Abstract
Tumor-associated macrophages (TAMs) are associated with poor prognosis in numerous human cancers and play important roles in tumor progression. Epithelial-mesenchymal transition (EMT) contributes to invasion and metastasis in cancer. However, the associations between TAMs and EMT are not clear in gastric cancer (GC). The present study was designed to investigate the effects of TAMs on EMT in human GC.
TAMs marker CD68 and EMT-related proteins were detected by immunohistochemistry (IHC) in human GC tissues and their clinical significance were evaluated.
A high level of infiltration of TAMs was associated with aggressive characteristics of tumor and an independent poor prognostic factor in human GC tissues. Infiltration of TAMs was also associated with EMT-related proteins in human GC tissues.
Our findings suggest that the high level of infiltration TAMs was associated with aggressive features of GC and is an independent poor prognostic factor in GC patients. TAMs are associated with EMT induction in human GC tissues. The level of TAMs infiltration may be used as a prognostic factor and even a therapeutic target in GC.
INTRODUCTION
Gastric cancer (GC) is one of the most common cancers in China, and the second most common cause of cancer deaths worldwide.1 Despite recent advances in diagnostic and treatment strategies, overall survival (OS) among GC patients is still poor, owing to high incidences of invasion and metastasis.2 It is necessary to clarify the mechanism of invasion and metastasis to improve the prognosis of patients with GC.
Increasing evidence suggests that epithelial-mesenchymal transition (EMT) contributes to invasion and metastasis in cancer.3–5 EMT is a process by which epithelial tumor cells lose epithelial features and gain mesenchymal phenotypes. During EMT, expression levels of the adhesion molecule E-cadherin are decreased, whereas N-cadherin and vimentin levels are increased.6 It is considered as the key step by which tumor cells gain the higher ability of invasive and metastatic. Tumor cells take advantage of EMT as an intermediary phenotype to achieve self-renewal and adapt to microenvironments.7,8 As EMT represents a crucial step in tumor progression, it is of interest to identify and characterize the mechanisms regulating this step.
It is generally accepted that tumor microenvironment (TME) play important roles in cancer develpoment and metastasis.9,10 TAMs are key orchestrators and a set of macrophages of TME.11,12 TAMs are associated with poor prognosis in numerous human cancers and play important roles in tumor progression.13 TAMs infiltration in tumors of GC patients is associated with more malignant phenotypes, including tumor angiogenesis, depth of invasion, nodal status, and clinical stages.14,15 Increasing evidence suggests that TAMs promote EMT in tumor cells.16–19 However, little is known about TAMs affect EMT in GC. There have been few studies of the correlation between TAM infiltration and the the expression of EMT markers in GC tissues. We speculated that TAMs might induce EMT and consequently promote GC cell migration and metastasis.
In this study, we examined the level of infiltrated TAMs and the expression of EMT markers in GC tissues using immunohistochemistry (IHC). The clinicopathological characteristics of GC and prognosis were demonstrated. Our findings demonstrate and further support an important link between TAMs and EMT in regulating GC metastasis.
MATERIALS AND METHODS
Ethics Statement
This study was approved by the Protection of Human Subjects Committee of First Affiliated Hospital, Xi’an Jiaotong University and complies with the Helsinki declaration.
Tissue Samples
We collected tissues specimens of 178 gastric cancer patients from consecutive surgical cases in Department of Surgical Oncology, The First Affiliated Hospital, Xi’an Jiaotong University and Department of Surgical Oncology, the 215th Hospital of Shaanxi province between 2004 and 2009. The patients included 125 male and 53 female patients (ranging from 25 to 81 years of age). All of the patients were assessed according to the system for staging primary tumor/regional lymph nodes/distant metastasis (TNM) described in the AJCC Cancer Staging Manual.20 None of these 178 patients received neoadjuvant or adjuvant chemotherapy before the operation.
Immunohistochemical Staining
The tissues specimens were fixed in neutral buffered formalin and embedded in paraffin wax. The sections of 4-mm thickness were cut and mounted on charged glass slides. Antigen retrieval was performed using citrate buffer at pH 6.0. Immunohistochemical staining was performed with anti-CD68 antibody (ZM-0060, Beijing Zhongshan Biotechnology, Beijing, China), and monoclonal antibodies against E-cadherin (ZA-0565–3) and vimentin (ZA-0511) were purchased from Beijing Zhongshan Biotechnology (Beijing, China). The streptavidin-peroxidase technique (SP-9001 Golden Bridge Int, Beijing, China) was used. An irrelevant rabbit antiserum served as a negative control. The sections were stained with 0.02% diaminobenzidine (DAB) solution followed by counterstaining with hematoxylin.
Evaluation of Immunohistochemical Analysis
The evaluation of CD68, E-cadherin and vimentin expression was performed independently by 2 experienced pathologists who were blinded to the clinical data with consensus. Sections were observed under a light microscope (Carl Zeiss Axio Scope. A1 microscope) at high magnification power (×400). TAMs density identified by CD68 expression were estimated (per mm2) at high power (×400) magnification from 5 areas per case.21 The average number of cells in each case was determined as follows: < mean as low density and >mean as high density.
The staining results of E-cadherin and vimentin were scored semiquantitatively by calculating the immunostaining intensity and the percentage of positive malignant cells. The percentage of positive malignant cells was determined in at least 5 areas under 400 × magnifications and averaged. The mean percentage was scored as follows: 0 (0–5%); 1(6–25%); 2 (26–50%); 3 (51–75%), and 4 (76–100%). The staining intensities were scored as follows: no coloring, 0 point; slightly yellow, 1 point; brownish-yellow, 2 points; and tan, 3 points. Finally, the staining score was obtained by calculating the product of the staining intensity and the positive cell percentage, where ≤ 5 was defined as low expression and ≥ 6 as high expression.
Statistical Analysis
Statistical analysis was performed using the SPSS software package (Version16.0, Chicago, IL). A chi-square test was used to test the association of CD68, E-cadherin and vimentin expression and the clinicopathological variables. The Spearman's rank correlation coefficient was used for analyzing the association of the E-cadherin and vimentin expression levels with the CD68 expression levels. Overall survival was defined as the time from the date of surgery to the date of the last follow-up or death from any cause. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. For multivariate analysis, the prognostic factors were analyzed using Cox's proportional hazard model. Student t test or 1-way ANOVA was used to compare the normally distributed variables. The results were considered statistically significant if P < 0.05 (P < 0.05∗, P < 0.01∗∗).
RESULTS
Infiltration of TAMs and Their Association With Clinicopathologic Features of Tumors
The clinicopathological characteristics of the GC patients were described in Table 1. Respective photomicrographs of immunohistochemical staining of CD68 are shown in Figure 1. High-expression of CD68 was closely related to the pTNM stage, nodal involvement, metastasis, Borrmann types, differentiation, and tumor diameter, but not to the patients’ gender, age, and the depth of invasion.
TABLE 1.
Correlation of CD68 Expression With Clinicopathologic Characteristics of Gastric Cancer
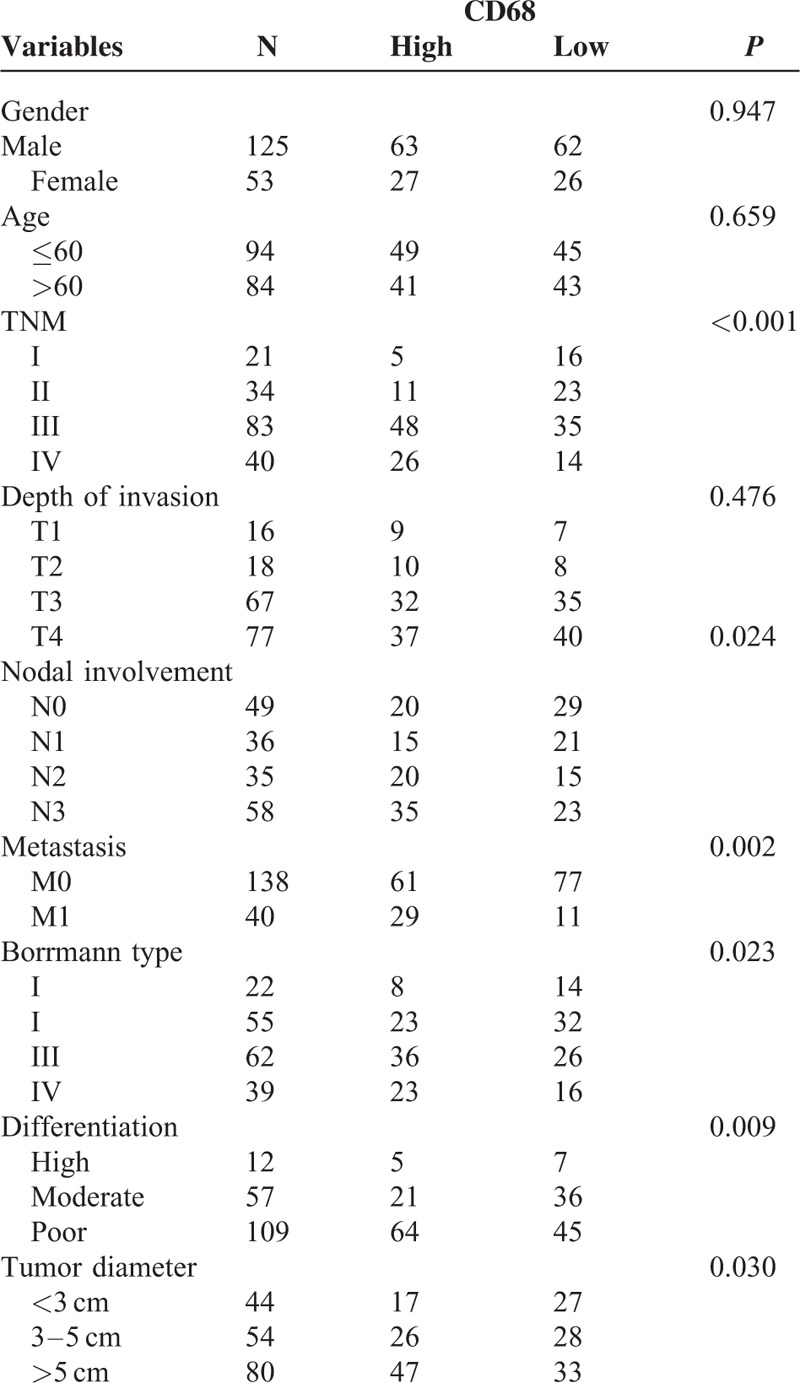
FIGURE 1.

Detection of CD68, E-cadherin, and Vimentin expression in GC tissue and adjacent normal tissue by IHC. Strong CD68 immunoreactivity was identified in poorly differentiated adenocarcinomas. E-cadherin expression was observed in normal gastric glands but not in GC tissue. Vimentin expression was not seen in normal tissue but was observed in GC tissue. GC = gastric cancer, IHC = immunohistochemistry.
Association of TAMs With Expression of EMT Markers
The relationships between expression of CD68 and the 2 EMT indicator proteins were calculated and have been outlined in Table 2. It was noted that epithelial protein loss frequencies in the 178 GC tissues were 66.02% for E-cadherin. Abnormal mesenchymal protein expression frequencies in the same samples were 23.30% for Vimentin. The result also showed that high expression of CD68 correlated with a loss of E-cadherin expression (r = 0.111, P < 0.001), and anomalous positivity of Vimentin (r = 9.111, P < 0.001) in clinical GC samples.
TABLE 2.
The Association Between CD68 With E-Cadherin and Vimentin

TAM Infiltration and Clinical Outcomes
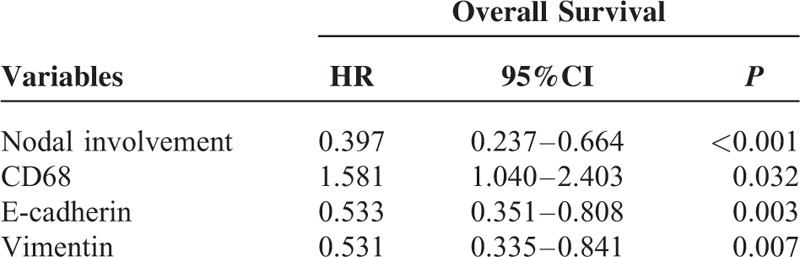
Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. The patients with high CD68 expression showed a more unfavorable prognosis than those with low expression (P < 0.001) (Figures 2A and Table 3). The low levels of E-cadherin expression had a statistically significant correlation with poor overall survival (P < 0.001) (Figure 2B and Table 3). The high levels of Vimentin expression had a statistically significant correlation with poor overall survival (P < 0.001) (Figure 2C and Table 3). Moreover, as seen in Table 4, multivariate Cox analysis showed that the expression level of CD68 was an independent prognostic factor for patients with GC (P < 0.05).
FIGURE 2.
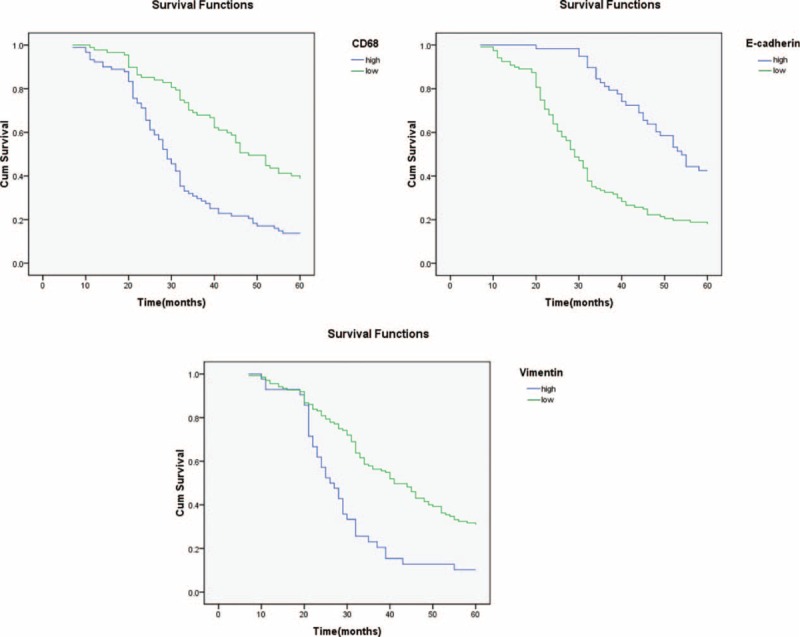
Expression of CD68 and associated EMT proteins predict poor prognosis of GC. Patients that overexpressed CD68 demonstrated shorter OS than those in the low expression group (P < 0.001). Patients with lower expression levels of E-cadherin had a worse OS than those with high E-cadherin expression levels (P < 0.001). Patients that were positive for vimentin expression exhibited poor survival rates compared with those who were negative for vimentin (P < 0.001). GC = gastric cancer, OS = overall survival.
TABLE 3.
Univariate Analysis for Overall Survival
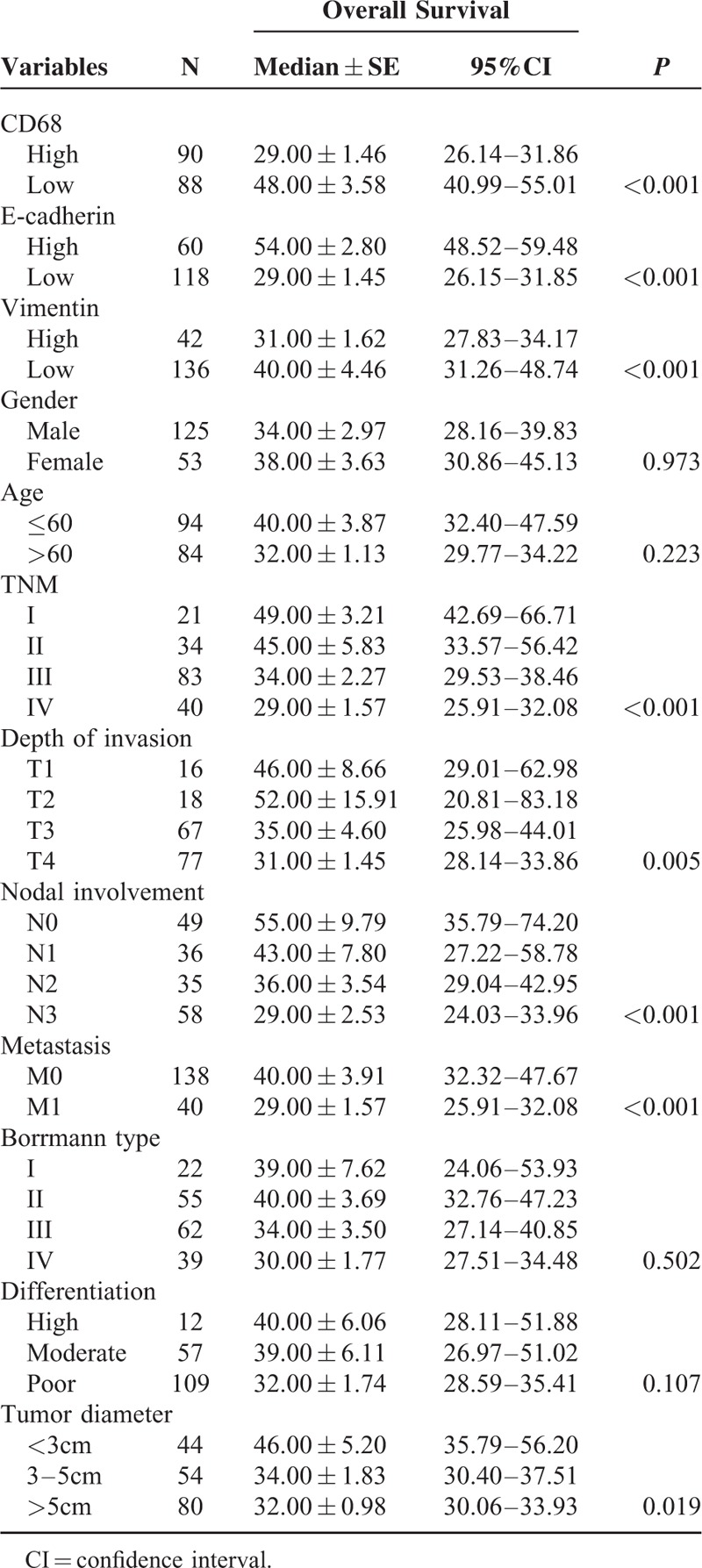
TABLE 4.
Multivariate Cox Proportional Hazards Analysis for Overall Survival
DISCUSSION
In recent decades, published reports have demonstrated that TAMs infiltration in GC is associated with more malignant phenotypes, including tumor angiogenesis, depth of invasion, nodal status, and clinical stages.14,15 It was demonstrated that TAMs could extend along lymphatic flow in the pre-metastatic lymph nodes of human GC.22 Zhang et al showed that infiltration of TAMs could predict OS of patients with GC after surgical resection.23 Lin et al and Wu et al reported that infiltration of TAMs correlate with disease progression and poor survival and thus can serve as a prognostic marker in GC.24,25 Infiltration of TAMs could also promote angiogenesis and lymphangiogenesis of GC.26 We have previous shown expression of RAP1B is associated with poor prognosis and promotes an aggressive phenotype in gastric cancer.27 However, whether or not infiltration of TAMs is involved in EMT of human GC needs further investigation.
In the present study, we found that high levels infiltration of TAMs in 51%(90/178) GC tissues. High levels infiltration of TAMs showed a close relationship with aggressive characteristics of tumor and an independent poor prognostic factor. E-cadherin was repressed in 66.02% (118/178) GC tissues. Vimentin immunoactivity was observed in 42 GC tissues where 36 of which CD68 was overexpressed. Overexpression of CD68 correlated with repression of E-cadherin, and expression of vimentin. In addition, CD68/vimentin expression significantly correlated with histological differentiation, lymph node metastasis, TNM stage, and poor prognosis. The character of EMT is that expression levels of the adhesion molecule E-cadherin are decreased, whereas N-cadherin and vimentin levels are increased.6 It is considered as the key step by which tumor cells gain the higher ability of invasive and metastatic. Our findings suggest that TAMs is associated with EMT induction in human GC tissues.
How TAMs affect GC progression by inducing EMT has not been clarified. In hepatocellular carcinoma, it was reported that TAMs promoted cancer stem cell-like properties via TGF-β1-induced EMT by secreted TGF-β1.16 In oral squamous cell carcinoma (OSCC), it was demonstrated that TAMs play a protumor role and promote EMT through activation of Gas6/Axl-NF-κB.17 In breast cancer, a recent study demonstrated that pterostilbene effectively suppresses the generation of CSCs and metastatic potentialunder the influence of TAMs via modulating EMT-associated signaling pathways, specifically NFκB/miR488 circuit.18 Liu CY et al reported TAMs promoted EMT in pancreatic cancer cells partially through TLR4/IL-10 signaling.19 It is suggested TAMs may induce invasiveness of GC by activating the β-catenin pathway.25 The mechanism of how TAMs affect GC progression by inducing EMT needs further investigation.
In summary, we showed that infiltration of TAMs was associated with aggressive features of GC and EMT. Infiltration of TAMs is an independent poor prognostic factor in GC patients. The level of TAMs infiltration may be used as a prognostic factor and even a therapeutic target in gastric cancer.
Footnotes
Abbreviations: EMT = epithelial-mesenchymal transition, GC = gastric cancer, IHC = immunohistochemistry, OS = overall survival, TAMs = tumor-associated macrophages, TME = tumor microenvironment.
JZ, YY, and YY equally contributed to this study.
Funding: this work was supported by the Scientific and Technological Planning project of Shaanxi Province (No. 2014KW23-02) and the Fundamental Research Funds for the Central Universities.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Catalano V, Labianca R, Beretta GD, et al. Gastric cancer. Crit Rev Oncol/Hematol 2009; 71:127–164. [DOI] [PubMed] [Google Scholar]
- 3.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010; 29:4741–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon CH, Kim MJ, Lee H, et al. PTTG1 oncogene promotes tumor malignancy via epithelial to mesenchymal transition and expansion of cancer stem cell population. J Biol Chem 2012; 287:19516–19527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Xiao D, Li G, et al. EphA2 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer cells. Oncogene 2014; 33:2737–2747. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871–890. [DOI] [PubMed] [Google Scholar]
- 7.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol 2009; 174:1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol 2010; 21 Suppl 7:vii89–vii92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allavena P, Sica A, Solinas G, et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol/Hematol 2008; 66:1–9. [DOI] [PubMed] [Google Scholar]
- 10.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124:263–266. [DOI] [PubMed] [Google Scholar]
- 11.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9:239–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol 2003; 22:773–778. [PubMed] [Google Scholar]
- 15.Ishigami S, Natsugoe S, Tokuda K, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res 2003; 23 (5a):4079–4083. [PubMed] [Google Scholar]
- 16.Fan QM, Jing YY, Yu GF, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett 2014; 352:160–168. [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Liu SY, Chou KC, et al. Tumor-associated macrophages promote oral cancer progression through activation of the Axl signaling pathway. Ann Surg Oncol 2014; 21:1031–1037. [DOI] [PubMed] [Google Scholar]
- 18.Mak KK, Wu AT, Lee WH, et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol Nutr Food Res 2013; 57:1123–1134. [DOI] [PubMed] [Google Scholar]
- 19.Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013; 93:844–854. [DOI] [PubMed] [Google Scholar]
- 20.Edge S, Compton BD, Fritz CC, et al. AJCC Cancer Staging Handbook. New York: Springer; 2010. [Google Scholar]
- 21.Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010; 10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go Y, Tanaka H, Tokumoto M, et al. Tumor-associated macrophages extend along lymphatic flow in the pre-metastatic lymph nodes of human gastric cancer. Annal Surg Oncol 2016; 23 Suppl 2:230–235. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Wang X, Shen Z, et al. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer 2015; 18:740–750. [DOI] [PubMed] [Google Scholar]
- 24.Lin CN, Wang CJ, Chao YJ, et al. The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC Cancer 2015; 15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu MH, Lee WJ, Hua KT, et al. Macrophage infiltration induces gastric cancer invasiveness by activating the beta-catenin pathway. PloS One 2015; 10:e0134122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu H, Xu JB, He YL, et al. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol 2012; 106:462–468. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Li M, Yan Y, et al. Expression of RAP1B is associated with poor prognosis and promotes an aggressive phenotype in gastric cancer. Oncol Rep 2015; 34:2385–2394. [DOI] [PubMed] [Google Scholar]


