Abstract
Accumulation of β-amyloid (Aβ) plaques is a pathological hallmark of Alzheimer disease. Aβ levels in animals and adults were reported to be associated with postoperative cognitive dysfunction (POCD). Our goal was to determine the plasma levels of Aβ in infants and young children after cardiac surgery with cardiopulmonary bypass (CPB).
Forty-two infants and young children aged from 1 to 35 months undergoing cardiac surgery with general anesthetics were prospectively enrolled from January to June 2014 at a tertiary medical center. Perioperative plasma samples were obtained, and Aβ42 and Aβ40 levels were measured using ELISA. Other clinical characteristics of the patients were also recorded.
Plasma levels of Aβ42 and Aβ40 decreased dramatically 2 hours after surgery and remained significantly lower 6 hours after operation. Baseline Aβ42 level correlated significantly with surgical intensive care unit (SICU) length of stay (LOS) and was an independent predictor for SICU LOS on multivariate analysis.
Cardiac surgery with CPB decreases plasma Aβ levels. Plasma levels of Aβ42 and Aβ40 might be used as novel biomarkers for predicting outcomes in the patient population.
INTRODUCTION
β-amyloid (Aβ) are peptides of 36 to 43 amino acids and the main component of the amyloid plaque in Alzheimer's disease (AD). It is widely accepted that Aβ oligomers are drivers of neurodegeneration and AD.1 The most common isoforms of Aβ are Aβ42 and Aβ40. They play important roles not only in AD but also in postoperative cognitive dysfunction (POCD).2 POCD is a complication following surgery that is characterized by a decline in cognitive functions such as memory, the ability to concentrate, and information processing. The symptoms of POCD vary among patients, but most complaints involve difficulties with memory, or in handling daily activities at home as well as at work.3 POCD has been associated with a higher risk of increased length of stay (LOS), postdischarge institutionalization, and mortality.4
Many clinical studies showed that POCD is often associated with cardiac surgery.5,6 Newman et al6 reported that the incidence of cognitive decline was 53% at discharge, 36% at 6 weeks, 24% at 6 months, and 42% at 5 years after coronary-artery bypass grafting. The use of cardiopulmonary bypass (CPB) has been described as a major contributor to the high incidence of POCD in this setting.7,8 It has long been assumed that cerebral embolism associated with CPB may account for POCD. Indeed, transcranial Doppler monitoring consistently demonstrates the presence of small particulate or air emboli during cardiac manipulations. In patients undergoing on-pump coronary artery bypass surgery, poor left ventricular function, elevated preoperative creatinine, prolonged ICU stay, and higher educational level have been determined as independent predictors of POCD occurrence.9 Alternatively, there are evidences to support that anesthesia induces POCD. Inhalational anesthetic isoflurane can induce caspase activation and apoptosis, enhance Aβ aggregation, and increase cytotoxicity.10,11 Zhang et al12 found isoflurane was associated with an increase of Aβ40 levels in cerebrospinal fluid (CSF) 24 hours after surgery and desflurane was associated with a decrease in CSF Aβ42 levels 2 hours after the surgery. Several studies using nuclear magnetic resonance (NMR) spectroscopy showed that smaller size anesthetic agents, such as isoflurane and desflurane, may cause greater Aβ oligomerization by interacting with residues on the peptide chain.13 Plasma Aβ42 and Aβ40 have been documented as markers for POCD.14 Therefore, it has been proposed that a combination of surgical trauma and anesthetic insult leads to a primary inflammatory response in the body, which results in neuroinflammation and Aβ accumulation in the CSF due to synaptic impairment. The net effect is an increase in the risk of developing POCD.15
POCD is well studied in adults but has been under-investigated in children. Since the first reported evidence of impaired cognition in children after halothane/nitrous oxide anesthesia,16 less than 10 studies on POCD in children have been published.17 Until now, no study examining Aβ42 and Aβ40 levels in children has been reported yet. In the present study, we hypothesized that Aβ42 and Aβ40 levels were altered after cardiac surgery with CPB in children. The patients in the present study were infants and young children less than 3 years old. Therefore, it was not feasible to assess POCD in this population. The primary endpoint of the study was to determine the effects of heart surgery with CPB on plasma levels of Aβ42 and Aβ40. The secondary endpoint was to study whether Aβ42 and Aβ40 levels were associated with adverse outcomes.
MATERIALS AND METHODS
Patient Population
This prospective study was conducted at the Children's Hospital, Zhejiang University. The protocol was approved by the Medical Ethics Committee of the Children's Hospital, Zhejiang University. Informed consents were obtained from the guardians or legal representatives of the patients before enrollment. Eligible participants were American Society of Anesthesiologists I to III patients aged ranging from 1 to 35 months of who had congenital heart disease requiring CPB under general anesthesia. The exclusion criteria included patients younger than 1 month and older than 36 months; patients born prematurely; patients with abnormal liver, renal function, or major chromosomal abnormalities; patients showing pulmonary inflammation before surgery; patients with pulmonary edema due to cardiac dysfunction and requiring extracorporeal membrane oxygenation support after the operation; and patients refusing to participate in the study.
Anesthesia and Cardiopulmonary Bypass Protocol
All patients were evaluated by standard echocardiography and/or cardiovascular angiography before surgery. The patients were orally intubated in the operating room. Anesthesia was managed according to a standard protocol, including induction with sevoflurane (2–5%) in oxygen, ketamine (1.0–2.0 mg/kg), midazolam (0.10–0.20 mg/kg), fentanyl (2–5 μg/kg), vecuronium (1 mg/kg) and maintenance with fentanyl (15–25 μg/kg) and sevoflurane (1–3%) in oxygen. Neuromuscular blockade was achieved with vecuronium (0.1 mg/kg, once every 60 minutes). The CPB circuit, which was identical for all patients, included a microporous hollow fiber membrane oxygenator (Dideco 901, Dideco, Mirandola, MO, Italy; Medtronic, Inc, Minnea-polis) and a Stockert III roll pump (Stockert Instrumente, Munich, Bavaria, Germany). Before aortic cannulation, 400 to 450 U/kg heparin was administered with the target kaolin-ACT value more than 450 seconds. The bypass circuit was primed with lactated Ringer solution, colloid (20% albumin, plasma 150 mL), mannitol (2.5 mL/kg), packed red blood cells (1.5 U), heparin (1000 IU for Dideco 901; 1250 IU for Medtronic, Inc.), and 5% sodium bicarbonate (5 mL/kg). Pump flow rates ranged from 3.0 to 2.0 L/min/m2. Core temperature was controlled at 30 to 32°C using a heat exchanger in the bypass circuit. At the end of CPB, in order to maintain the fluid balance, the modified ultrafiltration was used to remove the excess fluid in the body according to the hematocrit (maintenance of hematocrit >30%) and the monitored blood pressure (aortic blood pressure: 75–110/50–78 mm Hg; left atrial pressure: 5–12 mm Hg; right atrial pressure: 5–14 mm Hg according to the patient's age and weight).
Weaning From Mechanical Ventilation Protocol
The patients were transferred to the surgical ICU immediately after operation and subjected to mechanical ventilation (MC) using Servo i ventilators (Siemens, Munich, Germany). Patients were weaned from MV when they met the following criteria: stable hemodynamic profile, normal cardiac rhythm, adequate oxygenation on fraction of inspired oxygen ≤0.4, maintenance of pH > 7.35 and PaCO2 < 45 mm Hg, the level of consciousness consistent with adequate airway protective reflexes, absence of accessory respiratory muscle recruitment, and approval by the attending cardiac intensivists.
Data Collection and Definitions
Demographic and operative data were collected, including the age at surgery, weight, gender, Risk Adjusted Classification for Congenital Heart Surgery (RACHS-1), duration of CPB, aortic cross-clamp time, duration of MV, and the ratio of arterial oxygen pressure to the fraction of inspired oxygen (PaO2/FiO2). Additionally, an inotrope score was calculated at 24 hours following CPB. Furthermore, all patients were followed to determine surgical intensive care unit (SICU) LOS. No patient was lost during SICU observation.
Plasma Aβ40 and Aβ42 Measurement
For each patient, 1 mL of fresh blood was drawn into a vacuum tube containing EDTA at preoperation and at 0, 2, 6, 12, 24, 48, and 72 hours postoperation. After centrifugation at 3000 rpm for 5 minutes at 4°C, the plasma was divided into aliquots and frozen at −80°C until assay. Plasma Aβ levels were measured via commercial Aβ40 and Aβ42 ELISA kits (Invitrogen, Camarillo, CA) according to the manufacturer's instructions.
Statistical Analysis
Variables were presented as mean values and standard deviations if normally distributed, and otherwise as median values and interquartile ranges. Continuous data were compared using 1-way analysis of variants (ANOVA) or Kruskal–Wallis ANOVA with Dunn post hoc test as indicated. A Pearson correlation test was performed to determine the correlation between continuous data, and Spearman correlation test for MV time. Associations were determined using univariable analysis. Variables associated with postoperative SICU LOS at a P-value ≤0.15 were then included in a list of potential independent risk factors for multivariable linear regression analysis. All statistical analyses were performed using SPSS (SPSS 16.0 for Windows; SPSS, Chicago, IL). A P-value <0.05 was considered statistically significant.
RESULTS
Participants
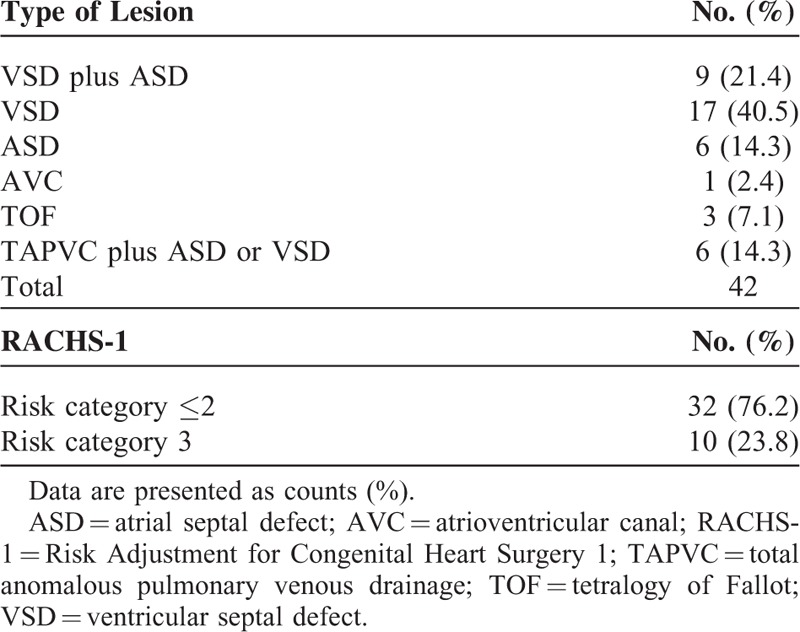
Forty-two infants and children younger than 3 years who underwent cardiac surgery with CPB were enrolled into the study. Demographic and operative data are shown in Table 1. Table 2 lists types of cardiac lesion and RACHS-1. The study procedures were well tolerated. All patients survived and were discharged.
TABLE 1.
Demographic and Operative Data of the Patients
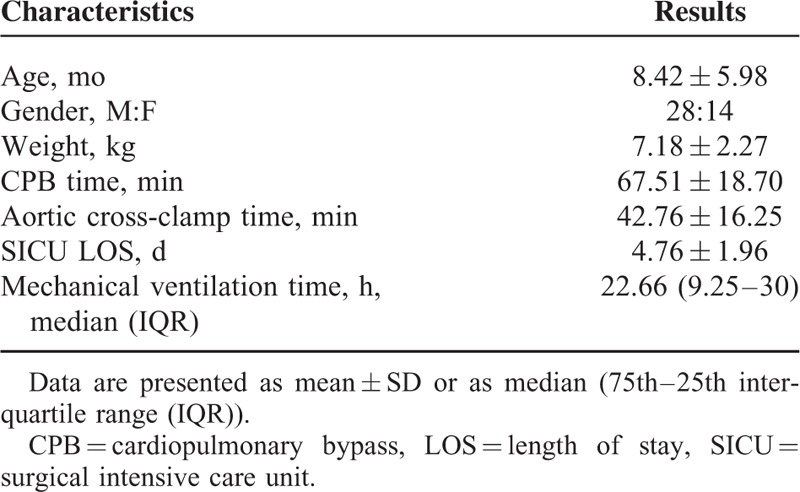
TABLE 2.
Cardiac Disease Classification and Corresponding Complexity of the Surgery
Plasma Aβ42 and Aβ40 Levels After Surgery
We first assessed the effects of cardiac surgery with CPB on plasma levels of Aβ42 and Aβ40 at baseline and at 0, 2, 6, 12, 24, 48 and 72 hours postoperation (Table 3). As shown in Figure 1, Aβ42 levels were significantly decreased at 2 hours postoperation as compared to baseline (3.49 ± 3.00 pg/mL vs 9.90 ± 7.78 pg/mL; P < 0.001) and remained significantly lower at 6 hours postoperation (5.07 ± 4.94 pg/mL; P < 0.01) (Figure 1A). Similarly, cardiac surgery with CPB resulted in a decrease over time in Aβ40 levels. Aβ40 levels were significantly reduced at 2 hours after surgery as compared to baseline (50.04 ± 37.18 pg/mL vs 109.14 ± 74.94 pg/mL; P < 0.001) and persisted at a lower levels at 6 hours after operation (67.65 ± 50.97 pg/mL; P < 0.01) (Table 3, Figure 1B). These findings demonstrate that cardiac surgery with CPB decreased the plasma Aβ levels at 2 and 6 hours after the surgery.
TABLE 3.
Perioperative PaO2/FiO2, plasma lactate and plasma Aβ42 and Aβ40 expression
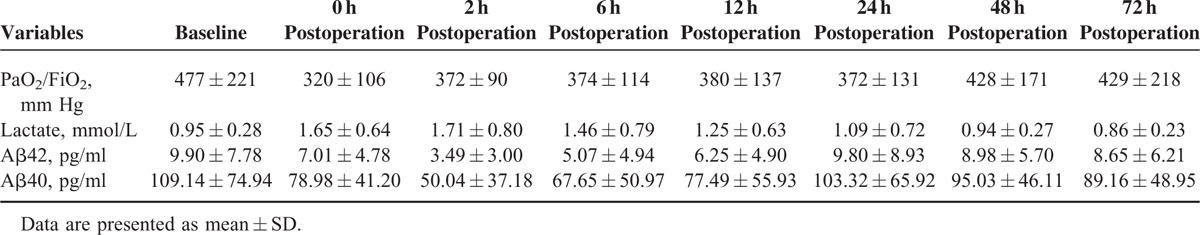
FIGURE 1.
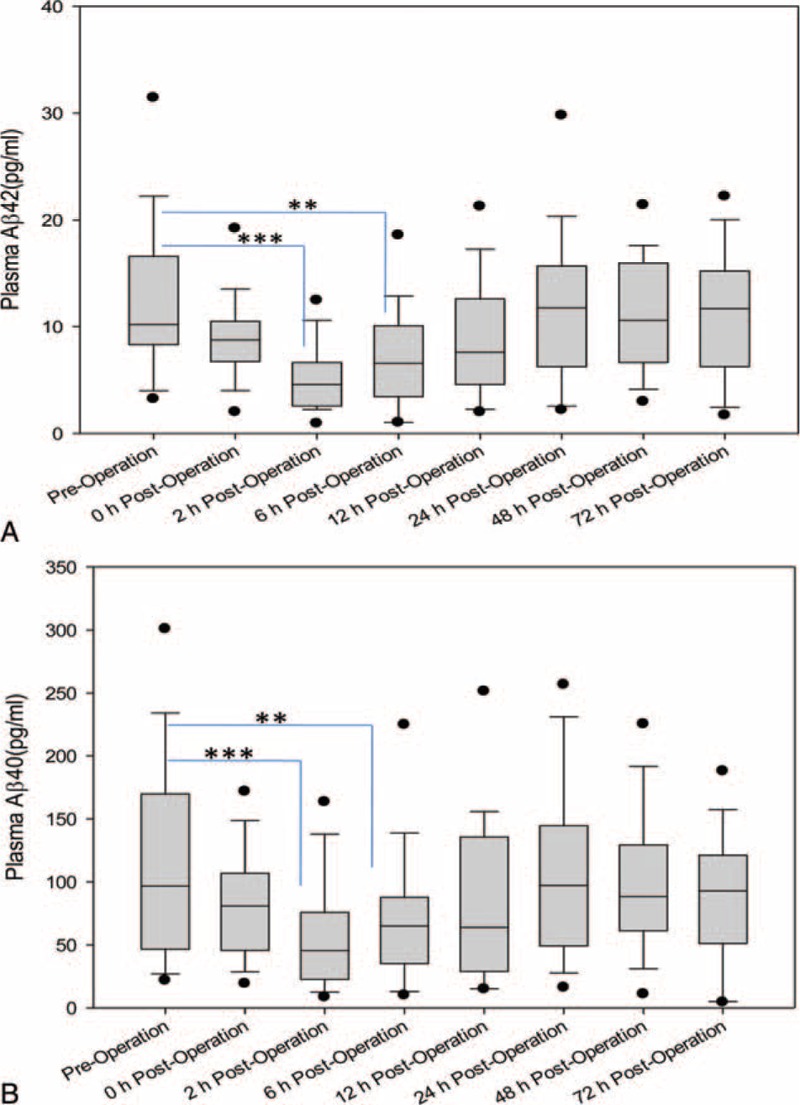
(A) Box-and-whisker plot of plasma Aβ42 levels following cardiac surgery with CPB. Distribution of Aβ42 levels among 42 patients at preoperation, 0, 2, 6, 12, 24, 48, and 72 hours postoperation. Box plots demonstrate median with interquartile ranges; error bars indicate 10th to 90th percentile values; and black dots show maximum and minimum levels. (B) Box-and-whisker plot of plasma Aβ40 levels following cardiac surgery with CPB. Distribution of 40 levels among 42 patients at preoperation, 0, 2, 6, 12, 24, 48, and 72 hours postoperation. Box plots demonstrate median with interquartile ranges; error bars indicate 10th to 90th percentile values; and black dots show maximum and minimum levels.
Correlation Between Baseline Aβ Levels and Clinical Parameters
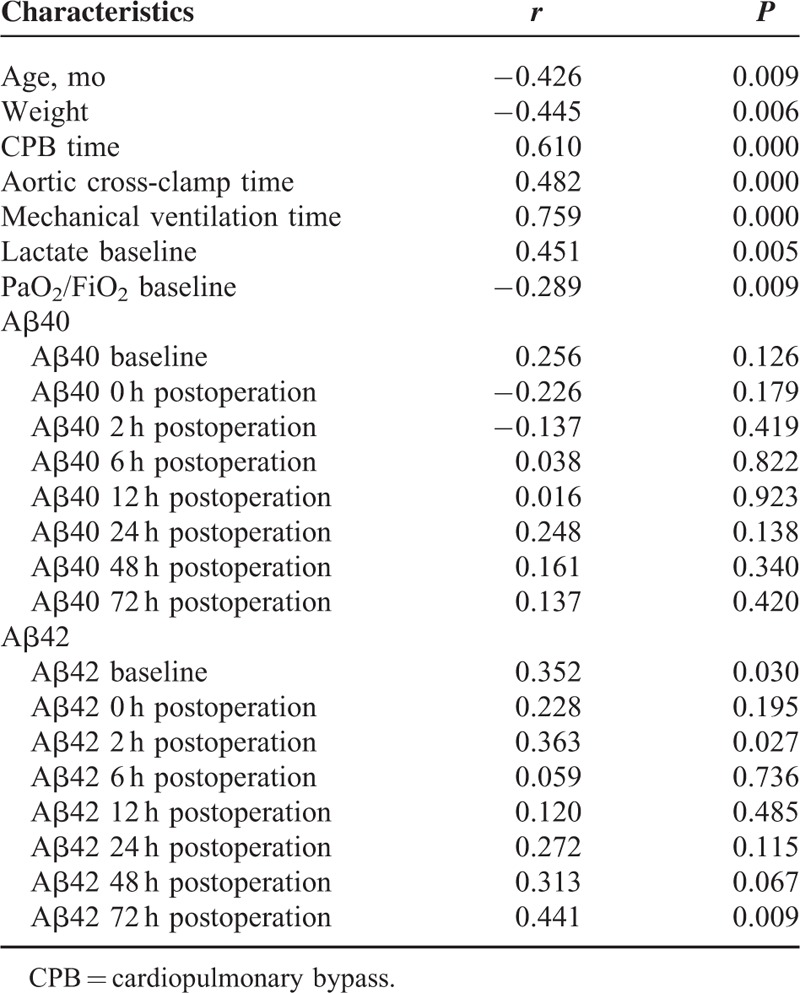
Since Aβ levels at baseline were reported to be correlated with clinical outcomes in adult patients with cardiac surgery,18 an univariate correlation analysis was performed between Aβ42/40 levels and clinical parameters including CPB time, aortic clamp time, MV time, age, weight, PaO2/FiO2 and SICU LOS. There was a significant correlation between Aβ42 level at baseline and MV time (r = 0.372, P = 0.022) as well as Aβ42 level immediately after surgery and MV time (r = 0.365, P = 0.026). There were also significant correlations between Aβ42 level at baseline and PaO2/FiO2 6 hours (r = −0.378, P = 0.019), 12 hours (r = −0.339, P = 0.037), as well as 24 hours (r = −0.330, P = 0.043) postoperation. At 2 hours postoperation, there was a significant correlation between Aβ42 level and the SICU LOS (r = 0.363, P = 0.027). However, Aβ40 level at baseline was only significantly correlated with PaO2/FiO2 (r = −0.336, P = 0.034) at 6 hours postoperation.
POCD after surgery has been associated with prolonged hospital LOS.19 Due to young age of the study patients, determination of POCD was not possible. Therefore, we sought to determine the association between SICU LOS and other clinical parameters using univariate correlation analysis. These parameters included age, weight, CPB time, aortic cross-clamp time, MV time, lactate baseline, PaO2/FiO2 ratio, Aβ40, and Aβ42 levels during the operation. There was a positive correlation between the SICU LOS and baseline Aβ42 levels (r = 0.352, P = 0.030) (Table 4). Other factors associated with SICU LOS included CPB time, aortic clamp time, MV, age, and weight, which has been documented in the literature.20
TABLE 4.
Factors Associated With Increased SICU LOS by Univariate Analysis
Independent Prognostic Values of Certain Factors
We also performed multivariate regression analysis to determine independent factors associated with increased SICU LOS (Table 4). All variables with a P ≤ 0.15 on univariate regression were included into the subsequent multivariate regression analysis. CPB time (P = 0.001), Aβ42 at baseline (P = 0.003), MV time (P = 0.005), and PaO2/FiO2 baseline (P = 0.020) were independent predictors of prolonged SICU LOS (Table 5). CPB time, MV time, and PaO2/FiO2 have been reported as predictors of long SICU LOS following cardiac surgery in children.21 Therefore, plasma levels of Aβ42 at baseline may serve as a new predictor of SICU LOS.
TABLE 5.
Potential Independent Risk Factors With SICU LOS by Stepwise Multiple Linear Regression Analysis

DISCUSSION
To our knowledge, this is the first report that cardiac surgery with CPB results in a rapid and significant decrease in plasma Aβ42 and Aβ40 levels in infants and young children at 2 and 6 hours postoperation. In addition, baseline Aβ42 level is an independent predicator for prolonged SICU LOS after surgery. These findings suggest POCD also occurs in these young patients as documented in the adult population.22
The reduction in Aβ42 and Aβ40 levels may result from the accumulation of Aβ peptide in the brain through the damaged blood–brain barrier (BBB) during and immediate after surgery with general anesthesia. In the present study, sevoflurane was used for both induction and maintenance phase of anesthesia. Sevoflurane recently has been shown to induce structural changes in brain vascular endothelial cells and increase BBB permeability.23 MMP-2 and 9 have also been demonstrated to increase the permeability of BBB by disrupting tight junction proteins in BBB.24 In an animal study with rats, surgery increased MMP-2 and MMP-9 protein expression and BBB permeability as evidenced by Evans blue leakage into the hippocampus. Furthermore, sevoflurane inhalation potentiated the effect of surgery on BBB.25 In patients, MRI-detected BBB disruption was reported after cardiac surgery.26 In previous studies, both cardiac surgery and anesthetics were demonstrated to increase Aβ levels in CSF.12,27 Unfortunately, the Aβ levels in CSF were not examined due to difficulties in obtaining the informed consent for lumbar puncture in the study population.
It has been demonstrated that the receptor for advanced glycation end-products (RAGE) mediated Aβ transport across the BBB and accumulation in the brain. In mice lacking RAGE expression, peripheral Aβ was not transported into the brain.28 RAGE was recognized as a receptor involved in Aβ-induced neuronal dysfunction.29 In children undergoing cardiac surgery necessitating CPB, our group showed that plasma soluble RAGE was immediately increased after surgery and enables prediction of acute lung injury.30 In adults under the same procedure, plasma soluble RAGE levels were increased significantly 2 hours postoperation and associated with prolonged LOS.31 In the present study, the reduction in plasma Aβ42 and Aβ40 levels occurred at 2 and 6 hours postoperation. Our results demonstrated that an increase in soluble RAGE levels is accompanied by a decrease in Aβ levels. These findings are consistent with the reports that RAGE is responsible for the Aβ transport to the brain.
We found that baseline Aβ42 but not Aβ40 has a predicative value for SICU LOS. It has been reported recently that CSF Aβ42, not Aβ40, predicts early-onset dementia in Parkinson disease.32 Aβ42 has an identical amino acid sequence with Aβ40, except for additional 2 amino acids at the C terminus. Aβ42 constitutes only 10% of total Aβ in the plasma.33 However, Aβ42 is a major component of senile plaques and cerebrovascular amyloid deposits.34 In vitro, Aβ42 solution forms soluble oligomers rapidly, whereas oligomerization of Aβ40 solution requires prolonged incubation. Furthermore, Aβ42 solution is more toxic to cultured human neuroblastoma SH-SY5Y cells than that of Aβ40.35 There are also differential changes in Aβ42 and Aβ40 with age. Insoluble Aβ42 in the brain increased progressively with age which helps to explain the occurrence of AD in the senior population.36
The mechanisms for POCD after cardiac surgery are not well understood. Some believe POCD is the result of cerebral inflammation caused by neuronal injuries and/or systemic inflammation. Biomarkers of neuronal injury such as neuron specific enolase and S100B have been correlated with POCD after cardiac surgery with CPB.37,38 Others propose that the underlying mechanisms may be similar to that of cognitive impairment in AD, which are believed to result from the accumulation of Aβ in the brain. It has been reported that plasma Aβ levels increase with age and are positively associated with cognitive impairment or AD.39 Preoperative plasma levels of Aβ42 and Aβ40 are associated with early POCD after cardiac surgery.18 Furthermore, cardiac surgery with CPB may induce increased postoperative Aβ levels in CSF.27 The present study showed that plasma Aβ levels were decreased immediately after surgery, which may result from the accumulation of Aβ in the brain. The increased Aβ levels in the brain might lead to POCD and prolonged SICU LOS. Indeed, we found plasma level of Aβ42 at baseline is an independent predictor of SICU LOS. Therefore, POCD may also be present in infants and young children after cardiac surgery with CPB.
Our study does have several limitations. First, we were unable to assess postoperative changes in cognitive function due to the young age of the patients. Therefore, the relationship between plasma Aβ levels and POCD development was not examined. Second, although plasma Aβ42 and Aβ40 levels before and after surgery were examined, the corresponding Aβ42 and Aβ40 levels in CSF were not ascertained due to the difficulties in obtaining informed consent in most of the study patients. Despite these limitations, our results support that levels of plasma Aβ42 and Aβ40 levels are decreased immediately following cardiac surgery with CPB. In addition, baseline Aβ42 levels might be an important biomarker for predicting outcomes following cardiac surgery with CPB. Further studies pertaining to the role of Aβ levels and POCD in older children following cardiac surgery with CPB are required.
Acknowledgments
We would like to thank Cheng Ding in the statistics department at Medical school, Zhejiang University for his assistance with this project.
Footnotes
Abbreviations: Aβ = β-amyloid, AD = Alzheimer disease, BBB = blood–brain barrier, CPB = cardiopulmonary bypass, CSF = cerebrospinal fluid, LOS = length of stay, MV = mechanical ventilation, NMR = nuclear magnetic resonance, PaO2/FiO2 = the ratio of arterial oxygen pressure to the fraction of inspired oxygen = POCD, postoperative cognitive dysfunction, RACHS-1 = Risk Adjusted Classification for Congenital Heart Surgery, RAGE = receptor for advanced glycation end-products, SICU = surgical intensive care unit.
This work was supported by Zhejiang Science and Technology Plan (2013C33147), the Zhejiang Health Bureau Plan (2013KYB154) to YH, the National Natural Science Foundation of China (81272139), and the National Science and Technology Support Program (2012BAI04B05) to QS.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci 2012; 15:349–357. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Dong Y, Wang H, et al. Age-dependent postoperative cognitive impairment and Alzheimer-related neuropathology in mice. Sci Rep 2014; 4:3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care 2011; 17:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009; 249:173–178. [DOI] [PubMed] [Google Scholar]
- 5.van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia 2012; 67:280–293. [DOI] [PubMed] [Google Scholar]
- 6.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001; 344:395–402. [DOI] [PubMed] [Google Scholar]
- 7.Selnes OA, McKhann GM. Neurocognitive complications after coronary artery bypass surgery. Ann Neurol 2005; 57:615–621. [DOI] [PubMed] [Google Scholar]
- 8.Gao L, Taha R, Gauvin D, et al. Postoperative cognitive dysfunction after cardiac surgery. Chest 2005; 128:3664–3670. [DOI] [PubMed] [Google Scholar]
- 9.Boodhwani M, Rubens FD, Wozny D, et al. Predictors of early neurocognitive deficits in low-risk patients undergoing on-pump coronary artery bypass surgery. Circulation 2006; 114 (Suppl. 1):I461–I466. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Culley DJ, Dong Y, et al. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol 2008; 64:618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology 2004; 101:703–709. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Tian M, Zheng H, et al. Effects of anesthetic isoflurane and desflurane on human cerebrospinal fluid Abeta and tau level. Anesthesiology 2013; 119:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal PK, Fodale V. Isoflurane and desflurane at clinically relevant concentrations induce amyloid beta-peptide oligomerization: an NMR study. Biochem Biophys Res Commun 2009; 379:716–720. [DOI] [PubMed] [Google Scholar]
- 14.Seppala TT, Herukka SK, Hanninen T, et al. Plasma Abeta42 and Abeta40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry 2010; 81:1123–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapila AK, Watts HR, Wang T, et al. The impact of surgery and anesthesia on post-operative cognitive decline and Alzheimer's disease development: biomarkers and preventive strategies. J Alzheimers Dis 2014; 41:1–13. [DOI] [PubMed] [Google Scholar]
- 16.Morgan SF, Furman EB, Dikmen S. Psychological effects of general anesthesia on five- to eight-year-old children. Anesthesiology 1981; 55:386–391. [DOI] [PubMed] [Google Scholar]
- 17.Millar K, Bowman AW, Burns D, et al. Children's cognitive recovery after day-case general anesthesia: a randomized trial of propofol or isoflurane for dental procedures. Paediatr Anaesth 2014; 24:201–207. [DOI] [PubMed] [Google Scholar]
- 18.Evered LA, Silbert BS, Scott DA, et al. Plasma amyloid beta42 and amyloid beta40 levels are associated with early cognitive dysfunction after cardiac surgery. Ann Thoracic Surg 2009; 88:1426–1432. [DOI] [PubMed] [Google Scholar]
- 19.Silbert BS, Scott DA, Evered LA, et al. A comparison of the effect of high- and low-dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology 2006; 104:1137–1145. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie M, Kuijpers M, Van Rossem M, et al. Determinants of intensive care unit length of stay for infants undergoing cardiac surgery. Congenit Heart Dis 2006; 1:152–160. [DOI] [PubMed] [Google Scholar]
- 21.Pagowska-Klimek I, Pychynska-Pokorska M, Krajewski W, et al. Predictors of long intensive care unit stay following cardiac surgery in children. Eur J Cardiothorac Surg 2011; 40:179–184. [DOI] [PubMed] [Google Scholar]
- 22.Goto T, Maekawa K. Cerebral dysfunction after coronary artery bypass surgery. J Anesth 2014; 28:242–248. [DOI] [PubMed] [Google Scholar]
- 23.Acharya NK, Goldwaser EL, Forsberg MM, et al. Sevoflurane and Isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res 2015; 1620:29–41. [DOI] [PubMed] [Google Scholar]
- 24.Feng S, Cen J, Huang Y, et al. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS ONE 2011; 6:e20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu N, Guo D, Wang H, et al. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res 2014; 1551:13–24. [DOI] [PubMed] [Google Scholar]
- 26.Merino JG, Latour LL, Tso A, et al. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol 2013; 34:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinsfelt B, Westerlind A, Blennow K, et al. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid beta. Acta Anaesthesiol Scand 2013; 57:82–88. [DOI] [PubMed] [Google Scholar]
- 28.Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003; 9:907–913. [DOI] [PubMed] [Google Scholar]
- 29.Origlia N, Righi M, Capsoni S, et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci 2008; 28:3521–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Chen Q, Shi S, et al. Plasma sRAGE enables prediction of acute lung injury after cardiac surgery in children. Crit Care 2012; 16:R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creagh-Brown BC, Quinlan GJ, Hector LR, et al. Association between preoperative plasma sRAGE levels and recovery from cardiac surgery. Mediators Inflamm 2013; 2013:496031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alves G, Lange J, Blennow K, et al. CSF Abeta42 predicts early-onset dementia in Parkinson disease. Neurology 2014; 82:1784–1790. [DOI] [PubMed] [Google Scholar]
- 33.Pomara N, Doraiswamy PM, Willoughby LM, et al. Elevation in plasma Abeta42 in geriatric depression: a pilot study. Neurochem Res 2006; 31:341–349. [DOI] [PubMed] [Google Scholar]
- 34.Roher AE, Lowenson JD, Clarke S, et al. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA 1993; 90:10836–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Agnaf OM, Mahil DS, Patel BP, et al. Oligomerization and toxicity of beta-amyloid-42 implicated in Alzheimer's disease. Biochem Biophys Res Commun 2000; 273:1003–1007. [DOI] [PubMed] [Google Scholar]
- 36.Miners JS, Jones R, Love S. Differential changes in Abeta42 and Abeta40 with age. J Alzheimers Dis 2014; 40:727–735. [DOI] [PubMed] [Google Scholar]
- 37.Grocott HP, Mackensen GB. Apolipoprotein E genotype and S100beta after cardiac surgery: is inflammation the link? Anesth Analg 2005; 100:1869–1870.author reply 1870. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen LS, Christiansen M, Hansen PB, et al. Do blood levels of neuron-specific enolase and S-100 protein reflect cognitive dysfunction after coronary artery bypass? Acta Anaesthesiol Scand 1999; 43:495–500. [DOI] [PubMed] [Google Scholar]
- 39.Fukumoto H, Tennis M, Locascio JJ, et al. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol 2003; 60:958–964. [DOI] [PubMed] [Google Scholar]


