Supplemental Digital Content is available in the text
Abstract
Few randomized clinical trials have evaluated the efficacy of ginseng in patients with type 2 diabetes mellitus (T2DM). The current meta-analysis evaluated the ginseng-induced improvement in glucose control and insulin sensitivity in patients with type-2 diabetes or impaired glucose tolerance.
Randomized clinical trials comparing ginseng supplementation versus control, in patients with T2DM or impaired glucose tolerance, were hand-searched from Medline, Cochrane, and Google Scholar databases by 2 independent reviewers using the terms “type 2 diabetes/diabetes/diabetic, impaired glucose tolerance, and ginseng/ginsenoside(s).” The primary outcome analyzed was the change in HbA1c, whereas the secondary outcomes included fasting glucose, postprandial glucose, fasting insulin, postprandial insulin, insulin resistance Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), triglycerides, total cholesterol, low density lipoprotein (LDL), and high density lipoprotein (HDL).
Of the 141 studies identified, 8 studies were chosen for the current meta-analysis. The average number of patients, age, and sex distribution among the groups were comparable. Results reveal no significant difference in HbA1c levels between the ginseng supplementation and the control groups (pooled standardized difference in means = −0.148, 95% CI: −0.637 to 0.228, P = 0.355). Ginseng supplementation improved fasting glucose, postprandial insulin, and HOMA-IR levels, though no difference in postprandial glucose or fasting insulin was observed among the groups. Similarly, triglycerides, total cholesterol, and LDL levels showed significant difference between the treatment groups, while no difference in HDL was seen. In addition, ginseng-related therapy was ineffective in decreasing the fasting glucose levels in patients treated with oral hypoglycemic agents or insulin.
The present results establish the benefit of ginseng supplementation in improving glucose control and insulin sensitivity in patients with T2DM or impaired glucose intolerance.
INTRODUCTION
The use of ginseng, a traditional Chinese medicine, is implicated in various indications including diabetes, cancer, heart disease, fatigue, immune boost, erectile dysfunction, high blood pressure, so on.1–7 There are 11 commercially available species of ginseng; however, the Asian (Panax ginseng) and the American (Panax quinquefolius L) ginseng are the 2 widely consumed varieties. The pharmacologically active component of ginseng is the triterpene β-glycosides, known as ginsenosides or panaxosides. More than 150 types of ginsenosides have been identified so far.8 Generally, extracts from the roots of the plant contain potent ginsenosides that can stabilize the glucose homeostasis in patients with type 2 diabetes mellitus (T2DM); however, the berries and leaves of ginseng have also been reported to lower blood glucose and decrease body weight.9,10
The efficacy of ginseng extract or powder on blood glucose control has been well documented in experimental models11,12 and on healthy individuals.13 However, there are very few randomized controlled trials assessing the safety and efficacy of ginseng extract in patients with type 2 diabetes or people with impaired fasting glucose or impaired glucose tolerance.14 Besides, most studies conducted in human subjects only evaluated the effect of a single oral dose, which was found to be significantly effective in lowering the blood glucose area under the curve, during the oral glucose tolerance test.15,16 Daily supplementation with fermented red ginseng lowered postprandial glucose levels in subjects with impaired fasting glucose or type 2 diabetes.17 Nevertheless, the efficacy of ginseng-related therapy varied considerably in the outcomes of long-term use, dose, formulation, and treatment duration. Interestingly, Reeds et al,8 reported the lack of efficacy of oral ginseng or ginsenoside Re therapy with regard to β-cell function or insulin sensitivity in overweight/obese subjects with impaired glucose tolerance or newly diagnosed diabetes.
The current meta-analysis was undertaken to evaluate the effect of ginseng or ginsenoside Re in improving the glucose homeostasis and insulin sensitivity in patients with type-2 diabetes, or with impaired glucose tolerance, who are at high risk for developing cardiovascular diseases. In addition, the use of ginseng extract as an adjunct therapy in T2DM will also be explored. Ginseng-related therapies include the use of ginseng, ginseng-extract, ginsenosides, and Chinese medicine with ginseng as the major ingredient (eg, Shenyan Kangfu tablets18).
METHODS
Search Strategy and Selection Criteria
We performed an updated literature search of the Medline, Cochrane, EMBASE, and Google Scholar databases until March 2, 2015, using the key words, “type 2 diabetes/diabetes/diabetic, impaired glucose tolerance, and ginseng/ginsenoside(s).” In addition, the reference lists of relevant studies were also hand-searched. Randomized controlled trials in patients with a diagnosis of either type 2 diabetes, or impaired glucose tolerance, and being treated with ginseng supplementation versus control or placebo were included in the current meta-analysis. Only those studies with a reported quantitative outcome were included.
We did not include cohort studies, letters, comments, editorials, case reports, proceedings, and personal communications for this review. We also excluded retrospective studies or those without any reported quantitative primary outcome.
Study Selection and Data extraction
Studies identified through the literature search were selected by 2 independent reviewers, and in case of ambiguity regarding eligibility a third reviewer was consulted. The following information/data were extracted from studies that met the inclusion criteria: name of the first author, the year of publication, study design, number of participants in each group, participants’ age and gender, treatment protocol, change in HbA1c, fasting glucose, postprandial glucose, fasting insulin, postprandial insulin, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), TG, total cholesterol, LDL, and HDL.
Quality Assessment
Included studies were assessed with the Cochrane Collaboration's tool for assessing risk.19 The supplemental Figure S2 represents the assessed outcomes for the 8 included studies.
Outcome Measures
The primary outcome measure was the change in HbA1c levels, while the secondary outcomes included, fasting glucose, postprandial glucose, fasting insulin, postprandial insulin, HOMA-IR, TG, total cholesterol, LDL, and HDL.
Statistical Analysis
For all the outcomes analyzed, both pre- and post-treatment measurements were summarized as mean ± standard deviation for each individual study and as comparisons of pooled estimates in the treatment group (comparison group) versus the control group. An effect size of std diff in the means of change in outcomes before and after treatment between the groups was presented as standard error, and 95% CI. An effect size with a std diff in means lower than 0 (<0) indicates that the comparison group has a greater decrease in outcome (ie, from pre- to post-treatment) than the control group; otherwise, the treatment group has a lesser change in the outcome (>0), compared with the control group. An effect size of 0 (=0) indicates that the change in outcomes was similar between the treatment and the control groups.
Heterogeneity among the studies was assessed by calculating Cochran Q and the I2 statistic, simultaneously. Either a Q statistic with P < 0.120 or an I2 statistic >50%21 was considered as obvious heterogeneity between studies. A random-effects model (DerSimonian-Laird method)22 was used when there was obvious heterogeneity among the studies, while a fixed-effects model was used (Mantel-Haenszel method) in case of homogenous studies. Sensitivity analysis was carried out based on the leave-one-out approach in the primary outcome, HbA1c, and in one of the secondary outcome, fasting glucose. Publication bias was assessed using funnel plots and Egger test for both the outcomes, and a P > 0.05 in the Egger test was considered statistically significant. All of the statistical analyses were performed using the Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
Ethics
This study did not involve human subjects, so informed consent was not required. In addition, no approval was required from an institutional review board.
RESULTS
Study Characteristics
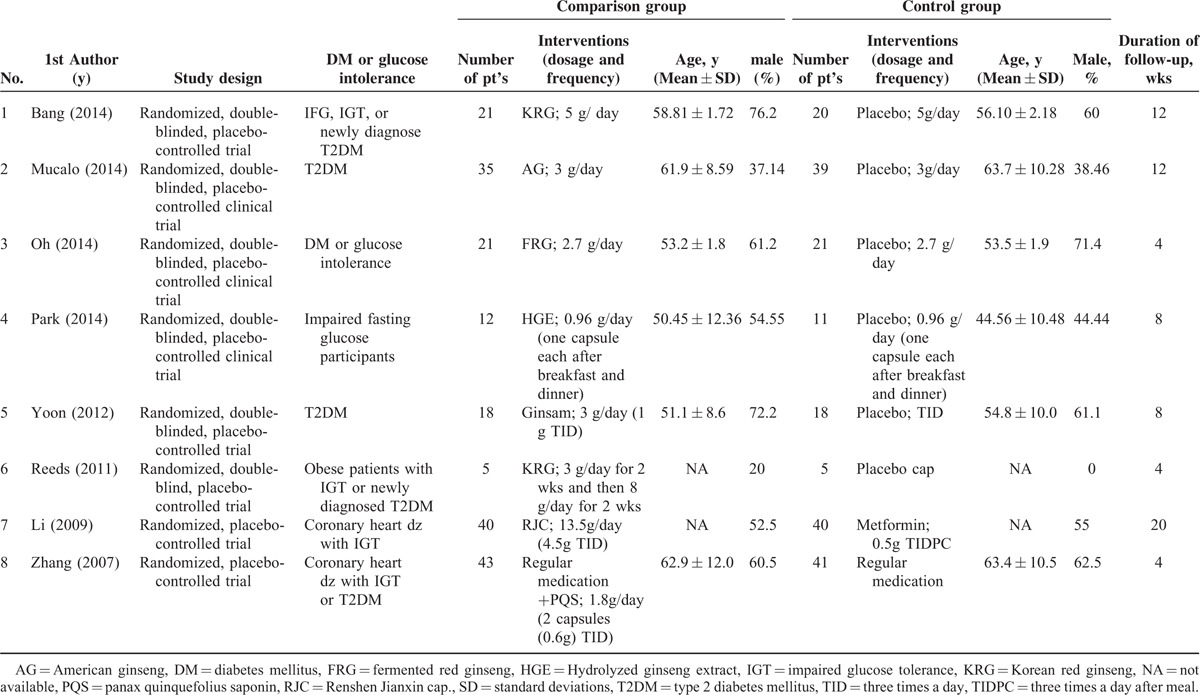
Of the 141 studies identified through the literature search, 8 studies were included in the final analysis.8,10,14,17,23–26 The flow chart for the selection of trials is shown in Figure 1. The study characteristics of the included trials are summarized in Table 1. The participant age was comparable among the treatment groups (comparison and control) within and between the studies (Table 1). The number of participants in the comparison group ranged from 5 to 43 (n = 195), while the number of participants in the control group ranged from 5 to 41 (n = 195) (Table 1). The mean follow-up time ranged from 4 to 20 weeks. Supplemental Table S1, represents the summary of all outcome measures of the studies included in the current meta-analysis.
FIGURE 1.
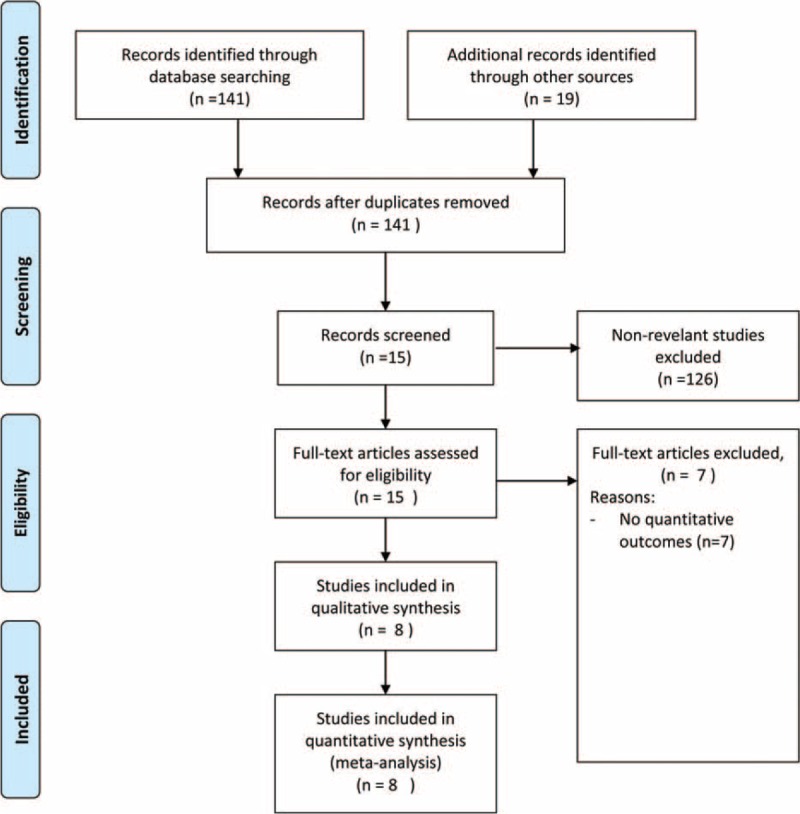
Flow chart for study selection.
TABLE 1.
Summary of basic characteristics of selected RCTs for meta-analysis
Study Outcomes
Primary Outcome
Change in HbA1c (%)
A total of 3 studies8,10,14 with reported changes in HbA1c levels from the baseline were evaluated. The change in the pooled std diff in the means of the HbA1c levels between the comparison and control groups is represented in Figure 2. A fixed model was applied because there was no observed heterogeneity among the studies with regard to HbA1c (Q = 0.505, I2 = 70%, P = 0.777). The pooled std diff in means of HbA1c levels did not show significant difference between the comparison and control groups (std diff in means = −0.148, 95% CI: −0.637 to 0.228, P = 0.355) (Figure 2).
FIGURE 2.
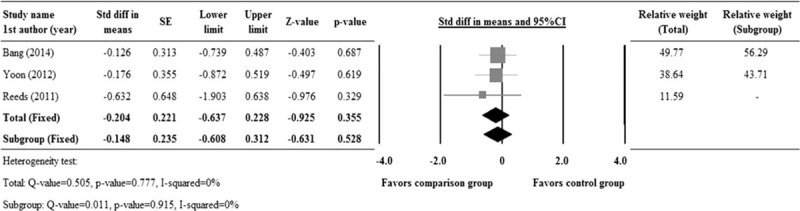
Forest plot showing changes in HbA1c levels, as compared between the comparison and control groups. 1st AU = first author, SE = standard error, Std diff = standardized difference, 95% CI: lower limit and upper limit.
Secondary Outcomes
Fasting Glucose, Postprandial Glucose, Fasting Insulin, Postprandial Insulin, HOMA-IR
There were 6 studies with complete records for fasting glucose, while 3 studies reported complete data for postprandial glucose, and 5 studies for fasting insulin, 2 studies for postprandial insulin, and 4 studies for HOMA-IR. The heterogeneity test indicates no significant heterogeneity among the studies, except for fasting and postprandial insulin, hence a fixed model was considered for fasting glucose, postprandial glucose, and HOMA-IR, whereas, a random model was considered for fasting and postprandial insulin (Fasting glucose, Q = 2.168, P = 0.825, I2 = 0%; Postprandial glucose, Q = 0.644, P = 0.725, I2 = 0%; Fasting insulin, Q = 23.636, P < 0.001, I2 = 83.08%; Postprandial insulin, Q = 8.08, P = 0.004, I2 = 87.62%; HOMA-IR, Q = 3.395, P = 0.335, I2 = 11.63%). The pooled std diff in means of fasting glucose, postprandial insulin, and HOMA-IR levels showed significant difference between the comparison and control groups (Fasting glucose, std diff in means = −0.306, 95% CI: −0.539 to −0.074, P = 0.01; postprandial insulin, std diff in means = −2.132, 95% CI: −3.706 to −0.558, P = 0.008; HOMA-IR, std diff in means = −0.397, 95% CI: −0.679 to −0.115, P = 0.006) (Figure 3A, D, E). However, no significant difference between the comparison and control groups was observed for postprandial glucose and fasting insulin. (Postprandial glucose, std diff in means = −0.338, 95% CI: −0.707 to 0.031, P = 0.072; fasting insulin, std diff in means = −0.615, 95% CI: −1.359 to 0.129, P = 0.105; Figure 3B, C).
FIGURE 3.
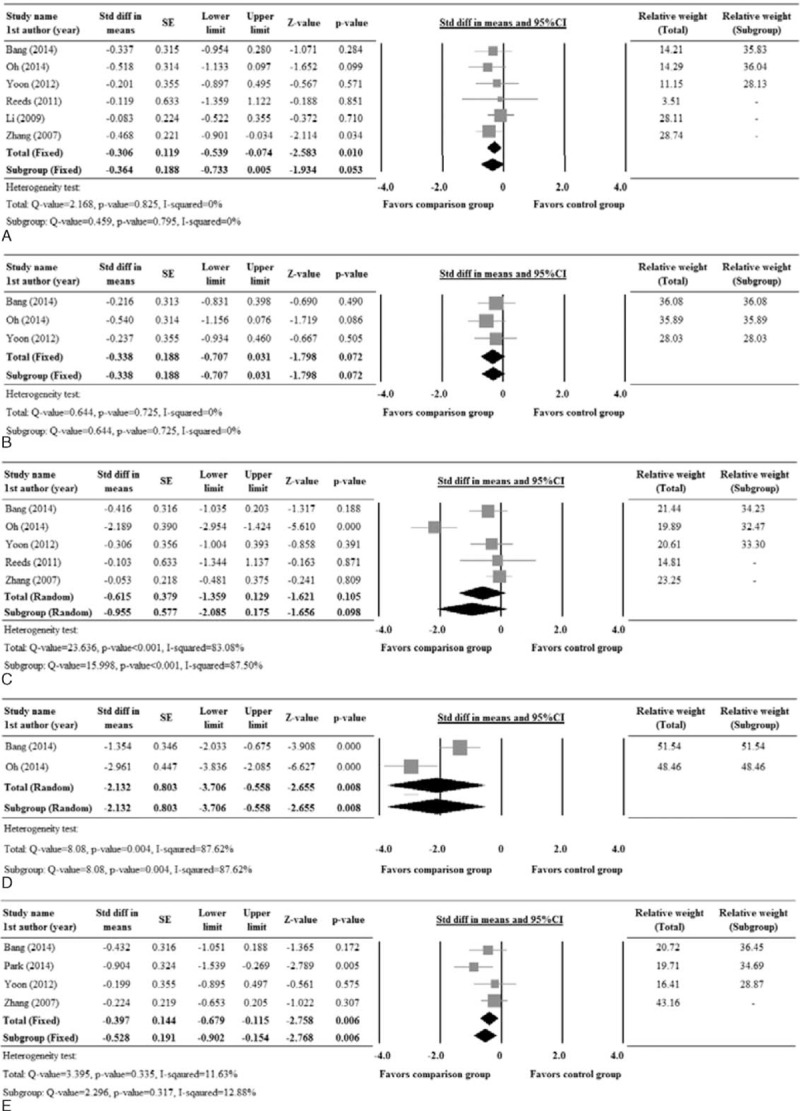
Forest plot showing the change in levels of (A) fasting glucose, (B) postprandial glucose, (C) fasting insulin, (D) postprandial insulin, and (E) HOMA-IR compared between patients who underwent comparison and control group treatments. 1st AU = first author, SE = standard error, Std diff = standardized difference, 5% CI, lower limit and upper limit.
TG, Total Cholesterol, LDL, HDL
Five studies with complete records for TG, total cholesterol, LDL, and HDL were evaluated for these secondary outcome measures. Analysis revealed significant heterogeneity among the studies except for TG. Hence, a random model was considered for TG, while a fixed effects model was considered for total cholesterol, LDL, and HDL (TG, Q = 3.714, P = 0.446, I2 = 0%; Total cholesterol, Q = 13.365, P = 0.010, I2 = 70.07%; LDL, Q = 12.70, P = 0.013, I2 = 68.47%; HDL, Q = 9.20, P = 0.056, I2 = 56.50%). The pooled std diff in means of TG, total cholesterol, and LDL levels showed significant difference between the comparison and control groups. However, no significant difference in HDL was seen between the 2 groups. (TG, std diff in means = −0.669, 95% CI: −0.949 to −0.389, P < 0.001; total cholesterol, std diff in means = −0.969, 95% CI: −1.542 to −0.396, P = 0.001; LDL, std diff in means = −0.730, 95% CI: −1.275 to −0.185, P = 0.009; HDL, std diff in means = −0.261, 95% CI: −0.709 to 0.188, P = 0.255; Figure 4A–D).
FIGURE 4.
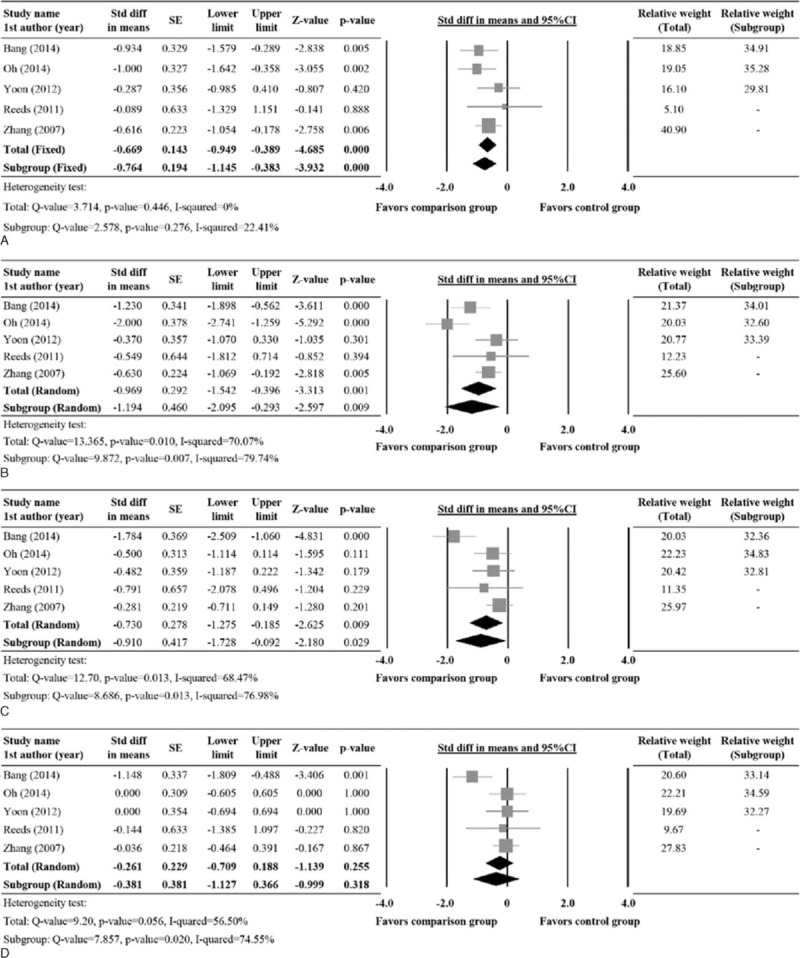
Forest plot showing the change in levels of (A) TG, (B) Total cholesterol, (C) LDL, and (D) HDL compared between patients underwent comparison and control group treatments. 1st AU = first author, diff = difference, SE = standard error, Std = standardized, TG = triglycerides, 95% CI: lower limit and upper limit.
Subgroup Analysis
There were 5 studies10,14,17,23,24 that included patients who did not receive any oral hypoglycemic agent or insulin injections. The subgroup analysis revealed a significant difference between the groups for secondary outcomes, postprandial insulin, HOMA-IR, TG, total cholesterol, and LDL (Postprandial insulin, std diff in means = −2.132, 95% CI: −3.706 to −0.558, P = 0.008; HOMA-IR, std diff in means = −0.528, 95% CI: −0.902 to −0.154, P = 0.006; TG, std diff in means = −0.764, 95% CI: −1.145 to −0.383, P < .001; total cholesterol, std diff in means = −1.194, 95% CI: −2.095 to −0.293, P = 0.009; LDL, std diff in means = −0.910, 95% CI: −1.728 to −0.092, P = 0.029) (Figures 3D, E, 4A–C).
It should be noted that the pooled std diff in the means of HOMA-IR showed significant difference between the comparison and control group, either in the total or subgroup analysis. However, the change in HOMA-IR was significantly different between the groups in only 1 study, Park et al24 (Figure 3E), indicating that this study influenced the overall results and is not reliable as the other outcomes analyzed.
Sensitivity Analysis
The sensitivity analysis for changes in HbA1c and fasting glucose levels were performed using the leave-one-out approach. Changes in the combined std diff in the means of HbA1c and fasting glucose levels between the groups, with each one of the studies removed in turn remained nonsignificant and were similar to that of the pooled estimate from all studies combined (Figure 5), indicating no obvious influence by any individual study on the pooled estimate. The sensitivity analysis suggests that the current results for HbA1c and fasting glucose are robust (Figure 5A, B).
FIGURE 5.
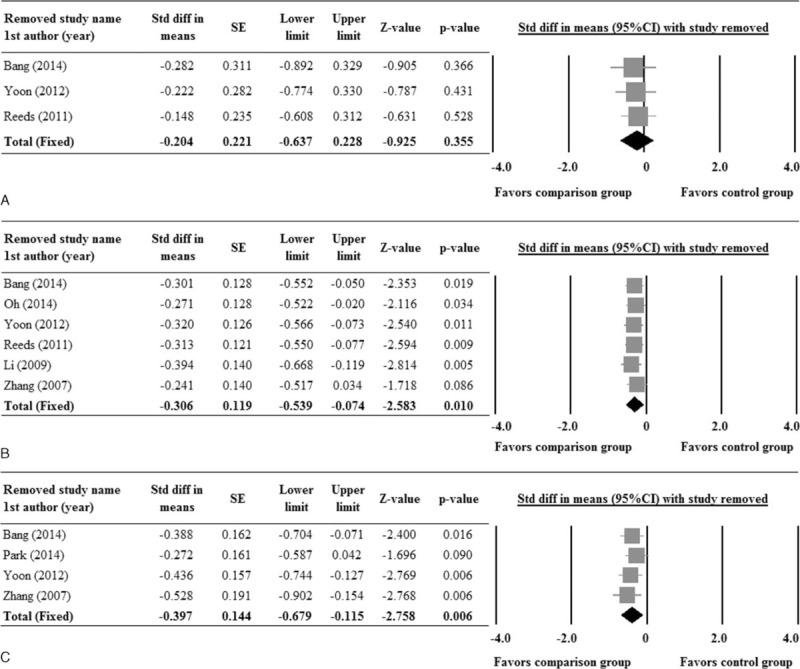
Sensitivity analysis for (A) HbA1c, (B) fasting glucose, and (C) HOMA-IR, using leave-one-out approach. 1st AU = first author, diff = difference, HOMA-IR = insulin resistance, SE = standard error, Std = standardized, 95% CI: lower limit and upper limit.
Publication Bias
Publication bias analysis was performed for fasting glucose using funnel plot (Figure 6) and Egger test. The Egger test indicates no publication bias for fasting glucose among the included studies (1-tailed P = 0.438). The publication bias analysis was not performed for the primary outcome, HbA1c, because more than 5 studies are required to detect funnel plot asymmetry.27
FIGURE 6.
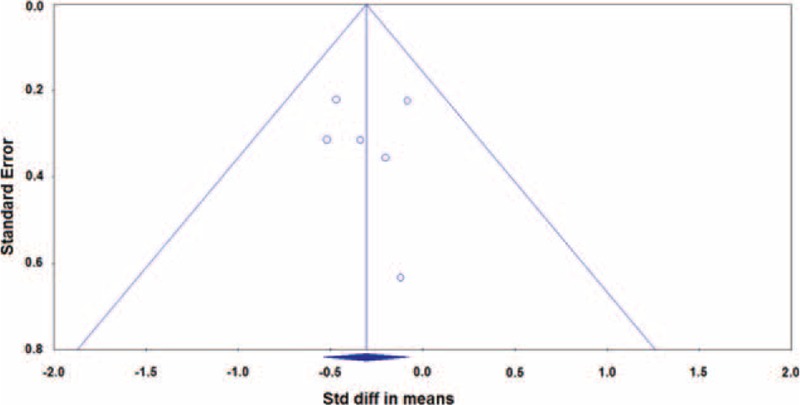
Funnel plots for clinical remission rate in fasting glucose. The 1-tailed P value from Egger test was derived as 0.438. diff = difference, Std = standardized.
Quality Assessment
The result of quality assessment is shown in the supplemental Figure S2. All of the studies included had randomization and had a low risk of selection bias, performance bias, attrition bias, and reporting bias (A), and overall, the included studies had good quality (B).
DISCUSSION
The current meta-analysis demonstrates the efficacy of ginseng-related therapy in postprandial insulin levels, HOMA-IR, TG, total cholesterol, and LDL in patients with T2DM or impaired glucose tolerance, with no effect on HbA1c or HDL levels. While ginseng-related therapy is effective in reducing the fasting glucose in all patients, it was found to be ineffective in patients undergoing insulin treatment or taking oral hypoglycemic agents (Figure 3A). Furthermore, postprandial glucose and fasting insulin levels were not different between the ginseng supplementation and the control group.
Our results reveal no difference in the HbA1c levels between the treatment and the control group (Figure 2). It has been shown elsewhere that, while ginseng supplementation is beneficial in lowering insulin resistance in T2DM patients, no significant change in the biomarkers of antioxidant defense or oxidant stress had been demonstrated.29 Similar to our results, Vuksan et al2 also observed no changes in the primary endpoint, HbA1c. Our current results are also in agreement with the previous reports,28,29 in which significant improvement in fasting blood glucose and HOMA-IR levels were observed with ginseng supplementation in subjects with or without diabetes. In addition, we have also demonstrated ginseng-induced improvement in postprandial insulin and glucose levels, TG, total cholesterol, and LDL levels (Figures 3, 4).
The present meta-analysis is the first updated review to evaluate the efficacy of ginseng-related therapy in patients with type 2 diabetes or glucose intolerance. Furthermore, our results demonstrate for the first time, an improved lipid profile (TG, total cholesterol, and LDL) associated with ginseng-related therapy in patients with T2DM or impaired glucose tolerance. Moreover, ginseng-related therapy was more effective in terms of HbA1c and fasting glucose levels, in drug naïve participants than those taking antidiabetic medications, probably due to the masking of the effect of ginseng by antidiabetic medications. It could also be due to the psychological attributes and better diet control in patients who are newly diagnosed with T2DM. However, the subgroup analysis revealed a significant difference between the groups for secondary outcomes such as postprandial insulin, HOMA-IR, TG, total cholesterol, and LDL (Figures 3, 4). This might be because most studies excluded patients taking glucose-lowering medications or insulin injections, as it will be difficult to accurately determine the glucose-lowering efficacy of ginseng extracts alone.14 Nonetheless, reports elsewhere indicate that supplementation with Korean red ginseng rootlet preparation maintained good glycemic control and improved plasma glucose and insulin levels beyond the usual therapy in people with well-controlled T2DM.2 This discrepancy could be attributed to the differences in formulations, the dose, and duration of treatment, as well as the method of extraction and the part of the plant, variable ages, and species of ginseng contributing to the variability in composition, thus affecting the efficacy of ginseng products.9,10,24 Ginseng extracts used in the studies included varied considerably (Korean red ginseng, American ginseng, fermented red ginseng, hydrolyzed extract, Ginsam, Renshen Jianxin cap., and panax quinquefolius saponin; Table 1), and with difference in species and methods of extraction, the active constituents and efficacy may vary.
Studies included in the present review also varied to a great extent in the dose (0.96–13.5 g/day), and duration of treatment (4–20 weeks). The pooled data for HbA1c was not significantly different between the treatment and the control groups, which could be attributed to the shorter duration of therapy. It is unclear whether a short duration of intervention has any clinical significance. The effect of ginseng on HbA1c might have been significant with longer treatment duration than 3 months, which equals to half life of red blood cells. Of the 8 studies included, only one of them (Yoon et al.) reported improvement in HbA1c,10 where ginsam at different doses showed moderate improvement in HbA1c level along with a significant reduction in fasting glucose levels in drug naïve patients with T2DM.
Although the mechanisms underlying the hypoglycemic effect of ginseng have not been fully elucidated, the possible mechanisms include modulations of, insulin production and secretion; glucose metabolism; glucose uptake; and inflammatory pathway.30 Further, ginsenosides are shown to activate AMP-activated protein kinase (AMPK) pathway.31–33 It has been suggested that ginsenosides may decrease the ATP biosynthesis, resulting in a change in the AMP: ATP ratio, which might further activate the AMPK pathway. Activation of AMPK pathway has been proposed as the mechanism for the suppression of hepatic gluconeogenesis and steatosis.30
Consistent with published reports on the safety of ginseng extract,2,34 none of the included studies reported any serious adverse events. The traditional Chinese medicine recommends a dose of 3 g/day as safe and efficacious.35 Mucalo et al23 assessed the safety of ginseng extracts in terms of kidney, liver or hemostatic function, and suggested that the long-term use of American ginseng extract is totally safe in a high cardiovascular disease risk population of patients with T2DM.
There are several limitations to the present analysis, major limitation being that in patients who were glucose intolerant or T2DM without OHA/insulin, the blood sugar may be fairly regulated with diet control alone. Patients with newly diagnosed T2DM may have better compliance and better diet control too. Thus the efficacy of ginseng-related therapy may be masked. In addition, the small sample size of the included studies may also be a major limiting factor. Besides, the labels and dosage of ginseng-related therapies were varied, as discussed earlier, leading to further bias in the analysis. Future studies comprising a larger cohort of patients with uncontrolled blood sugar are needed to validate the current results on HbA1c. Although, ginseng-related therapy can increase insulin sensitivity in our study, more clinical trials should be designed to evaluate its efficacy as an adjunct therapy in diabetes mellitus and whether it can effectively reduce the dosage of antidiabetic medications, which are often associated with significant risk, including hypoglycemia, lactic acidosis and weight gain.36
In summary, the current results suggest that the ginseng-related therapy exert better glycemic control, with an excellent safety profile. Furthermore, it might be a better option in drug-naïve diabetic patients, rather than as an adjunct therapy in patients on anti-diabetic medications. The present analysis did not show an improvement in the pooled HbA1c levels, which might be attributed to variations in the species and dosage of ginseng-related therapy, along with the shorter treatment duration. We propose a standardized treatment regimen with duration greater than 3 months, before any strong conclusions can be drawn, on the safety and efficacy of ginseng extracts as a dietary supplement in patients with diabetes mellitus or glucose intolerance.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: HOMA-IR = insulin resistance, OGTT = oral glucose tolerance test, SD = standard deviation, SE = standard error, T2DM = type 2 diabetes mellitus, TG = triglycerides.
This study was supported by the National Clinical Key Specialty Construction Project of Geriatrics Department and the Key Disciplines Construction Plan of Zhejiang Province Traditional Chinese Medicine (2012-XK-A20).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Vogler BK, Pittler MH, Ernst E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol 1999; 55:567–575. [DOI] [PubMed] [Google Scholar]
- 2.Vuksan V, Sung MK, Sievenpiper JL, et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis 2008; 18:46–56. [DOI] [PubMed] [Google Scholar]
- 3.Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 2009; 7:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stavro PM, Woo M, Heim TF, et al. North American ginseng exerts a neutral effect on blood pressure in individuals with hypertension. Hypertension 2005; 46:406–411. [DOI] [PubMed] [Google Scholar]
- 5.Stavro PM, Woo M, Leiter LA, et al. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension 2006; 47:791–796. [DOI] [PubMed] [Google Scholar]
- 6.Jang DJ, Lee MS, Shin BC, et al. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol 2008; 66:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev 2004; 9:259–274. [PubMed] [Google Scholar]
- 8.Reeds DN, Patterson BW, Okunade A, et al. Ginseng and ginsenoside Re do not improve beta-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care 2011; 34:1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets 2008; 8:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon JW, Kang SM, Vassy JL, et al. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: results of a double-blind, placebo-controlled study. J Diabetes Investig 2012; 3:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attele AS, Zhou YP, Xie JT, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes 2002; 51:1851–1858. [DOI] [PubMed] [Google Scholar]
- 12.Waki I, Kyo H, Yasuda M, Kimura M. Effects of a hypoglycemic component of ginseng radix on insulin biosynthesis in normal and diabetic animals. J Pharmacobiodyn 1982; 5:547–554. [DOI] [PubMed] [Google Scholar]
- 13.De Souza LR, Jenkins AL, Sievenpiper JL, et al. Korean red ginseng (Panax ginseng C.A. Meyer) root fractions: differential effects on postprandial glycemia in healthy individuals. J Ethnopharmacol 2011; 137:245–250. [DOI] [PubMed] [Google Scholar]
- 14.Bang H, Kwak JH, Ahn HY, et al. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food 2014; 17:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuksan V, Sievenpiper JL, Koo VY, et al. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med 2000; 160:1009–1013. [DOI] [PubMed] [Google Scholar]
- 16.Sievenpiper JL, Sung MK, Di Buono M, et al. Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. J Am Coll Nutr 2006; 25:100–107. [DOI] [PubMed] [Google Scholar]
- 17.Oh MR, Park SH, Kim SY, et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. BMC Complement Altern Med 2014; 14:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Mu W, Zhai J, et al. The key role of Shenyan Kangfu tablets, a Chinese patent medicine for diabetic nephropathy: study protocol for a randomized, double-blind and placebo-controlled clinical trial. Trials 2013; 14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JGS. Cochrane handbook for systematic reviews of interventions Version 5.1. 0 [updated March 2011]. . Chichester, West Sussex, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 20.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997; 127:820–826. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 23.Mucalo I, Jovanovski E, Vuksan V, et al. American ginseng extract (Panax quinquefolius l.) is safe in long-term use in type 2 diabetic patients. Evid Based Complement Alternat Med 2014; 2014: 969168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SH, Oh MR, Choi EK, et al. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J Ginseng Res 2014; 38:239243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li AM, Zhao J. Effect of renshen jianxin capsule for alleviating insulin resistance in patients with coronary heart disease and glucose tolerance impairment. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009; 29:830–833. [PubMed] [Google Scholar]
- 26.Zhang Y LS, Liu YY. Effect of panax quinquefolius saponin on insulin sensitivity in patients of coronary heart disease with blood glucose abnormality. Zhongguo Zhong Xi Yi Jie He Za Zhi 2007; 27:1066–1069. [PubMed] [Google Scholar]
- 27.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shishtar E, Sievenpiper JL, Djedovic V, et al. The effect of ginseng (the genus panax) on glycemic control: a systematic review and meta-analysis of randomized controlled clinical trials. PLoS One 2014; 9:e107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma SW, Benzie IF, Chu TT, et al. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes Obes Metab 2008; 10:1125–1127. [DOI] [PubMed] [Google Scholar]
- 30.Yuan HD, Kim JT, Kim SH, et al. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res 2012; 36:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan HY, Yuan HD, Jung MS, et al. Ginsenoside Re lowers blood glucose and lipid levels via activation of AMP-activated protein kinase in HepG2 cells and high-fat diet fed mice. Int J Mol Med 2012; 29:73–80. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Yuan HD, Chung SH. Ginsenoside Rg1 suppresses hepatic glucose production via AMP-activated protein kinase in HepG2 cells. Biol Pharm Bull 2010; 33:325–328. [DOI] [PubMed] [Google Scholar]
- 33.Yuan HD, Kim do Y, Quan HY, et al. Ginsenoside Rg2 induces orphan nuclear receptor SHP gene expression and inactivates GSK3beta via AMP-activated protein kinase to inhibit hepatic glucose production in HepG2 cells. Chem Biol Interact 2012; 195:35–42. [DOI] [PubMed] [Google Scholar]
- 34.Choi J, Kim TH, Choi TY, et al. Ginseng for health care: a systematic review of randomized controlled trials in Korean literature. PLoS One 2013; 8:e59978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dharmananda S. The nature of ginseng. Traditional use, modern research, and the question of dosage. HerbalGram 2002; 54:34–51. [Google Scholar]
- 36.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002; 287:360–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


