Abstract
Diffusion tensor imaging (DTI) is an increasingly used noninvasive imaging tool. However its long-term clinical utility is unclear. Parkinson's disease (PD) is a common neurodegenerative disease.
We prospectively examined a cohort of 46 Parkinson's disease (PD) patients who underwent diffusion tensor imaging (DTI) of the brain at baseline and 6 years later on a 1.5 Tesla scanner using a standardized protocol. DTI parameters of mean diffusivity (MD) and fractional anisotrophy (FA) were extracted using regions-of-interest (ROIs) analysis from various brain regions.
Compared to the baseline scan, MD increased in all brain regions (P < 0.0001). FA increased in the substantia nigra and posterior putamen, but decreased in the frontal white matter (P < 0.0001). Linear regression analysis demonstrated that the MD in the anterior putamen increased 11.6 units (95% CI = [4.71, 18.43]) (P = 0.0003) for every unit increase of United PD Rating Scale (UPDRS).
Our 6-year prospective longitudinal study demonstrated increased diffusivity in all brain regions and that in the anterior putamen correlated with disease progression. Serial diffusion data may be useful as an additional objective in vivo biomarker for motor progression in PD.
INTRODUCTION
Diffusion tensor imaging (DTI) is a noninvasive magnetic resonance imaging (MRI)-based technique that is increasingly used to characterize microstructural brain changes in Parkinson's disease (PD).1–5 Mean diffusivity (MD) and fractional anisotropy (FA) are common DTI measures extracted from the diffusion tensor which measure the magnitude of water diffusion and directional dependence of diffusivity respectively.1–6 Many DTI studies on PD are based on a cross-sectional design at 1 time point.1–5 Although longitudinal nuclear imaging studies have been conducted in PD,7,8 radiation risk, cost, and infrastructural support limit their clinical utility. Systematic longitudinal DTI studies in PD over a considerable time interval are lacking. We hypothesize that change in DTI parameters in nigrostriatal structures may be useful correlates with motor progression.
To address current gaps in the literature, we conducted a prospective DTI MRI brain study of a cohort of 46 PD patients over a 6-year period to determine if the change in DTI MD and FA for specific brain regions correlates with disease progression.
MATERIALS AND METHODS
Patients and Brain MRI scans
The study received approval from the institutional ethics committee (year of approval 2011, grant number 2002/009/d/C) and all patients gave written informed consent. The patients were diagnosed with mild to moderate PD at recruitment by movement disorders neurologists based on the United Kingdom Brain Bank criteria and were part of an earlier DTI study.2 They were evaluated using the United Parkinson Disease Rating Scale (UPDRS) motor scores over a 6-year period. They underwent brain imaging twice (at baseline and 6 years later) on a 1.5T MR scanner (Siemens Avanto, Erlangen, Germany), using a standardized imaging protocol.2 Briefly, the diffusion data was obtained using an echo planar imaging sequence with 12 directions, b-values of 0 and 800 s/mm2, TE/TR = 90/4300 ms, 1.2 × 1.2 × 4 mm3 voxel size and 4 averages. For both MRI, patients were scanned during their “on” stage to reduce motion, with the scan tilt parallel to the anterior-posterior commissural (AC-PC) lines. The MR scans were reviewed to exclude pathology in the regions of interest.
Image Analysis
FA and MD values were obtained using the DTI Taskcard on the Leonardo workstation (Siemens, Erlangen, Germany). Two raters, blinded to the clinical severity and disease progression, independently placed regions of interest (ROIs) using standardized techniques and atlas-based reference maps on the brain structures (Figure 1). ROIs (40.2 mm3) were drawn in the substantia nigra on the slice below which the red nucleus was most prominently as seen on the b0 image. ROIs were also placed in the caudate (44.2 mm3), anterior putamen (106 mm3), posterior putamen (106 mm3), and ventrolateral thalamus (106 mm3) on the slice immediately above the AC-PC line. For the frontal white matter, ROIs (350 mm3) were placed on the slice where the lateral ventricles are no longer seen.
FIGURE 1.
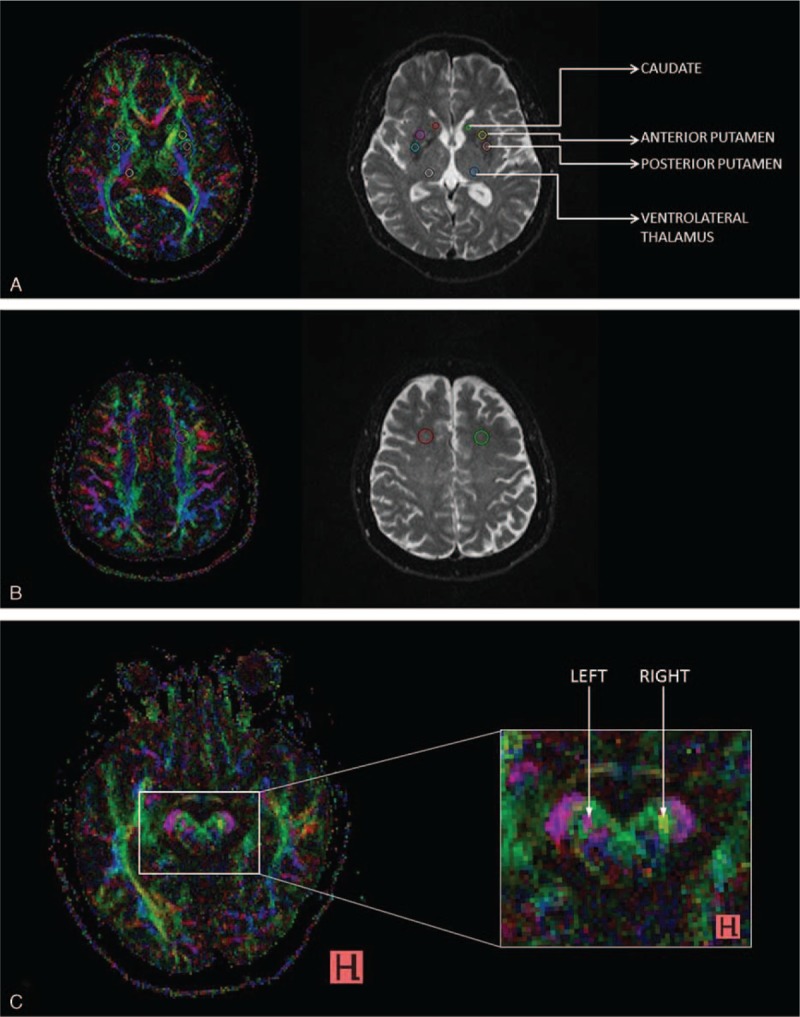
AB colour FA maps (left) and corresponding b0 images (right), depicting placement of regions of interest (ROIs) in the (A) caudate, anterior, and posterior putamen, ventrolateral thalamus, (B) frontal white matter and C, (C) substantia nigra.
All statistical analyses were done using R 3.0.2 (www.r-project.org) with a 2-sided significance level of 0.05. Intraclass correlation coefficient values comparing the DTI parameters obtained by the 2 raters were calculated. The averaged value for each ROI was used for subsequent analysis. The Wilcoxon signed-rank sum test was performed to evaluate the change in MD and FA. Linear regression analysis was carried out to evaluate the relationship of the change in MD and FA and change in the UPDRS motor score between baseline and year 6 scan with adjustments for age and gender.
RESULTS
The mean age of the PD patients was 70.1 years ± 9.3 and 47.8% were men. The median duration of PD at presentation was 3 years, inter quarter range (1.25, 5). The median interval between scans was 6.3 years. The inter-rater agreement was excellent with intraclass correlation coefficients >0.8 for all ROI DTI parameters.
Changes in MD and FA in all brain regions from the baseline to the year 6 scan were shown in Table 1. Compared to the baseline scan, MD increased in all brain regions (P < 0.0001) 6 years later. FA decreased in the frontal white matter, but increased in the substantia nigra and posterior putamen (P < 0.0001) and thalamus (P < 0.005). Linear regression analysis (after adjustments for age, gender, and duration of disease) demonstrated that the MD increased 11.6 units (95% CI = [4.71, 18.43]) (P = 0.0003) for every unit increase of UPDRS motor score (Tables 2 and 3).
TABLE 1.
Difference in MD and FA Between Baseline and 6th Year
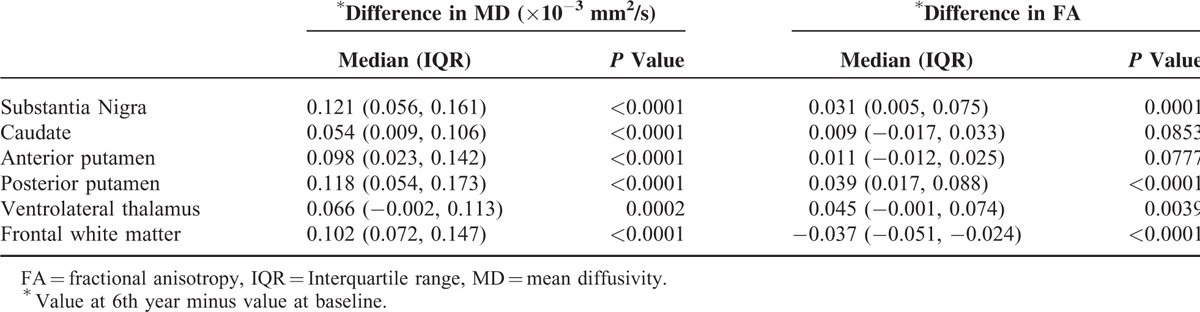
TABLE 2.
Regression Analysis of Change in MD With Change in UPDRS Motor Score ∗Adjusted by Age, Gender, and Duration of Disease
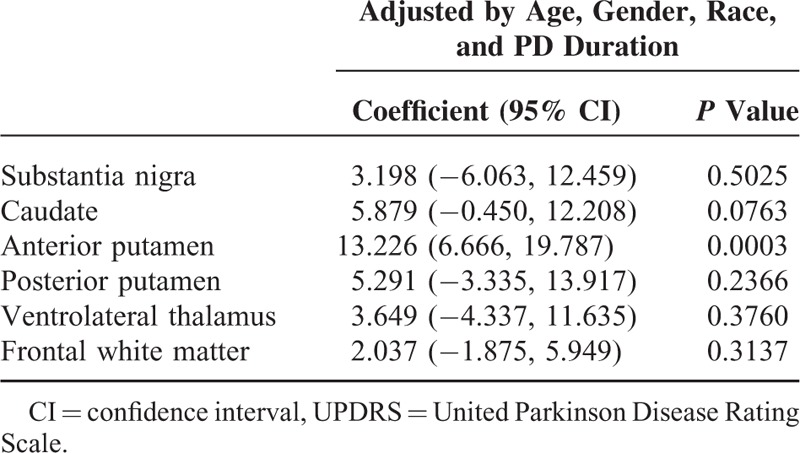
TABLE 3.
Regression Analysis of Change in FA Change With Change in UPDRS Motor Score ∗Adjusted by Age, Gender, and Duration of Disease

DISCUSSION
Our results showed an increase in MD over 6 years in all the brain regions that were examined. Increase in diffusivity largely reflects tissue loss and extracellular fluid accumulation. In addition, we found that an increase in anterior putaminal MD correlated with progression of motor scores (ROI analysis blinded from UPDRS motor evaluation) over the 6-year period. Péran et al4 also found increase in diffusivity in the striatal subregions in PD. Two previous longitudinal 6-(18 fluoro)-L-dopa positron emission tomographic studies7,8 showed progressive and nonlinear dopaminergic hypofunction in the putamen in PD patients over a 5-year follow-up. However, no correlation between the studied parameters with motor scales was found in these studies.
FA reflects microstructural integrity, with reductions implying diminished tissue integrity.1–6 Cross-sectional studies have reported lower FA values in the substantia nigra (SN) in PD compared to controls,1–5 some correlating with motor scales. Ironically, we found a temporal increase in FA >6 years in the SN in our cohort. FA also increased in other subcortical nuclei: the caudate, putamen, and thalamus. Ferritin-bound iron has been found to affect DTI parameters,6,9 with FA increasing as iron concentrations increase, whereas MD was insensitive to these changes.9 Iron deposition in SN and basal ganglia of PD patients10 may explain the FA increase in part.9 Differential patterns of mineralization of the deep gray nuclei add to the complexity and may account for the subnuclear regional FA differences.10 The lack of association in the posterior putamen compared to the anterior putamen in our study could be explained by the potential confounder from more iron deposition in the posterolateral putamen compared to anteromedial putamen. A study in 14 PD patients showed increased iron in caudal putamen but not in anterior putamen over a 3-year period.11
Besides iron, selective elimination of myelinated fibers traversing gray nuclei, targeted loss of specific dendritic connections, gliosis and tissue compaction are other postulated mechanisms explaining increases in FA.10 Clearly, FA changes are complex and multifactorial, susceptible to iron effects and other complex striatal microstructural reorganization induced by dopaminergic denervation. In contrast, the frontal white matter, which is remote from the nigrostriatal circuitry and contains little iron, showed a distinctly different pattern of FA decrease and MD increase over time. In cross-section studies,8 Zhan et al demonstrated decreased FA in frontal white matter in PD compared with health controls. This suggests that white matter integrity is disturbed in PD. The decreased FA decrease in the frontal white matter in PD patients in our longitudinal study corroborates this hypothesis.
Our study has some potential limitations. We did not have age matched control data. However, as the main objective of the study was to identify DTI parameters that can correlate with clinical disease progression in PD, the patients were used as their own controls. Furthermore, for long-term longitudinal studies, it will be difficult to find 1 for 1 matching since no individual will be alike for the potential confounding variables over time. As this is an observation study, 1 cannot prove a cause effect association. Our regression analysis showed a correlation between anterior putamen diffusivity and progression of motor scores over a 6-year period. This suggests that mean diffusivity in anterior putamen may be a noninvasive imaging marker for disease progression. Nuclear imaging (18F fluoro-L-dopa) studies over 5 years have shown that the disease process in PD first affects posterior putamen, followed by the anterior putamen and the caudate nucleus.8
In conclusion, our longitudinal 6-year study involving a large cohort of PD patients demonstrated that increase in anterior putaminal diffusivity correlated with disease progression. Our findings also suggest that serial diffusivity changes are more consistent than anisotropy changes and may be useful as an additional objective in vivo biomarker for evaluating disease progression in PD. Further longitudinal multimodal MRI studies to evaluate the utility of both diffusion and brain-iron changes in PD in monitoring disease progression and response to therapy will be useful.
Acknowledgment
We would like to thank Siemens Medical Solutions, Singapore for their technical assistance in this study.
Footnotes
Abbreviations: AC-PC lines = anterior–posterior commissural, DTI = diffusion tensor imaging, FA = fractional anisotrophy, MD = mean diffusivity, MRI = magnetic resonance imaging, PD = Parkinson's disease, ROIs = regions-of-interest, SN = substantia nigra, UPDRS = United Parkinson Disease Rating Scale.
Author contributions: L-LC, E-KT, and HR designed the study. K-MN, C-SY, and L-LC did the image analysis. H-HL performed the statistical analysis. L-LC and E-KT wrote the manuscript. All the authors were involved in the interpretation of the data and revision of the manuscript. L-LC supervised the study.
Financial Disclosures: E-KT has received honoraria from Elsevier and Wiley publishers. The corresponding author declares that the authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research; that the authors have full access to all of the data; and that the authors have the right to publish any and all data, separate and apart from the guidance of any sponsor.
Funding: National Medical Research Council (translational clinical research programme in Parkinson's disease), Singapore; Singhealth Foundation. EK-T is supported by the STaR award. The funders have no role in the preparation of the manuscript.
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology 2013; 80:857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan LL, Rumpel H, Yap K, et al. Case control study of diffusion tensor imaging in Parkinson's disease. J Neurol Neurosurg Psychiatry 2007; 78:1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan W, Kang GA, Glass GA, et al. Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Mov Disord 2012; 27:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Péran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain 2010; 133:3423–3433. [DOI] [PubMed] [Google Scholar]
- 5.Vaillancourt DE, Prodoehl J, Abraham I, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 2009; 72:378–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfefferbaum A, Adalsteinsson E, Rohlfing T, et al. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol Aging 2010; 31:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brück A, Aalto S, Rauhala E, et al. A follow-up study on 6-[18F]fluoro-L-dopa uptake in early Parkinson's disease shows nonlinear progression in the putamen. Mov Disord 2009; 24:1009–1015. [DOI] [PubMed] [Google Scholar]
- 8.Nurmi E, Ruottinen HM, Bergman J, et al. Rate of progression in Parkinson's disease: a 6-[18F]fluoro-L-dopa PET study. Mov Disord 2001; 16:608–615. [DOI] [PubMed] [Google Scholar]
- 9.Rulseh AM, Keller J, Tintěra J, et al. Chasing shadows: what determines DTI metrics in gray matter regions? An in vitro and in vivo study. J Magn Reson Imaging 2013; 38:1103–1110. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Butros SR, Shuai X, et al. Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility weighted imaging. AJNR Am J Neuroradiol 2012; 33:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulla M, Bonny JM, Ouchchane L, et al. Is R2∗ a new MRI biomarker for the progression of Parkinson's disease? A longitudinal follow-up. Plos One 2013; 8:e57904. [DOI] [PMC free article] [PubMed] [Google Scholar]


