Google Scholar]
- 11.Szabó-Taylor KÉ, Nagy G, Eggleton P, et al. Oxidative stress in rheumatoid arthritis. Studies on Arthritis and Joint Disorders 2013; New York: Springer, 145–167. [Google Scholar]
- 12.Li C, Peng Y, Mao B, et al. Thioredoxin reductase: a novel, independent prognostic marker in patients with hepatocellular carcinoma. Oncotarget 2015; 6:17792–17804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustacich D, Powis G. Thioredoxin reductase. Biochem J 2000; 346:1–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi Y, Taniguchi-Ueda Y, Mori K, et al. A novel promotor sequence is involved in the oxidative stress-induced expression of the adult T-cell leukemia-derived factor (ADF)/human thioredoxin (TRX) gene. Nucl Acids Res 1996; 24:2746–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurice MM, Nakamura H, van der Voort EAM, et al. Evidence for a role of an altered redox state in hyporesponsiveness of synovial T cells in rheumatoid arthritis. J Immunol 1997; 158:1458–1465. [PubMed] [Google Scholar]
- 16.Lemarechal H, Allanore Y, Chenevier-Gobeaux C, et al. High redox thioredoxin but low thioredoxin reductase activities in the serum of patients with rheumatoid arthritis. Clin Chim Acta 2006; 367:156–161. [DOI] [PubMed] [Google Scholar]
- 17.Jikimoto T, Nishikubo Y, Koshiba M, et al. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol Immunol 2002; 38:765–772. [DOI] [PubMed] [Google Scholar]
- 18.Aletaha D, Neogi T, Silman A, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69:1580–1588. [DOI] [PubMed] [Google Scholar]
- 19.Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38:44–48. [DOI] [PubMed] [Google Scholar]
- 20.Datta S, Kundu S, Ghosh P, et al. Correlation of oxidant status with oxidative tissue damage in patients with rheumatoid arthritis. Clin Rheumatol 2014; 33:1557–1564. [DOI] [PubMed] [Google Scholar]
- 21.Mantle D, Falkous G, Walker D. Quantification of protease activities in synovial fluid from rheumatoid and osteoarthritis cases: comparison with antioxidant and free radical damage markers. Clin Chim Acta 1999; 284:45–58. [DOI] [PubMed] [Google Scholar]
- 22.Bohanec Grabar P, Logar D, Tomsic M, et al. Genetic polymorphisms modifying oxidative stress are associated with disease activity in rheumatoid arthritis patients. Dis Markers 2009; 26:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veselinovic M, Barudzic N, Vuletic M, et al. Oxidative stress in rheumatoid arthritis patients: relationship to diseases activity. Mol Cell Biochem 2014; 391:225–232. [DOI] [PubMed] [Google Scholar]
- 24.Altindag O, Karakoc M, Kocyigit A, et al. Increased DNA damage and oxidative stress in patients with rheumatoid arthritis. Clin Biochem 2007; 40:167–171. [DOI] [PubMed] [Google Scholar]
- 25.Mapp PI, Grootveld MC, Blake DR. Hypoxia, oxidative stress and rheumatoid arthritis. Br Med Bull 1995; 51:419–436. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol 1997; 15:351–369. [DOI] [PubMed] [Google Scholar]
- 27.Wruck CJ, Fragoulis A, Gurzynski A, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis 2011; 70:844–850. [DOI] [PubMed] [Google Scholar]
- 28.Halliwell B. Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis 1995; 54:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah D, Wanchu A, Bhatnagar A. Interaction between oxidative stress and chemokines: possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis. Immunobiology 2011; 216:1010–1017. [DOI] [PubMed] [Google Scholar]
- 30.Leaver SK, MacCallum NS, Pingle V, et al. Increased plasma thioredoxin levels in patients with sepsis: positive association with macrophage migration inhibitory factor. Intensive Care Med 2010; 36:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morand EF, Leech M, Weedon H, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: clinical correlations. Rheumatology 2002; 41:558–562. [DOI] [PubMed] [Google Scholar]
- 32.Radstake TRDJ, Sweep FCGJ, Welsing P, et al. Correlation of rheumatoid arthritis severity with the genetic functional variants and circulating levels of macrophage migration inhibitory factor. Arthritis Rheum 2005; 52:3020–3029. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Ann Rev Immunol 1996; 14:397–440. [DOI] [PubMed] [Google Scholar]
- 34.Tak PP, Zvaifler NJ, Green DR, et al. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today 2000; 21:78–82. [DOI] [PubMed] [Google Scholar]
- 35.Kullmann F, Judex M, Neudecker I, et al. Analysis of the p53 tumor suppressor gene in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 1999; 42:1594–1600. [DOI] [PubMed] [Google Scholar]
- 36.Powis G, Oblong JE, Gasdaska PY, et al. The thioredoxin/thioredoxin reductase redox system and control of cell growth. Oncol Res 1994; 6:539–544. [PubMed] [Google Scholar]
- 37.Kabuyama Y, Kitamura T, Yamaki J, et al. Involvement of thioredoxin reductase 1 in the regulation of redox balance and viability of rheumatoid synovial cells. Biochem Biophys Res Commun 2008; 367:491–496. [DOI] [PubMed] [Google Scholar]


