Abstract
The purpose of this study was to investigate the risk factors for central and lateral neck lymph node metastases in papillary thyroid carcinoma (PTC) and multifocal papillary thyroid carcinoma (MPTC), particularly when associated with Hashimoto thyroiditis (HT).
A retrospective analysis of 763 consecutive patients who underwent total thyroidectomy with bilateral central neck dissection in the First Affiliated Hospital, College of Medicine, Zhejiang University between October 2011 and October 2014 was conducted. All patients had formal histological diagnoses of HT. Multivariable logistic regression analysis was performed to identify risk factors of neck lymph node metastases.
Our study identified 277 PTC patients with HT and showed comparatively low rates of central lymph node metastases (CLNM) compared with the PTC patients without HT (37.2% versus 54.7%, P < 0.001). There were no statistically significant differences in lateral lymph node metastases (LLNM) (P = 0.656). Neck lymph node metastases were histologically proven in 127 (45.8%) patients with PTC with HT, including 103 CLNM and 24 LLNM. There were no significant differences in LLNM between the MPTC-associated HT and classic MPTC cases; however, a significantly reduced risk of CLNM was observed in the MPTC-associated HT compared with the MPTC cases (35.7% versus 72.4%, respectively, P < 0.001). In the multivariate analysis, HT was identified as an independent alleviating factor for CLNM in all PTC patients (odds ratio, 0.369; 95% confidence interval (CI), 0.261 to 0.521; P < 0.001) and in MPTC patients (odds ratio, 0.227; 95% CI, 0.126–0.406; P < 0.001). A cut-off of thyroid peroxidase antibody >140 IU/mL was established as the most sensitive and specific level for the prediction of MPTC based on receiver operating characteristic curve analyses. Thyroid peroxidase antibody, age, tumor size, and multifocality exhibited the ability to predict CLNM in PTC with HT patients with an area under the curve of 81.1% based on a multivariate model.
Hashimoto thyroiditis was associated with increased prevalences of multifocality and capsular invasion. In contrast, HT was associated with a reduced risk of CLNM in PTC and MPTC patients, which indicated a potential protective effect. We found that the prognostic prediction model was applicable for predicting multifocality and CLNM in PTC patients with HT.
INTRODUCTION
The incidence of thyroid carcinoma has rapidly increased in recent years, and this condition affects approximately 5 per 100,000 to 15 per 100,0001 persons. Approximately 70% to 80% of thyroid cancers are papillary thyroid carcinomas (PTCs),2 which exhibited a relatively benign clinical course. Hashimoto thyroiditis (HT) is the most common form of autoimmune thyroid disease, and the incidence of this disease is approximately 2% of the general population.3 The relationship between PTC and HT has been controversial. Some investigations have reported HT is a risk factor for PTC, whereas other studies have not observed a positive correlation.4 The frequency of the association between PTC and HT ranges from 9% to 58% in a series of studies.5
Recently, studies have also focused on the influence of HT on the prognoses of PTC patients associated with HT. Some investigators have reported that PTC patients with lymphocytic thyroiditis tend to exhibit a lower frequency of lymph node metastases, less advanced disease, and better prognoses. In contrast, other studies have demonstrated that PTC coexisting with HT is more likely to be bilateral and multifocal.6,7 Meta-analyses have indicated that HT is present in 26% of multifocal patients compared with 21% of unifocal patients.6 Interestingly, some studies have revealed that multifocal cases are associated with an increased incidence of central lymph node metastases (CLNM)8; however, other studies have found no difference between unifocal and multifocal PTC.9 Overall, the associations of lymph node metastasis and multifocal papillary thyroid carcinoma (MPTC) with HT are not fully understood.
Although some studies have investigated the clinicopathologic factors of PTC with HT, the prognostic significance of HT in MPTC remains unclear. It is uncertain whether coexisting with HT in PTC and MPTC patients represents a good prognosis or is simply the concurrence of both diseases. It is therefore reasonable to evaluate the influence of HT on lymph node metastases in PTC and MPTC patients. Notably, most previous studies evaluated PTC with HT. We hypothesize that the presence of HT may be associated with less risk of neck lymph node metastases (NLNM) and a better prognosis. The aim of this study was to evaluate the clinicopathologic features of MPTC with and without associated HT. Additionally, we sought to investigate the risk factors associated with NLNM in MPTC that are concomitant with HT.
MATERIALS AND METHODS
General Information
This study was approved by ethical committees of the First Affiliated Hospital. The informed consent requirement was obtained from each patient. A retrospective review of the Thyroid Disease Diagnosis and Treatment Center database of the First Affiliated Hospital of the College of Medicine of Zhejiang University was performed. The data from the 3-year period between October 2011 and October 2014 were analyzed. All 763 PTC patients underwent total thyroidectomy with bilateral central neck dissection. The male-to-female ratio of the patients was 185:578, and the mean age was 43.98 ± 11.96 years (15–79 years). The patients comprised 277 subjects with histologically proven HT (36.3%) and 486 patients without HT (63.7%; Table 1). Of the 234 MPTC patients, 129 were observed concomitant with HT (55.1%) and 105 patients without HT (44.9%; Figure 1).
TABLE 1.
Clinicopathologic Characteristics of the Study Population∗ (n = 763)
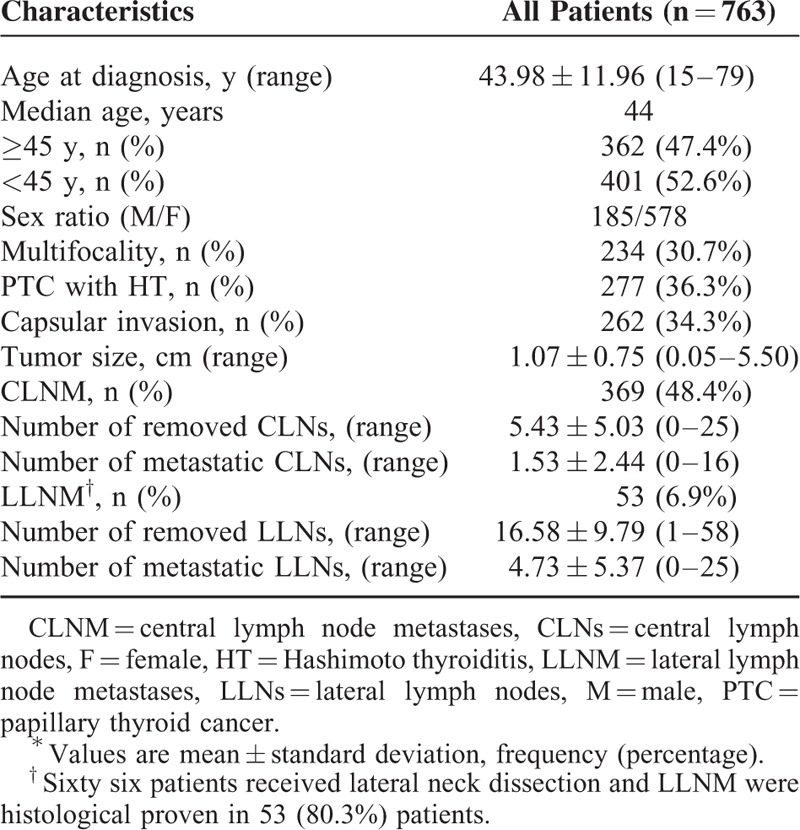
FIGURE 1.
Flow diagram of the study design. Among the 763 papillary thyroid carcinoma patients who underwent total thyroidectomy with bilateral central neck dissection. Hashimoto thyroiditis was present in 277 patients and absent in 486 patients.
The clinical assessments performed for all patients included assays of thyroid hormone, checks of the serum thyrotropin and thyroid peroxidase antibody (TpoAb) levels, ultrasonic examination of the thyroid and computed tomography examinations of the neck, which were performed preoperatively. Ultrasound-guided fine-needle aspiration (FNA) biopsies were performed in 547 patients. Thyroidectomies were performed in the patients with high levels of suspicion of malignancy based on prior thyroid FNA and ultrasound examinations. The surgical procedures for the thyroidectomies with bilateral central neck dissection were selected based on the following factors: the presence of suspected malignancy based on thyroid nodules larger than 1 cm with or without contralateral thyroid nodules, suspected malignancy based on the presence of thyroid nodules less than 1 cm with contralateral thyroid nodules larger than 0.5 cm, and multifocal PTC or bilateral PTC. Patients with Graves’ disease were excluded. Lateral lymph node dissections were performed in 66 patients who underwent preoperative ultrasonography, computed tomography, or FNA of the lateral lymph nodes due to potential metastasis.
Receiver operating characteristic curves were used to examine the abilities to predict MPTC and CLNM using a multivariate model. A binary logistic regression was performed to identify independent prognostic predictors of CLNM. Finally, we identified four variables, that is, TpoAb, age, tumor size at the time of diagnosis and multifocality. Then a logistic regression analysis of the multivariate model using 4 variables was performed to calculate the prediction probability. Receiver operating characteristic curves were drawn based on data from a classification table using prediction probability ranged from 0 to 1. The predictive value of the established model was assessed by calculating the area under the curve (AUC).
Statistical Analysis
Quantitative data are displayed as the mean ± the SD. Statistical analyses were performed using the χ2 test, Fisher exact test, and the t test, as appropriate. Differences with P values <0.05 were defined as statistically significant. The statistical analyses were performed with the SPSS 22.0 program (SPSS, Chicago, IL) for Windows. The sample size was statistically determined using PASS 11.0 (NCSS, LLC).
RESULTS
Comparisons of the Clinicopathologic Features of the Papillary Thyroid Carcinomas Patients With and Without Hashimoto Thyroiditis
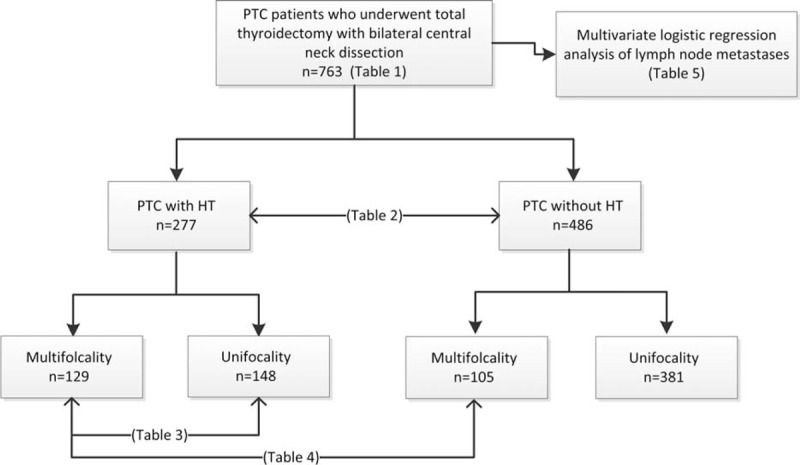
Among the 763 patients in this study, HT was present in 277 patients (36.3%) and absent in 486 (63.7%) patients. Central lymph node metastases were histologically proven in 389 patients (48.4%) and lateral lymph node metastases (LLNM) were in 53 patients (6.9%; Table 1). The male-to-female ratios of the patients with and without HT were 1:4.04 and 1:2.74, respectively (P = 0.033). Compared with the PTC patients without HT, the PTC patients with HT tended to exhibit multifocality (46.6% versus 21.6%, P < 0.001) and greater capsular invasion (43.3% versus. 29.2%, P < 0.001). However, there were no statistically significant differences in age (<45 versus ≥45 years, P = 0.333), tumor size (≤1 versus > 1 cm, P = 0.238), or serum thyrotropin levels (P = 0.068). Additionally, the numbers of CLNM and LLNM were evaluated. Approximately 37.2% (103/277) of the PTC patients with HT exhibited CLNM, and 54.7% (266/486) of the PTC patients without HT exhibited CLNM (P < 0.001). There were no statistically significant differences in LLNM (P = 0.656), the numbers of metastatic lateral lymph nodes (P = 0.455) or laterally removed lymph nodes (P = 0.059; Table 2).
TABLE 2.
Results of the Correlation Between Hashimoto Thyroiditis and Clinicopathological Variables∗
Hashimoto Thyroiditis, Multifocal Carcinoma, and Lymph Node Metastases
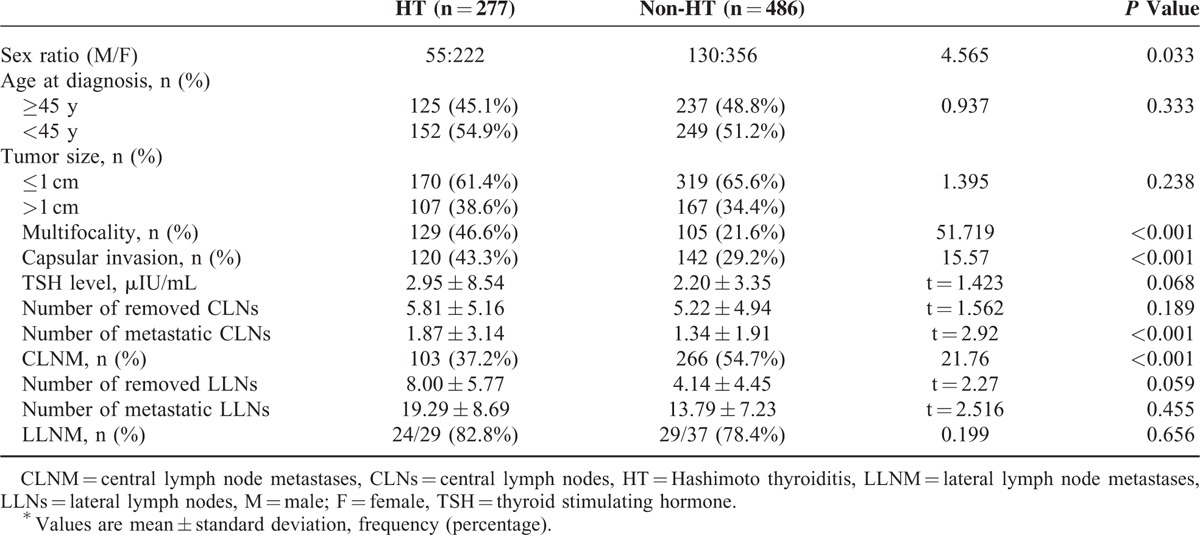
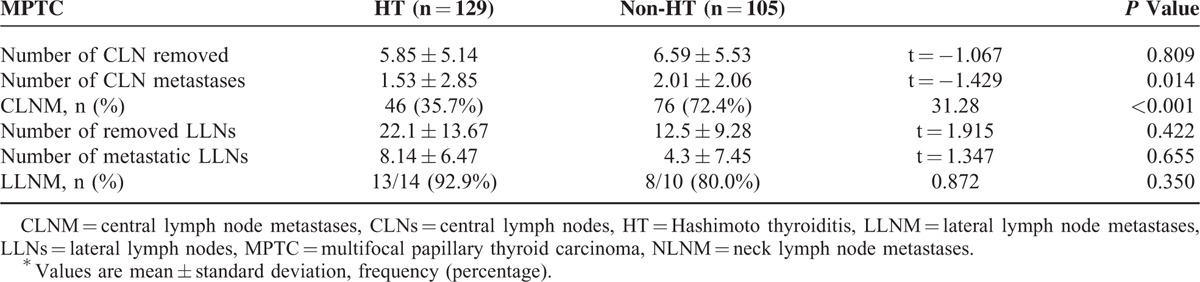
Compared to the MPTC with HT (129 patients), the unifocal PTC with HT (148 patients) were significantly associated with the number of metastatic central lymph nodes (P = 0.003). However, there was no difference in the CLNM and LLNM (P = 0.624 and P = 0.151, respectively) (Table 3). Multifocal papillary thyroid carcinomas without HT were observed in 105 patients. In contrast, metastasis to the central compartment was noted in 46 (35.7%) MPTC patients with HT and 76 (72.4%) MPTC patients without HT (P < 0.001). No significant differences were observed in LLNM (P = 0.350). The 9 patients with confirmed central compartment metastases were more likely to have LLNM (100%) in MPTCs with HT than the 7 patients without HT (77.8%; Table 4).
TABLE 3.
Comparison of Neck Lymph Node Metastases of Papillary Thyroid Cancer With Hashimoto Thyroiditis According to the Presence of Multifocality∗
TABLE 4.
Comparison of Neck Lymph Node Metastases of Multifocal Papillary Thyroid Carcinoma According to the Presence of Hashimoto Thyroiditis∗
Risk Factors for Lymph Node Metastases in the Papillary Thyroid Carcinomass and Multifocal Papillary Thyroid Carcinomas
A preoperative multivariate logistic regression analysis that included sex (male/female), age (≥45/<45 years), tumor size (>1 cm/≤1 cm), capsular invasion (present/absent), HT presence (present/absent) and multifocality (present/absent) was used to assess whether these factors were associated with NLNM in PTCs. We found that an age greater than or equal to 45 years (OR = 2.050; 95% CI, 1.504–2.793, P < 0.001), tumor size >1 cm (OR = 2.708; 95% CI, 1.831–4.006, P < 0.001), and the presence of HT (OR = 0.369; 95% CI, 0.261–0.521, P < 0.001) were noted to be significantly associated with CLNM in PTCs. However, no variables were found to be significantly associated with LLNM (Table 5). We next investigated the risk factors associated with CLNM in MPTCs. Hashimoto thyroiditis was a significant independent alleviating factor for CLNM in MPTCs, with an odds ratio of 0.227 (95% CI, 0.126–0.406, P < 0.001; Table 6).
TABLE 5.
Multivariate Logistic Regression Analysis of Variables Associated With Central and Lateral Lymph Node Metastases in All Patients
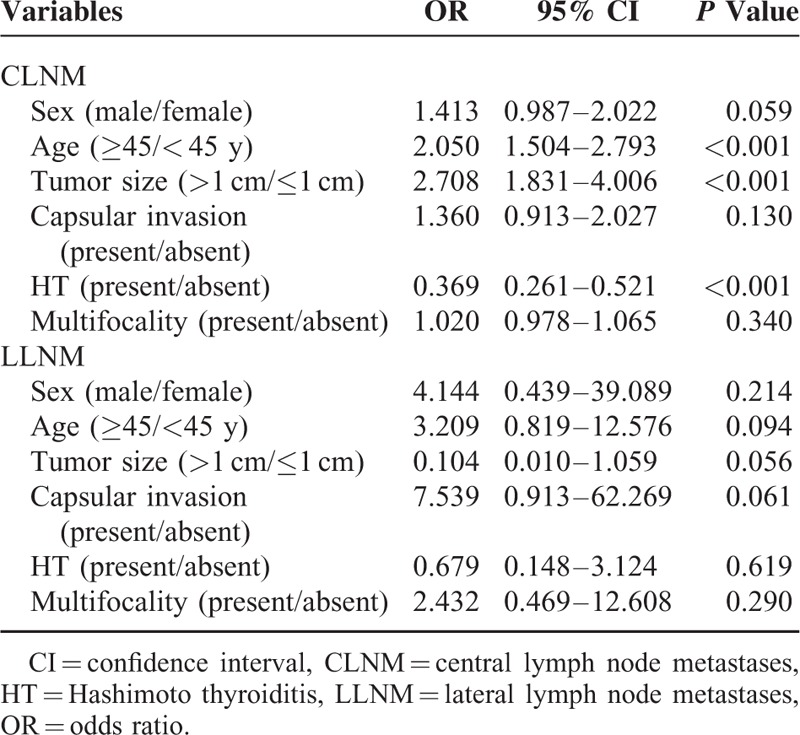
TABLE 6.
Multivariate Logistic Regression Analysis of Central Lymph Node Metastases in Multifocal Papillary Thyroid Carcinoma Patients
Receiver Operating Characteristic Curve Analyses for Multifocal Papillary Thyroid Carcinoma and Central Lymph Node Metastases
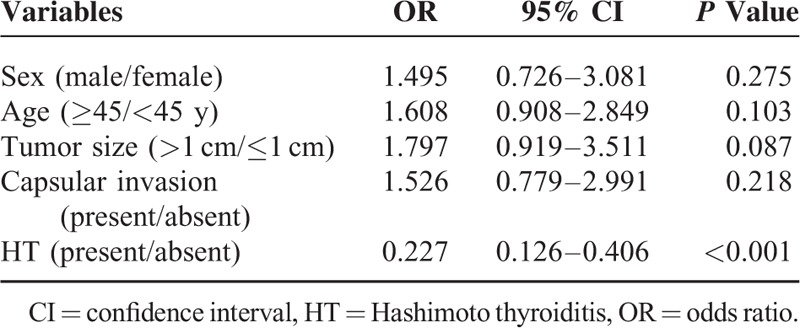
Receiver operating characteristic curves were used to predict multifocality (Figure 2A and B). The AUCs and p values that were calculated for the PTC and PTC with HT patients for multifocality were 54.5% (P < 0.05) and 84.3% (P < 0.0001), respectively, which indicated that the TpoAb value could accurately predict multifocality in patients with PTC with HT. Thyroid peroxidase antibody, age, tumor size and the presence of multifocality were included in the multivariate logistic regression analysis (Table 7). We created the multivariate model to calculate the probability for predicting CLNM. Receiver operating characteristic curve analysis was also performed to predict CLNM in both the PTC and PTC with HT patient (AUCs: 73.4% and 81.1%, respectively, P < 0.0001; Figure 2C and D).
FIGURE 2.
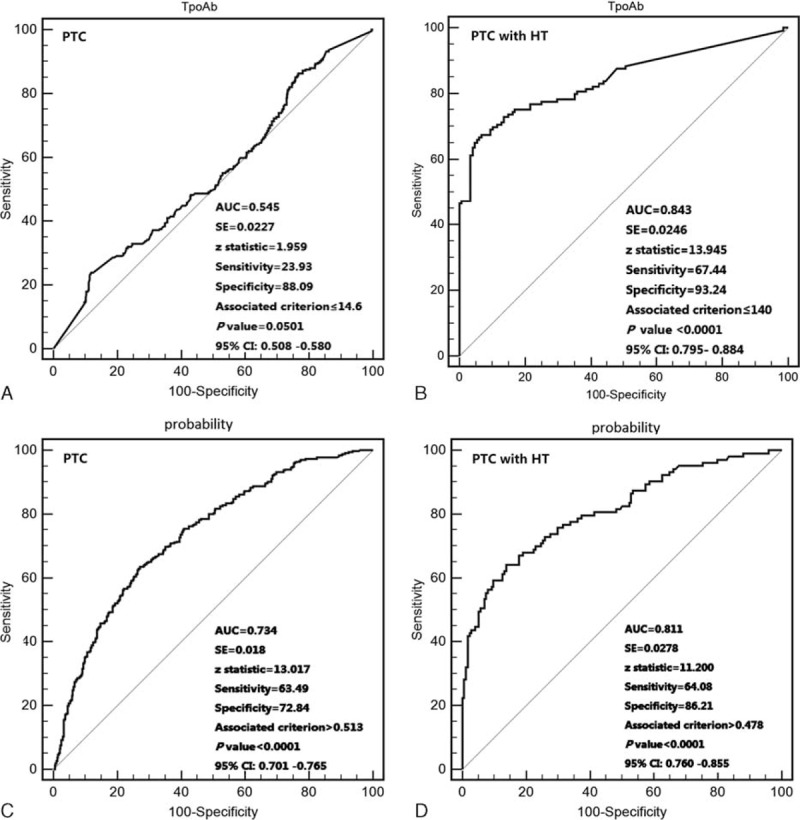
Receiver operating characteristic curve analyses of thyroid peroxidase antibody for predicting multifocal papillary thyroid carcinoma (A and B). Receiver operating characteristic curve analyses for prediction of central lymph node metastases using the multivariate model (C and D). This Receiver operating characteristic curve using probability cut offs from 0 to 1 (see methods). (A and C): All papillary thyroid cancer patients (n = 763). (B and D): papillary thyroid cancer patients with HT (n = 277).
TABLE 7.
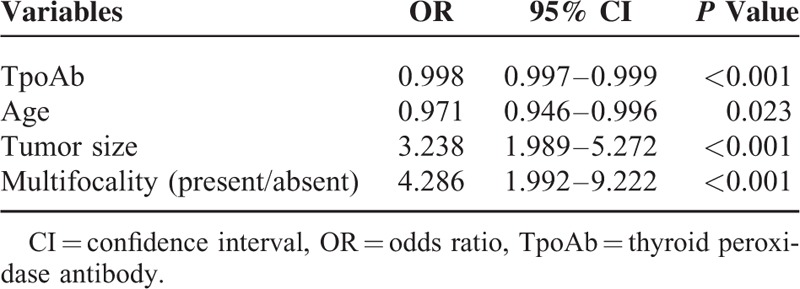
Multivariate Analyses of Variables for the Prediction of Central Lymph Node Metastases in Papillary Thyroid Carcinoma Patients With Hashimoto Thyroiditis
DISCUSSION
Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis and was first described by Hakaru Hashimoto in 1912. Hashimoto thyroiditis is the most common form of autoimmune thyroid disease.10 The incidence of HT is approximately 0.3 to 1.5 cases per 1000 persons every year.3 The main pathological abnormalities include lymphocytic infiltration and oxyphilic changes that can lead to fibrous variants and parenchymal atrophy.11 Hashimoto thyroiditis is widely considered to be associated with thyroid dysfunction and the development of thyroid nodules.12 Papillary thyroid carcinoma is another of the most common endocrine malignancies, and the incidence of this condition has rapidly increased in recent years.13 The relationship between PTC and HT was first described in 1955 by Dailey.14 Since that time, numerous studies have focused on this link, but it still remains a controversial issue. In systematic literature reviews, the average prevalences of PTC in patients with HT have been found to be 1.2% in FNAB studies and 27.56% in thyroidectomy studies.15 In contrast, one meta-analysis demonstrated that the coexistence of HT was significantly associated with PTCs compared with benign lesions and other thyroid carcinomas. This finding implies that HT may indeed be a risk factor for the development of PTC.6 Although some clinical studies have reported a positive correlation between HT and PTC, the immunological mechanisms of this association remain unknown.
Tumor multifocality is typically present in PTC patients, and the prevalence of multifocality ranges from 18% to 87%.16,17 Although high-resolution ultrasonography and FNAB have been used to diagnose MPTC, some small tumor foci are frequently found postthyroidectomy.17 The association between lymph node metastasis and MPTC remains controversial. Some studies have indicated that multifocality results in an increased incidence of CLNM8 but other studies have found no difference between unifocal and multifocal PTC.9
In the present study, we found the rate of HT in patients with PTC was 36.3% and that in the patients with MPTC, the rate of HT was 55.1%. Recently, studies of this topic similarly demonstrated that the prevalence of PTC in patients with HT have been found to be 29.4%∼58.3% in the same region.18,19 The rate of HT was higher among the patients with MPTC compared with those with PTC. In contrast, our study revealed that multifocal papillary thyroid cancer is approximately twice as frequent as PTCs in patients with Hashimoto thyroiditis, which suggests that HT may predispose patients to the development of MPTC. Capsular invasion was more frequently noted than CLNM in PTCs with HT. However, the tumor sizes, ages at the time of diagnosis, and the frequencies of lateral lymph node metastasis in PTCs with and without HT were similar. Our study revealed that a TpoAb > 140 IU/mL predicted the occurrence of MPTC; thus, surgeons should give more attention to tumor multifocality when they encounter histologically proven Hashimoto thyroiditis and PTCs during surgery.
Although PTC is generally an indolent disease that has an excellent prognosis,20 reducing the risk of recurrence and postoperative complications is important. Certain clinicopathological features, such as age at the time of diagnosis, tumor size, extrathyroidal extension, multiple centricity, lymph node metastasis, and distant metastasis, are capable of predicting recurrences in patients treated with surgery.21–26 There are no recent unambiguous clinical studies that have indicated whether HT predisposes patients to cancer recurrence. Several articles have reported that the presence of HT in PTC is associated with smaller tumor size, less tumor invasion, a lower frequency of lymph node metastasis, less advanced TNM stage, and better prognosis.7,27–29 In contrast, other studies have reported that PTCs that coexist with HT are more likely to be bilateral and multifocal, resulting in a higher frequency of lymph node metastases.30–32
We noticed that the frequency of CLNM in PTC patients with HT was less than that in the patients without HT and that the difference was more significant in MPTC patients. Moreover, there were no statistically significant differences in LLNM. When lymph node metastasis was present in the central compartment of the necks of patients with MPTC, 100% (9/9) of the HT patients exhibited lateral lymph node metastasis involvement, whereas 77.8% (7/9) of the patients without HT exhibited such involvement. In the multivariable analysis, the presence of HT was noted as significantly associated with CLNM in patients with MPTC, which was indicative of a complicated interaction of lymph node metastases in PTC and HT. We used a multivariate model that involved TpoAb, age, tumor size at the time of diagnosis, and multifocality. The sensitivity and specificity of CLNM in the PTC patients (cutoff probability value: 0.513) were 63.5% and 72.8%, respectively, and in the PTC with HT patients (cutoff probability value: 0.478), these values were 64.1% and 84.2%, respectively.
In recent years, the coexistence of thyroid cancer and HT has been of great concern, but the mechanisms related to the relationship between them have remained unclear. So far, the main molecular finding is the mutations of oncogene BRAF (B-type Raf kinase)V600E and the recombination of RET/PTC (Rearranged during transfection).33 Although RET/PTC-induced signaling of thyroid cells is mediated through the MAPK (Mitogen-activated protein kinase) pathway,34 it has been recognized that BRAF mutant is another stronger cause of aberrant activation of the pathway.35 Bozec A et al36 indicated that HT may be the activator of MAPK signaling pathway in the development of PTC. Many studies have investigated a significant association of BRAFV600E mutation with lymph node metastasis.37,38 Some studies revealed that CLNM was accompanied by high prevalence of BRAFV600E mutation.39,40 The relationship of BRAFV600E mutation with HT in PTC patients’ lymph node metastases remains unknown. In addition, other studies have reported that CST6 (Cystatin E/M) and CXCL14 (Chemokine (C-X-C motif) ligand 14) genes, which have a strong correlation with BRAFV600E mutation were associated with CLNM in PTC patients.41 Nonetheless, whether these genes and HT are linked in PTC patients is still unknown.
Whether HT represents a host immune response to PTC or just a chance occurrence remains unclear. Ehlers et al42 postulated several possible pathomechanisms and reported that thyroid carcinoma develop despite, or due to, immune responses. Hashimoto thyroiditis is a most common autoimmune thyroid disease, and the thyroid-specific immune response is one of the relevant pathological processes. In addition, several studies have demonstrated that cancer immunosurveillance process can eliminate malignant cells. The infiltration of CD4+, CD8+, and Th17 cells is important in the immune-escape mechanisms of PTC and result in a decreased immune response that leads to a favorable prognosis.43
Although few published studies have evaluated the association between CLNM of PTC patients with HT, we confirm the findings that HT in patients with PTC was associated with a low probability of CLNM. The strength of our study relates to the analysis of CLNM in MPTC patients, as well as the effects of HT on CLNM in MPTCs. We also indicated that the TpoAb value could predict multifocality in patients with PTC with HT and evaluated the ability of the model for predicting CLNM in PTC with HT patients. Furthermore, this model could be applied in clinical practice. Nonetheless, our study had several limitations. First, our study was a retrospective and from a single center. A multicenter prospective study will be more helpful for finding out intrinsic effects of HT on CLNM. Second, more research on the mechanism of the molecular biology is needed for explanation of the correlations observed in the future. Finally, we indicated that HT was associated with a reduced risk of CLNM in MPTC patients. Hashimoto thyroiditis was reported to be associated with improved prognosis in PTC patients with CLNM.7 Whether the similar influence in MPTC patients with CLNM remain unknown. Further follow-up study should be explored in future to evaluate the prognosis of HT in MPTC patients.
In conclusion, our results provide the consecutive analysis of the relationship between HT and PTC, demonstrated that HT may influence lymph node metastases in the necks of MPTC patients. Hashimoto thyroiditis was associated with multifocality and a reduced risk of CLNM, even in MPTC patients, which indicated a potential protective effect.
Footnotes
Abbreviations: CLNM = central lymph node metastases, HT = Hashimoto thyroiditis, LLNM = lateral lymph node metastases, MPTC = multifocal papillary thyroid carcinoma, NLNM = neck lymph node metastases, PTC = papillary thyroid cancer, TpoAb = thyroid peroxidase antibody.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cooper DS, Doherty GM, et al. American Thyroid Association Guidelines Taskforce on Thyroid N Differentiated Thyroid C. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009; 19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 2.Kwak JY, Kim EK, Kim JK, et al. Dual priming oligonucleotide-based multiplex PCR analysis for detection of BRAFV600E mutation in FNAB samples of thyroid nodules in BRAFV600E mutation-prevalent area. Head Neck 2010; 32:490–498. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto thyroiditis: a century later. Adv Anat Pathol 2012; 19:181–186. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metabol 2013; 98:474–482. [DOI] [PubMed] [Google Scholar]
- 5.Fugazzola L, Colombo C, Perrino M, et al. Papillary thyroid carcinoma and inflammation. Frontiers Endocrinol 2011; 2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Kim Y, Choi JW, et al. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol 2013; 168:343–349. [DOI] [PubMed] [Google Scholar]
- 7.Kim EY, Kim WG, Kim WB, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol 2009; 71:581–586. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Wang L, Yi P, et al. Risk factors for central lymph node metastasis of patients with papillary thyroid microcarcinoma: a meta-analysis. Int J Clin Expt Pathol 2014; 7:932–937. [PMC free article] [PubMed] [Google Scholar]
- 9.Schindler AM, van Melle G, Evequoz B, et al. Prognostic factors in papillary carcinoma of the thyroid. Cancer 1991; 68:324–330. [DOI] [PubMed] [Google Scholar]
- 10.Anil C, Goksel S, Gursoy A. Hashimoto's thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid 2010; 20:601–606. [DOI] [PubMed] [Google Scholar]
- 11.Bircan HY, Koc B, Akarsu C, et al. Is Hashimoto's thyroiditis a prognostic factor for thyroid papillary microcarcinoma? Eur Rev Med Pharmacol Sci 2014; 18:1910–1915. [PubMed] [Google Scholar]
- 12.Cipolla C, Sandonato L, Graceffa G, et al. Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg 2005; 71:874–878. [PubMed] [Google Scholar]
- 13.Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 2015; 47:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg 1955; 70:291–297. [DOI] [PubMed] [Google Scholar]
- 15.Jayaprakash K, Kishanprasad H, Hegde P, et al. Hashimotos thyroiditis with coexistent papillary carcinoma and non-Hodgkin lymphoma-thyroid. Ann Med Health Sci Res 2014; 4:268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy RP, Wang M, Jones TD, et al. Molecular evidence for the same clonal origin of multifocal papillary thyroid carcinomas. Clin Cancer Res 2006; 12:2414–2418. [DOI] [PubMed] [Google Scholar]
- 17.Mazeh H, Samet Y, Hochstein D, et al. Multifocality in well-differentiated thyroid carcinomas calls for total thyroidectomy. Am J Surg 2011; 201:770–775. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Dai J, Wu T, et al. The study of the coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol 2014; 140:1021–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Li H, Ji QH, et al. The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC cancer 2012; 12:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito Y, Miyauchi A. Nonoperative management of low-risk differentiated thyroid carcinoma. Curr Opin Oncol 2015; 27:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podnos YD, Smith D, Wagman LD, et al. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg 2005; 71:731–734. [DOI] [PubMed] [Google Scholar]
- 22.Yu XM, Wan Y, Sippel RS, et al. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg 2011; 254:653–660. [DOI] [PubMed] [Google Scholar]
- 23.Vrachimis A, Gerss J, Stoyke M, et al. No significant difference in the prognostic value of the 5th and 7th editions of AJCC staging for differentiated thyroid cancer. Clin Endocrinol 2014; 80:911–917. [DOI] [PubMed] [Google Scholar]
- 24.Kim KJ, Kim SM, Lee YS, et al. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol 2015; 22:125–131. [DOI] [PubMed] [Google Scholar]
- 25.Hyun SM, Song HY, Kim SY, et al. Impact of combined prophylactic unilateral central neck dissection and hemithyroidectomy in patients with papillary thyroid microcarcinoma. Ann Surg Oncol 2012; 19:591–596. [DOI] [PubMed] [Google Scholar]
- 26.Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid 2012; 22:1144–1152. [DOI] [PubMed] [Google Scholar]
- 27.Kurukahvecioglu O, Taneri F, Yuksel O, et al. Total thyroidectomy for the treatment of Hashimoto's thyroiditis coexisting with papillary thyroid carcinoma. Adv Ther 2007; 24:510–516. [DOI] [PubMed] [Google Scholar]
- 28.Repplinger D, Bargren A, Zhang YW, et al. Is Hashimoto's thyroiditis a risk factor for papillary thyroid cancer? J Surg Res 2008; 150:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KW, Park YJ, Kim EH, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck 2011; 33:691–695. [DOI] [PubMed] [Google Scholar]
- 30.Consorti F, Loponte M, Milazzo F, et al. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto's thyroiditis. Eur Surg Res 2010; 45:333–337. [DOI] [PubMed] [Google Scholar]
- 31.Kebebew E, Treseler PA, Ituarte PH, et al. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg 2001; 25:632–637. [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Choi YJ, Yun JS. Features of papillary thyroid microcarcinoma in the presence and absence of lymphocytic thyroiditis. Endocr Pathol 2010; 21:149–153. [DOI] [PubMed] [Google Scholar]
- 33.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007; 28:742–762. [DOI] [PubMed] [Google Scholar]
- 34.Ciampi R, Nikiforov YE. RET/PTC rearrangements and BRAF mutations in thyroid tumorigenesis. Endocrinology 2007; 148:936–941. [DOI] [PubMed] [Google Scholar]
- 35.Melillo RM, Castellone MD, Guarino V, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest 2005; 115:1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Bozec A, Lassalle S, Hofman V, et al. The thyroid gland: a crossroad in inflammation-induced carcinoma? An ongoing debate with new therapeutic potential. Curr Med Chem 2010; 17:3449–3461. [DOI] [PubMed] [Google Scholar]
- 37.Zeng RC, Jin LP, Chen ED, et al. Potential relationship between Hashimoto's thyroiditis and BRAF mutation status in papillary thyroid cancer. Head Neck 2015. [DOI] [PubMed] [Google Scholar]
- 38.Jin L, Sebo TJ, Nakamura N, et al. BRAF mutation analysis in fine needle aspiration (FNA) cytology of the thyroid. Diagn Mol Pathol 2006; 15:136–143. [DOI] [PubMed] [Google Scholar]
- 39.Kebebew E, Weng J, Bauer J, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 2007; 246:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oler G, Ebina KN, Michaluart P, Jr, et al. Investigation of BRAF mutation in a series of papillary thyroid carcinoma and matched-lymph node metastasis reveals a new mutation in metastasis. Clin Endocrinol 2005; 62:509–511. [DOI] [PubMed] [Google Scholar]
- 41.Oler G, Camacho CP, Hojaij FC, et al. Gene expression profiling of papillary thyroid carcinoma identifies transcripts correlated with BRAF mutational status and lymph node metastasis. Clin Cancer Res 2008; 14:4735–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlers M, Schott M. Hashimoto's thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends Endocrinol Metabol 2014; 25:656–664. [DOI] [PubMed] [Google Scholar]
- 43.Cunha LL, Morari EC, Guihen AC, et al. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol 2012; 77:918–925. [DOI] [PubMed] [Google Scholar]


