Abstract
We investigated the change of the corticospinal tract (CST) in the unaffected hemisphere by the change of the dominant hand in stroke patients, using diffusion tensor tractography (DTT).
Forty-eight stroke patients with right-hand dominance were recruited. The patients were assigned to 3 groups: group A (12 patients)—right-hand dominance was maintained after the right-hand weakness, group B (17 patients)—right-hand dominance changed to the left-hand dominance after the right-hand weakness, and group C (19 patients)—right-hand dominance was maintained after the left-hand weakness had developed. The function of the unaffected upper extremity was evaluated using the grip strength (GS), Manual Function Test (MFT), Purdue Pegboard Test (PPT), and modified Barthel Index (MBI). DTT was performed twice (1st DTT, 2nd DTT), and the fractional anisotropy (FA), apparent diffusion coefficient (ADC), and voxel number (VN) of the CST in the unaffected hemisphere were measured.
In group B, the VN on 2nd DTT was significantly increased compared with the 1st DTT, and all other clinical data (GS, MFT, PPT, and MBI) showed a significant increase between 1st and 2nd DTT (P < 0.05). The change of the VN showed moderate correlation with the change of the GS (r = 0.499, P < 0.05), PPT (r = 0.531, P < 0.05), and MBI (r = 0.551, P < 0.05).
We found that the fiber number of the CST in the unaffected hemisphere was increased by the change of the dominant hand in stroke patients. We believe that our results have important implications in terms of neurorehabilitation.
INTRODUCTION
Motor weakness is a major sequela in stroke patients; ∼80% of stroke patients experience motor weakness and, among these, only 20% of patients show complete recovery.1 In particular, the upper extremity, which is important to activities for daily living, remains in a nonfunctional state at 6 months after stroke onset in 30% to 60% of stroke patients.2,3 On the other hand, ∼80% of humans have right-hand dominance and 45% to 50% of strokes are known to occur in the left hemisphere.4–6 As a result, a significant portion of stroke patients are obliged to change their dominant hand after stroke. Change of the dominant hand in the adult brain might be accompanied by change of neural tracts in the brain. Therefore, elucidation of change of neural tracts following change of the dominant hand might be useful in neurorehabilitation and neuroscience.
The corticospinal tract (CST), a major neural tract for motor function in the human brain, is mainly concerned with execution of movement of the hand, particularly fine motor activities.7,8 Therefore, the change of the CST in the unaffected hemisphere might be prominent when the dominant hand is changed following stroke. The development of diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has enabled 3-dimensional reconstruction and estimation of the CST.9 Many studies using DTT have reported on the CST in stroke patients.10–16 However, the majority of these studies have focused on the CST in the affected hemisphere and only a few studies have reported on change of the CST in the unaffected hemisphere.10–16 No study on CST change in the unaffected hemisphere by change of the dominant hand in stroke patients has been reported so far.
In this study, we investigated the change of the CST in the unaffected hemisphere by the change of the dominant hand in stroke patients, using DTT.
SUBJECTS AND METHODS
Subjects
Forty-eight consecutive stroke outpatients (31 men, 17 women; mean, 52.94 ± 10.59 years, 25 patients: intracranial hemorrhage and 23 patients: cerebral infarct) were recruited according to the following criteria: (1) first-ever stroke; (2) age: 20 to 79 years; (3) show hemiparesis after stroke onset; (4) show right-hand dominance determined using the Edinburg Handedness inventory before stroke onset17; (5) no history of peripheral nerve injury or musculoskeletal disease (e.g., arthritis, musculotendinous injury, or bone fracture) in the unaffected upper extremity. Patients with severe cognitive problem (Mini-Mental State Examination score of <24) were excluded. The patients were assigned to 3 groups: group A—right-hand dominance was maintained due to mild weakness after development of dominant (right) hand weakness at stroke onset, group B—right-hand dominance changed to left-hand dominance due to severe weakness after development of dominant (right) hand weakness at stroke onset, and group C—right-hand dominance was maintained after development of nondominant (left) hand weakness at stroke onset; group A—12 patients (6 men, mean age; 51.50 ± 11.82 years, 5 patients: intracranial hemorrhage and 7 patients: cerebral infarct); group B—17 patients (12 men, mean age; 53.47 ± 9.28 years, 10 patients: intracranial hemorrhage and 7 patients: cerebral infarct); and group C—19 patients (13 men, mean age; 53.53 ± 11.59 years, 10 patients: intracranial hemorrhage and 9 patients: cerebral infarct). The patient provided signed, informed consent, and the study protocol was approved by Yeungnam University hospital Institutional Review Board.
Clinical Evaluation
The grip strength (GS), Manual Function Test (MFT), Purdue Pegboard Test (PPT), and modified Barthel Index (MBI) were used for evaluation of function of the unaffected side. The GS was evaluated using a dynamometer (Jamar Hydraulic Hand Dynamometer, model-5030J1). Maximum grip strength in shoulder adduction and elbow flexion position was evaluated. The score was the average of measured values (unit: kg) by 3 trials.18 The MFT is composed of 32 test (total score 32) items for evaluation of arm motions and manipulative activities. Arm motion activity consisted of elevation (forward and lateral) and touching (the occiput and dorsum with the palm). Manipulative activity consisted of grasping, pinching, carrying a cube, and pegboard. Each item was scored by either 0 (failure) or 1 (success).19 The PPT consists of 5 separate tests: right hand; left hand; both hands; right plus left plus both hands; and assembly. We evaluated only the unaffected hand function that estimated the number of pins performed by the patient within 30 seconds.20 We used the average peg counts by 3 trials. The MBI consisted of 10 items (range of score is 0–100). Higher score indicates more independence in ADL.21 The reliability and validity of the GS, MFT, PPT, and MBI are well-established.18,20–24 All evaluations were performed 2 times on the day of DTI scan, respectively (first evaluation: 1st DTT and follow-up evaluation: 2nd DTT).
Diffusion Tensor Tractography
A 1.5-T Philips Gyroscan Intera system equipped with a synergy-L Sensitivity Encoding head coil was used for DTI scanning twice (1st DTT: mean 23 ± 15.40 days after onset, 2nd DTT: mean 472 ± 449.17 days after onset). Sixty-seven slices were acquired parallel to the anterior commissure-posterior commissure line for each of the 32 noncollinear and noncoplanar diffusion-sensitizing gradients. DTI scanning parameters were as follows: matrix: 128 × 128 matrix, repetition time: 10,726 ms, echo time: 76 ms, parallel imaging reduction factor: 2, echo-planar imaging factor: 67, number of excitations: 1, and slice thickness: 2.3 mm.
Affine multiscale registration was used for removal of eddy current-induced image distortions and motion artifacts.25 The Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl) was used for preprocessing of DTI datasets. The CST was reconstructed using DTI-Studio software (CMRM, Johns Hopkins Medical Institute). For 3-dimensional reconstruction of the CST, 2 regions of interest (ROIs) were placed as follows: the first ROI—the anterior blue portion (the CST area on the color map) of the upper pons, and the second ROI—the anterior blue portion (the CST area on the color map) of the lower pons.26 Fiber tracking was performed using a fractional anisotropy (FA) threshold of >0.2 and direction threshold <60°.27 The FA, apparent diffusion coefficient (ADC), and voxel number (VN) of the CST in the unaffected hemisphere were measured.
Data Analysis
SPSS software (v.17.0; SPSS, Chicago, IL) was used for data analysis. The Kruskal–Wallis test was used due to the small number of patients. Demographic data of patients and DTT parameters in terms of the FA, ADC, and VN at baseline (1st DTT) were compared between 3 groups to determine homogeneity of the group assignment of patients. The chi-square test was used for sex and stroke type. The Wilcoxon sighed-rank test was used to determine change in the CST between 1st DTT and 2nd DTT in each group and the change in clinical data (GS, MFT, PPT, and MBI) in group B. Spearman correlation analysis was used to determine correlation between the VN and clinical data in group B. The significant level of the P value was set at 0.05.
RESULTS
No significant differences in the demographic data and 1st DTT parameters (FA, ADC, and VN) were observed between 3 groups (P > 0.05) (Table 1). In group B, the VN on 2nd DTT was significantly increased compared with that of 1st DTT (P < 0.05). However, all other DTT parameters in 3 groups showed no significant difference between 1st and 2nd DTT (P > 0.05) (Table 2) (Figure 1).
TABLE 1.
Demographic and First Diffusion Tensor Tractography Data of the Patients
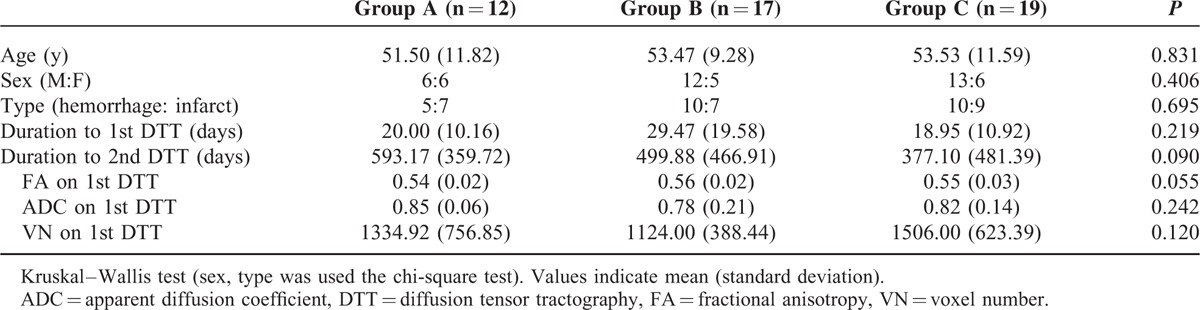
TABLE 2.
First and Second Diffusion Tensor Tractography Data in the 3 Groups
FIGURE 1.
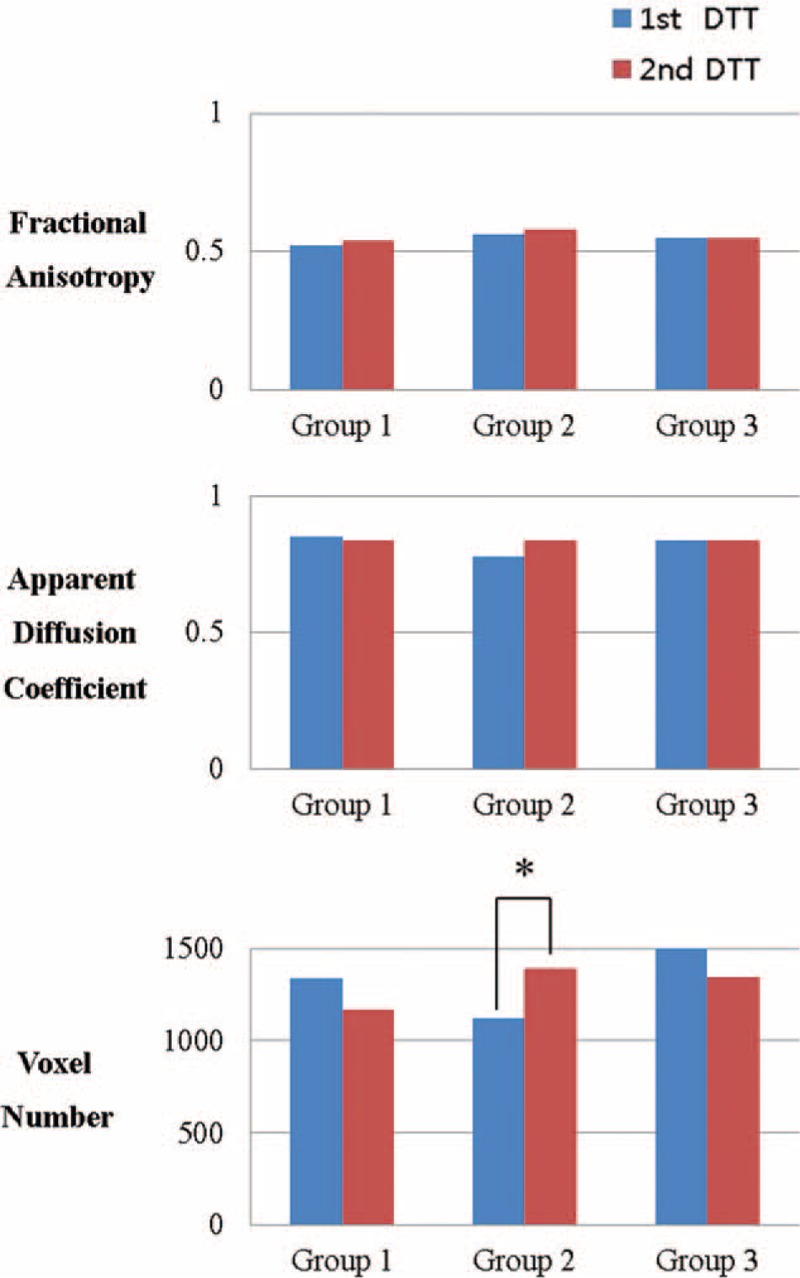
Comparison of the fractional anisotropy, apparent diffusion coefficient, and voxel number of the corticospinal tract in the unaffected hemisphere in each group. The voxel number increased significantly in group B.
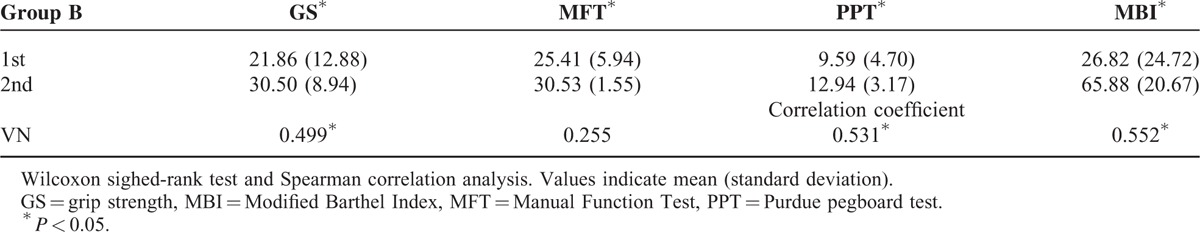
In group B, all clinical data in terms of the GS, MFT, PPT, and MBI were significantly increased between 1st and 2nd DTT (P < 0.05). The change of the VN showed moderate correlation with the change of the VN: GS (r = 0.499, P < 0.05), PPT (r = 0.531, P < 0.05), and MBI (r = 0.551, P < 0.05).28 However, no significant correlation was observed with the change of the MFT (P > 0.05) (Table 3).
TABLE 3.
Comparison of Clinical Data Between First and Second Diffusion Tensor Tractography in Group B
DISCUSSION
In this study, change of the CST in the unaffected hemisphere by the change of the dominant hand following stroke was investigated using DTT. Our results were as follows: first, in group B patients, whose dominant hand changed to the left hand after stroke, the VN of the CST in the unaffected hemisphere was increased on 2nd DTT compared with 1st DTT without significant change in the FA and ADC values, whereas patients in the other groups (groups A and C) showed no significant change in terms of all DTT parameters (FA, ADC, and VN); second, all clinical data (GS, MFT, PPT, and MBI) showed significant improvement on 2nd DTT compared with 1st DTT in group B patients; and third, the change of the VN of the unaffected CST in group B patients showed significant correlation with the change of the GS, PPT, and MBI.
The FA value represents the degree of directionality of water diffusion, whereas the ADC value indicates the magnitude of water diffusion.29,30 The VN is determined by the included number of voxels in a neural tract.31 Therefore, the increased VN without change in the FA and ADC values in the unaffected CST in group B suggests increment of total number of neural fibers of the CST in the unaffected hemisphere following change of the dominant hand to the left hand. In other words, the CST in the unaffected hemisphere in group B patients showed a compensatory and proliferative phenomenon in terms of the number of neural fibers by the increment of usage of the originally nondominant hand following change of handedness. As a result, the total fiber number of the CST in the unaffected hemisphere was increased.
In the left hand of group B patients, the functional improvement in terms of the GS, MFT, PPT, and MBI with the change of the dominant hand, and the clinical correlation of the functional improvement of the unaffected side in terms of the GS, PPT, and MBI with the VN of the unaffected CST appears to be consistent with the above-mentioned results. The result showing correlation of the increment of the VN in the unaffected CST in group B patients with the increment of the GS and PPT, indicating grip strength and fine motor ability, respectively, appears to coincide with previous studies reporting association of the main function of the CST with the grip power and fine motor ability of the hand.7,8,32–35 However, no correlation of the MFT with the increment of the VN in the unaffected CST in group B patients appears to be ascribed to the characteristics of the MFT, which is the test for proximal part as well as distal part in upper extremity.19 On the other hand, the result showing that the change of the MBI, which indicates the degree of activities of daily living including leg function suggests that patients should participate more actively in their own activity of daily living with residual function to change the dominant hand.21,24 This result might suggest that change of the dominant hand is necessary to patient's volition as well as CST compensation in the unaffected hemisphere.
Since the introduction of DTI, many studies have reported on an injured CST in stroke patients.10–16 However, the majority of these studies have focused on recovery of the CST and association of the injured CST in the affected hemisphere with motor function.10–14 Only a few studies have reported on change of the CST in the unaffected hemisphere.15,16 In 2010, Kwak et al investigated change of the CST in the unaffected hemisphere at the early stage of stroke in 53 patients with intracerebral hemorrhage using DTT and found that the VN of the unaffected CST was increased significantly compared with normal subjects.15 In 2012, Jang et al investigated change of the anterior CST in the unaffected hemisphere of 32 chronic stroke patients using DTT and reported that the VN of the anterior CST in the unaffected hemisphere showed negative correlation with motor function of the affected extremities.16 Thus, to the best of our knowledge, this is the first study on change of the CST in the unaffected hemisphere by change of the dominant hand following stroke. However, limitations of this study should be considered; first, the relatively small number of patients; second, the high standard deviation of duration to 2nd DTT from onset; third, the unequal proportions of hemorrhage and infarct in 3 groups; and fourth, limitations of DTT analysis; DTT technique might be operator dependent and regions of fiber complexity and crossing may cause underestimation of reconstruction of a neural tract.36,37 Therefore, further studies to overcome the above-mentioned limitations should be encouraged.
In conclusion, we found that the fiber number of the CST in the unaffected hemisphere was increased by change of the dominant hand in stroke patients. We believe that our results have important implications in terms of neurorehabilitation. In detail, when stroke patients are obliged to change their dominant hand due to severe injury of the CST in the affected hemisphere or when showing a low functional state in the unaffected hand after change of the dominant hand, clinicians and therapists can induce rapid change of the dominant hand or functional improvement of the changed dominant hand by application of modalities to increase the fiber number of the CST in the unaffected hemisphere. Therefore, further research on modalities to increase the fiber number of the CST should be encouraged.
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, CST = corticospinal tract, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, FA = fractional anisotropy, GS = grip strength, MBI = modified Barthel Index, MFT = Manual Function Test, PPT = Purdue Pegboard Test, VN = voxel number.
Funding: this work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- 1.Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009; 8:741–754. [DOI] [PubMed] [Google Scholar]
- 2.Page SJ, Levine P, Leonard A, et al. Modified constraint-induced therapy in chronic stroke: results of a single-blinded randomized controlled trial. Phys Ther 2008; 88:333–340. [DOI] [PubMed] [Google Scholar]
- 3.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair 2008; 22:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macciocchi SN, Diamond PT, Alves WM, et al. Ischemic stroke: relation of age, lesion location, and initial neurologic deficit to functional outcome. Arch Phys Med Rehabil 1998; 79:1255–1257. [DOI] [PubMed] [Google Scholar]
- 5.Sze KH, Wong E, Or KH, et al. Factors predicting stroke disability at discharge: a study of 793 Chinese. Arch Phys Med Rehabil 2000; 81:876–880. [DOI] [PubMed] [Google Scholar]
- 6.Annett M. Laterality and types of dyslexia. Neurosci Biobehav Rev 1996; 20:631–636. [DOI] [PubMed] [Google Scholar]
- 7.Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. J Rehabil Med 2014; 46:193–199. [DOI] [PubMed] [Google Scholar]
- 8.Jang SH, Kim K, Kim SH, et al. The relation between motor function of stroke patients and diffusion tensor imaging findings for the corticospinal tract. Neurosci Lett 2014; 572:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999; 45:265–269. [DOI] [PubMed] [Google Scholar]
- 10.Cho SH, Kim DG, Kim DS, et al. Motor outcome according to the integrity of the corticospinal tract determined by diffusion tensor tractography in the early stage of corona radiata infarct. Neurosci Lett 2007; 426:123–127. [DOI] [PubMed] [Google Scholar]
- 11.Jung YJ, Jang SH. The fate of injured corticospinal tracts in patients with intracerebral hemorrhage: diffusion tensor imaging study. AJNR Am J Neuroradiol 2012; 33:1775–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei W, Bai L, Wang J, et al. A longitudinal study of hand motor recovery after sub-acute stroke: a study combined FMRI with diffusion tensor imaging. PLoS One 2013; 8:e64154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rong D, Zhang M, Ma Q, et al. Corticospinal tract change during motor recovery in patients with medulla infarct: a diffusion tensor imaging study. Biomed Res Int 2014; 2014:524096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X, Schlaug G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front Hum Neurosci 2015; 9:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak SY, Yeo SS, Choi BY, et al. Corticospinal tract change in the unaffected hemisphere at the early stage of intracerebral hemorrhage: a diffusion tensor tractography study. Eur Neurol 2010; 63:149–153. [DOI] [PubMed] [Google Scholar]
- 16.Jang SH, Kwon HG. Change of the anterior corticospinal tract on the normal side of the brain in chronic stroke patients: diffusion tensor imaging study. Somatosens Mot Res 2015; 32:25–30. [DOI] [PubMed] [Google Scholar]
- 17.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113. [DOI] [PubMed] [Google Scholar]
- 18.Bertrand AM, Fournier K, Wick Brasey MG, et al. Reliability of maximal grip strength measurements and grip strength recovery following a stroke. J Hand Ther 2015. [DOI] [PubMed] [Google Scholar]
- 19.Jung HY, Yoon JS, Park BS. Recovery of proximal and distal arm weakness in the ipsilateral upper limb after stroke. NeuroRehabilitation 2002; 17:153–159. [PubMed] [Google Scholar]
- 20.Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol 1948; 32:234–247. [DOI] [PubMed] [Google Scholar]
- 21.Jung HY, Park BK, Shin HS, et al. Development of the Korean version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med 2007; 31:283–297. [Google Scholar]
- 22.Hamilton GF, McDonald C, Chenier TC. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther 1992; 16:215–219. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Kondo T, Suzukamo Y, et al. Reliability and validity of the Manual Function Test in patients with stroke. Am J Phys Med Rehabil 2009; 88:247–255. [DOI] [PubMed] [Google Scholar]
- 24.Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol 1989; 42:703–709. [DOI] [PubMed] [Google Scholar]
- 25.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 Suppl 1:S208–219. [DOI] [PubMed] [Google Scholar]
- 26.Jang SH. Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med J 2011; 52:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci 2004; 3:11–17. [DOI] [PubMed] [Google Scholar]
- 28.Evans JD. Straightforward Statistics for the Behavioral Sciences. Pacific Grove: Brooks/Cole Publishing Company; 1996. [Google Scholar]
- 29.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34:51–61. [DOI] [PubMed] [Google Scholar]
- 30.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging 2008; 27:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Jang SH, Chang CH, Lee J, et al. Functional role of the corticoreticular pathway in chronic stroke patients. Stroke 2013; 44:1099–1104. [DOI] [PubMed] [Google Scholar]
- 32.Parikh P, Davare M, McGurrin P, et al. Corticospinal excitability underlying digit force planning for grasping in humans. J Neurophysiol 2014; 111:2560–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gagne M, Schneider C. Dynamic changes in corticospinal control of precision grip during wrist movements. Brain Res 2007; 1164:32–43. [DOI] [PubMed] [Google Scholar]
- 34.Bonnard M, Gallea C, De Graaf JB, et al. Corticospinal control of the thumb-index grip depends on precision of force control: a transcranial magnetic stimulation and functional magnetic resonance imagery study in humans. Eur J Neurosci 2007; 25:872–880. [DOI] [PubMed] [Google Scholar]
- 35.Cho HM, Choi BY, Chang CH, et al. The clinical characteristics of motor function in chronic hemiparetic stroke patients with complete corticospinal tract injury. NeuroRehabilitation 2012; 31:207–213. [DOI] [PubMed] [Google Scholar]
- 36.Lee SK, Kim DI, Kim J, et al. Diffusion-tensor MR imaging and fiber tractography: a new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 2005; 25:53–65.discussion 66-58. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Sakai K, Akazawa K, et al. MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009; 8:165–174. [DOI] [PubMed] [Google Scholar]


