Abstract
We report on patients with post-traumatic fatigue and hypersomnia who showed injury of the lower portion of the ascending reticular activating system (ARAS) between the pontine reticular formation (RF) and the intralaminar thalamic nucleus (ILN) following mild traumatic brain injury (TBI), using diffusion tensor tractography (DTT).
Two patients with mild TBI resulting from a car accident were enrolled in this study. Patient 1 was a 51-year-old woman showed abnormalities as 6.9 (cut off: 3.7 points) and 18 (cut off: 10) on the Fatigue Severity Scale and the Epworth Sleepiness Scale at 11 months after onset. Patient 2 was a 64-year-old woman who revealed abnormalities on the Fatigue Severity Scale and the Epworth Sleepiness Scale with 6.8 and 19 at 3 months after onset.
In both patients, the upper ARAS in which the neural connectivity of the ILN to the cerebral cortex did not show significant abnormalities. However, we observed the narrowing of the left dorsal lower ARAS between the pontine RF and the ILN in both patients and the tearing (patient 1) and narrowing (patient 2) of the left ventral lower ARAS between the pontine RF and the hypothalamus.
Injuries of the dorsal and ventral lower ARAS were demonstrated in patients with fatigue and hypersomnia following mild TBI. We believe that these injuries of the ARAS might be a pathogenetic mechanism of fatigue and hypersomnia in patients with TBI.
INTRODUCTION
Before the introduction of diffusion tensor imaging (DTI), accurate evaluation of the ascending reticular activating system (ARAS) in the live human brain was limited. Recently, diffusion tensor tractography (DTT), which is derived from DTI, has enabled 3-dimensional reconstruction and estimation of the ARAS in the human brain.1–4 As a result, a few recent studies using DTT have reported on injury of the ARAS in patients with severe traumatic brain injury (TBI).5,6 However, no study on injury of the ARAS in patients with mild TBI has been reported.
Post-traumatic fatigue and hypersomnia are common sequelae following TBI.7–9 Previous studies have reported close association between fatigue and sleepiness in patients with TBI.7,8 The pathogenetic mechanism of post-traumatic fatigue and hypersomnia has not been clearly elucidated, although several studies have suggested an injury of the brainstem or ARAS.9–11
In the present study, using DTT, we report on patients with post-traumatic fatigue and hypersomnia who showed injury of the dorsal (between the pontine reticular formation [RF] and the intralaminar thalamic nucleus [ILN]) and ventral (between the pontine RF and the hypothalamus) lower portion of the ARAS following mild TBI.
CASE REPORT
Subjects
Two patients were enrolled according to the following inclusion criteria: (1) loss of consciousness (LOC) for 30 min or less, post-traumatic amnesia (PTA) for <24 h and Glasgow Coma Scale (GCS) score of 13 to 15 recorded 30 min postinjury or later upon presentation for health care,12 (2) notify hypersomnia and fatigue since the onset of TBI, (3) no visible lesion on T1-weighted, T2-weighted, fluid attenuated inversion recovery, and T2-weighted gradient recall echo images, and (4) no history of previous head trauma. All patients provided signed, informed consent, and Yeungnam University hospital Institutional Review Board approved the study protocol.
Patient 1 was a 51-year-old woman who had suffered head trauma resulting from a car accident. While driving her sedan, the driver side of her car collided with a mini-bus; consequently, her head hit the car seat. The patient showed LOC (5 min) or PTA (10 min) at the time of head trauma, and her GCS was 15 when she arrived at the hospital. At the time of DTI scanning (11 months after onset), she showed abnormalities as 6.9 (cut off: 3.7 points) and 18 (cut off: 10), respectively, on the Fatigue Severity Scale and the Epworth Sleepiness Scale.13–16
Patient 2 was a 64-year-old woman who had suffered head trauma resulting from a car accident. While driving in a passenger seat of a sedan, her car was hit from front by a sedan. As a result, her head hit the steering wheel. The patient experienced LOC for ∼5 min without PTA. Her GCS score was 15 when she arrived at the hospital. At the time of DTI scanning (3 months after onset), she showed abnormalities on the Fatigue Severity Scale and the Epworth Sleepiness Scale with 6.8 (cut off: 3.7 points) and 19 (cut off: 10), respectively.13–16
Diffusion Tensor Imaging
Diffusion tensor imaging data were acquired at 11 months (patient 1) and 3 months (patient 2) after onset of head trauma using a 1.5 T with 32 noncollinear diffusion sensitizing gradients by single-shot echo-planar imaging. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; TR = 10,398 ms; TE = 72 ms; parallel imaging reduction factor = 2; echo-planar imaging factor = 59; b = 1000 s/mm2; and a slice thickness of 2.5 mm. The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library was used for analysis of DTI data. Eddy current correction was applied to correct the head motion effect and image distortion. FMRIB Diffusion Software with routines option (0.5 mm step lengths, 5000 streamline samples, curvature thresholds = 0.2) was used for fiber tracking.17 Three portions of the ARAS were reconstructed by selection of fibers passing through region of interest (ROI) as follows:2–4 the dorsal lower ARAS, between the pontine RF (ROI 1) and the ILN (ROI 2),2 the ventral lower ARAS, between the pontine RF (ROI 1) and the hypothalamus (ROI 2),4 and the upper ARAS, in which the neural connectivity of the ILN (ROI 1) to the cerebral cortex was analyzed.3 The tract volume and the narrowest area of dorsal and ventral lower ARAS were measured by counting voxels.
In both patients, the upper ARAS in which the neural connectivity of the ILN to the cerebral cortex did not show significant abnormalities. However, we observed the narrowing of the left dorsal lower ARAS between the pontine RF and the ILN in both patients and the tearing (patient 1) and narrowing (patient 2) of the left ventral lower ARAS between the pontine RF and the hypothalamus. The tract volume of the dorsal and ventral lower ARAS were 280/322 (right) and 149/172 (left) voxels in the patient 1, and 320/201 (right) and 185/49 (left) voxels in the patient 2. In addition, compared with the right hemisphere (100%), the narrowest area of left hemisphere in the dorsal and ventral lower ARAS on an axial slice were as follows—the patient 1: 7 voxels (29.2%, right: 24 voxels) and 11 voxels (26.8%, right: 41 voxels), and the patient 2: 15 voxels (33.3%), right: 45 voxels) and 3 voxels (15%, right:20 voxels) (Figure 1).
FIGURE 1.
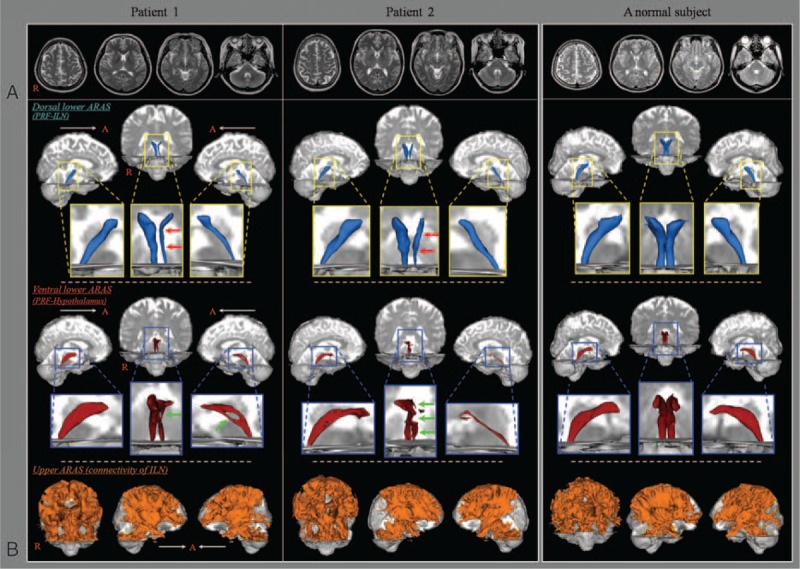
(A) T2-weighted brain MR images at 11 months (patient 1) and 3 months (patient 2) after onset show no abnormal lesion. (B) Results of diffusion tensor tractography (DTT). The narrowing (red arrows) of the left dorsal lower ascending reticular activating system (ARAS) between the pontine reticular formation and intralaminar thalamic nucleus in both patients, and the tearing (patient 1, green arrow) and narrowing (patient 2, green arrows) of the left ventral lower ARAS between the pontine reticular formation and the hypothalamus are observed compared with the right side of each patient and both sides of a normal subject (45 year-old woman). ARAS = ascending reticular activating system, DTT = diffusion tensor tractography, ILN = intralaminar thalamic nucleus, MR = magnetic resonance, PRF = pontine reticular formation.
DISCUSSION
In the present study, using DTT, we evaluated the 3 portions of the ARAS: the upper ARAS in which the neural connectivity of the intralaminar thalamic nucleus to the cerebral cortex, the dorsal lower ARAS, between the pontine RF and the ILN, and the ventral lower ARAS, between the pontine RF and the hypothalamus. We found that the dorsal and ventral lower ARAS was torn or narrowed in both patients. The post-traumatic fatigue and hypersomnia in these patients might be mainly attributed to the injury of the left dorsal and ventral lower ARAS.
Since introduction of DTI, a few studies have reported on injury of the ARAS in patients with TBI.5,6 In 2013, Edlow et al (2013) reported on a patient with coma following severe TBI who showed complete disruption of white matter pathways connecting brainstem arousal nuclei to the basal forebrain and thalamic nuclei, and partial disruption of the pathways connecting the thalamus and basal forebrain to the cerebral cortex.5 Jang et al (2015) recently demonstrated recovery of the injured lower portion of the ARAS between the pontine RF and the ILN in a patient with severe TBI.6 On the other hand, descending reticular activating system, which is involved in the generation of movement using mediating the spinal motoneurons, could also be related to the state of consciousness. In 2013, Tapia et al suggested that arousal state was related to muscular activation which was mediated by the descending reticular activating system.18 Therefore, to the best of our knowledge, this is the first study to demonstrate injury of the lower portion of the ARAS between the pontine RF and the ILN in patients with mild TBI. Nevertheless, limitations of this study should be considered. First, because it is a case report, this study is limited; therefore, conduct of further studies comprising a large number of patients would be necessary. Second, despite being a powerful anatomic imaging tool, because regions of fiber complexity and crossing can prevent full reflection of the underlying fiber architecture, DTI may underestimate or overestimate the fiber tracts.19
In conclusion, this study demonstrated injuries of the dorsal and ventral lower portions of the ARAS in patients with fatigue and hypersomnia following mild TBI. We believe that these injuries of the ARAS might be a pathogenetic mechanism of fatigue and hypersomnia in patients with TBI. Conduct of further studies on this topic should be encouraged .
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2015R1A2A2A01004073).
Footnotes
Abbreviations: ARAS = ascending reticular activating system, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, GCS = Glasgow Coma Scale, ILN = intralaminar thalamic nucleus, LOC = loss of consciousness, PTA = post-traumatic amnesia, RF = reticular formation, ROI = region of interest, TBI = traumatic brain injury.
The authors have no conflicts of interest to disclose
REFERENCES
- 1.Edlow BL, Takahashi E, Wu O, et al. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 2012; 71:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo SS, Chang PH, Jang SH. The ascending reticular activating system from pontine reticular formation to the thalamus in the human brain. Front Hum Neurosci 2013; 7:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang SH, Lim HW, Yeo SS. The neural connectivity of the intralaminar thalamic nuclei in the human brain: a diffusion tensor tractography study. Neurosci Lett 2014; 579:140–144. [DOI] [PubMed] [Google Scholar]
- 4.Jang SH, Kwon HG. The ascending reticular activating system from pontine reticular formation to the hypothalamus in the human brain: a diffusion tensor imaging study. Neurosci Lett 2015; 590:58–61. [DOI] [PubMed] [Google Scholar]
- 5.Edlow BL, Haynes RL, Takahashi E, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol 2013; 72:505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang SH, Kim SH, Lim HW, et al. Recovery of injured lower portion of the ascending reticular activating system in a patient with traumatic brain injury. Am J Phys Med Rehabil 2015; 94:250–253. [DOI] [PubMed] [Google Scholar]
- 7.Beaulieu-Bonneau S, Morin CM. Sleepiness and fatigue following traumatic brain injury. Sleep Med 2012; 13:598–605. [DOI] [PubMed] [Google Scholar]
- 8.Ponsford JL, Ziino C, Parcell DL, et al. Fatigue and sleep disturbance following traumatic brain injury—their nature, causes, and potential treatments. J Head Trauma Rehabil 2012; 27:224–233. [DOI] [PubMed] [Google Scholar]
- 9.Zasler ND, Katz DI, Zafonte RD. Brain injury medicine: principles and practice; chapter 42. Fatigue: assessment and treatment. 2nd edn.New York, NY: Demos Medical Pub; 2013. [Google Scholar]
- 10.Menzler K, Belke M, Unger MM, et al. DTI reveals hypothalamic and brainstem white matter lesions in patients with idiopathic narcolepsy. Sleep Med 2012; 13:736–742. [DOI] [PubMed] [Google Scholar]
- 11.Yassin W, Sugihara G, Oishi N, et al. Hypothalamic-amygdalar-brainstem volume reduction in a patient with narcolepsy secondary to diffuse axonal injury. J Clin Sleep Med 2015; 11:581–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995; 45:1253–1260. [DOI] [PubMed] [Google Scholar]
- 13.Bloch KE, Schoch OD, Zhang JN, et al. German version of the Epworth Sleepiness Scale. Respiration 1999; 66:440–447. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz JE, Jandorf L, Krupp LB. The measurement of fatigue: a new instrument. J Psychosom Res 1993; 37:753–762. [DOI] [PubMed] [Google Scholar]
- 15.Norrie J, Heitger M, Leathem J, et al. Mild traumatic brain injury and fatigue: a prospective longitudinal study. Brain Inj 2010; 24:1528–1538. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine. The international classification of sleep disorders: diagnostic and coding manual. 2nd ednWestchester, Ill: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 17.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- 18.Tapia JA, Trejo A, Linares P, et al. Reticular activating system of a central pattern generator: premovement electrical potentials. Physiol Rep 2013; 1:e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada K, Sakai K, Akazawa K, et al. MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009; 8:165–174. [DOI] [PubMed] [Google Scholar]


