Abstract
Otitis media (OM) has numerous presentations in children. Together with conventional medical therapies aimed to prevent and/or treat OM, a rising number of complementary and alternative medicine (CAM) treatment options can be offered. Since OM is common in children, parents may ask healthcare professionals about possible CAM therapies. Many physicians feel that their knowledge is limited regarding these therapies, and that they desire some information. Therefore, we conducted a literature review of CAM therapies for OM, taking into account that many of these treatments, their validity and efficacy and have not been scientifically demonstrated.
We performed a search in MEDLINE (accessed via PubMed) using the following terms: “CAM” in conjunction with “OM” and “children. Retrieved publications regarding treatment of OM in children which included these terms included randomized controlled trials, prospective/retrospective studies, and case studies.
The following CAM options for OM treatment in children were considered: acupuncture, homeopathy, herbal medicine/phytotherapy, osteopathy, chiropractic, xylitol, ear candling, vitamin D supplement, and systemic and topical probiotics. We reviewed each treatment and described the level of scientific evidence of the relevant publications.
The therapeutic approaches commonly associated with CAM are usually conservative, and do not include drugs or surgery. Currently, CAM is not considered by physicians a potential treatment of OM, as there is limited supporting evidence. Further studies are warranted in order to evaluate the potential value of CAM therapies for OM.
INTRODUCTION
Alternative medicine is any practice claiming to possess the healing effects of conventional medicine, but does not originate from evidence-based scientific methods.1 It consists of a range of healthcare practices, products, and therapies, ranging from being biologically plausible but not scientifically tested, to being directly contradicted by evidence, or even harmful or toxic. Complementary medicine is an alternative medicine used in conjunction with conventional medicine, in a belief that it may be synergistic.2
Complementary and alternative medicine (CAM) is popular worldwide. Expenditure on CAM experts visits and therapies in children is constantly growing.3 The main reasons for choosing CAM therapies are that CAM attempts to provide a personalized approach to the sick child, parents’ disappointment with conventional medicine, personal or professional recommendations, and parents’ previous experience. Despite significant expenditures on testing CAM, including $124 million spent by the U.S. Government in 2014,4 <5% of therapies were tested in children. Only few have shown effectiveness, leading physicians to question their efficacy.
Otitis media (OM) includes a spectrum of diseases, which range from middle ear fluid collection (OM with effusion, OME), to purulent fluid behind the tympanic membrane (acute otitis media, AOM) and recurrent AOM (RAOM). Many countries published different guidelines for OM treatment. Currently, CAM therapies are either ignored or discouraged those guidelines, even in countries where CAM is popular.5,6
Due to OM high prevalence, physicians may be asked their opinion regarding CAM therapies for pediatric OM, but they may often feel uncomfortable advising parents due to lack of knowledge. In order to fill the knowledge gap, we sought to review scientifically the knowledge gained regarding CAM therapies for pediatric OM.
METHODS
We used the terms “complementary medicine” and/or “alternative medicine” in conjunction with “otitis media” and “children” in our MEDLINE search (accessed via PubMed), and The Cochrane Library, from January 1980 to September 2015. Randomized controlled trials (RCTs), prospective/retrospective studies, and case reports in the English language reporting on any CAM treatment in the context of pediatric OM were included. If the article was not published in English, we relied on the abstract as it appeared in the search engine. No authors declared conflict of interest. Ethics committee approval was not requested because it is not needed for systematic reviews of the literature according to the Israeli and Italian laws.
RESULTS
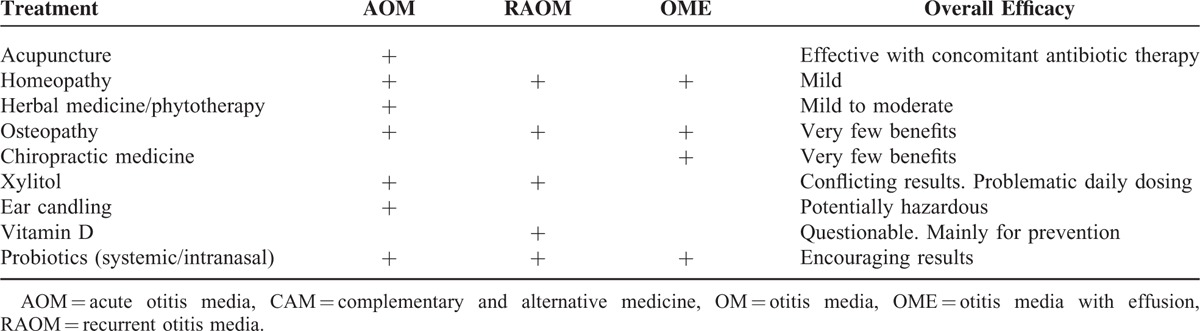
Table 1 summarizes the evidence gathered regarding the efficacy of different CAM treatment options for OM in children.
TABLE 1.
Reported CAM Treatment Options for OM, According to OM Type
ACUPUNCTURE
Acupuncture (needle puncturing) derives from traditional Chinese medicine and involves inserting thin needles into the body at specific points.2 According to acupuncture, the body's energy force, chi (qi), differentiates a corpse from a live human being. Acupuncture balances and enhances chi to bring the body into a healthy state. The auricle harbors numerous locations which are punctured for the treatment of many diseases. Yet, 4 specific locations around the external canal are believed to be the primary gatekeepers of the ear's energy, and they are punctured in OM cases (Figure 1). There is little understanding why acupuncture may be beneficial, but it is suggested that it has immunomodulatory properties that may play a role in clearance of middle ear fluid.7
FIGURE 1.
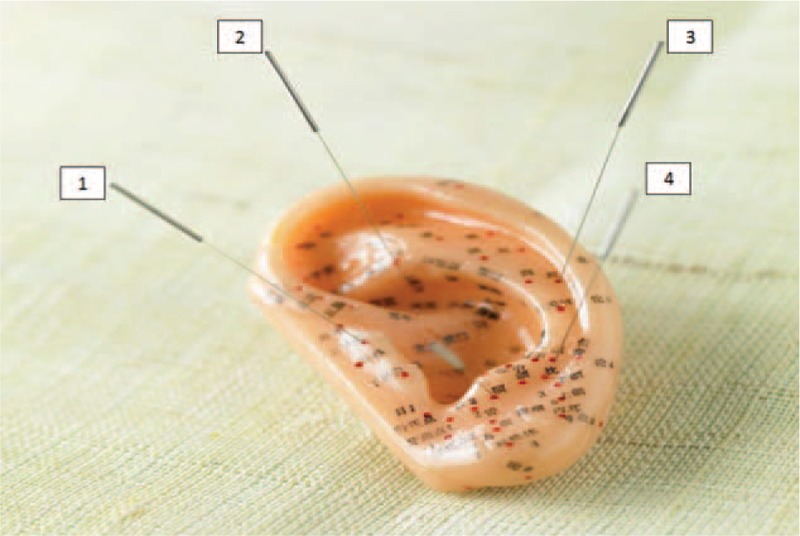
Key locations for relieving ear pain in otitis media (OM) in an ear model: (1) tragus, (2) base of the triangular fossa, (3) mid-helix, and (4) antitragus.
Few studies concerning acupuncture for the treatment of OM in humans have only been published, but not in English.8–10 In 2 studies in canines, acupuncture was evaluated for the treatment of recurrent otitis episodes. At first, animals with otitis were randomized to receive conventional therapy, and either sham acupuncture or “directed” acupuncture. Over the subsequent year, majority of the dogs in the acupuncture group were otitis-free.11 The same authors reported that acupuncture may enhance the effect of antibiotic treatment for otitis in dogs.12 However, since the reported type of otitis is unclear, and given that otitis externa usually affects canines, these conclusions may not be attributable to humans.
HOMEOPATHY
Homeopathy is based on the “like cures like” (similia similibus curentur) doctrine: a substance that causes the symptoms of a disease in healthy people would cure similar symptoms in sick people.1 Homeopaths generally prescribe remedies that have a “symptom picture,” which they consider most closely equates to the constellation of the patient's symptoms.
Most remedies combine an extract of a natural substance, combined with a synthetic compound, which enhances the therapeutic effect. A list of homeopathic remedies for OM treatment is shown in Table 2.
TABLE 2.
Common Homeopathic Remedies for Otitis Media

Research into the effects of homeopathic treatment for OM is scant, and its quality is limited. The first prospective cohort study comparing the use of homeopathy for RAOM with conventional treatment was reported in 1997: 71% children from the homeopathic group had fewer OM episodes, whereas 57% of the conventional group received treatment for OM.13 The unequal numbers between the homeopathic (103) and conventional group,14 and the absence of randomization considerably weakened the study's validity. Two subsequent RCTs also showed promising results. The 1st compared homeopathic and placebo for AOM in 75 children from Seattle who presented with otalgia and tympanic membrane bulging of ≤36 hours duration. A significant decrease in symptoms at 24 and 64 hours after treatment were observed in the homeopathy group, and there were fewer treatment failures in this group after 5 days, 2 weeks, and 6 weeks, but they were not statistically significant.15 In another study from Jaipur, India, 81 young children with AOM were randomly assigned to conventional (antipyretics, analgesics) and homeopathy treatment groups. Nearly all children in the conventional group eventually required antibiotics, compared to none in the homeopathy group. The number of children experiencing “cure” suggested that early homeopathic treatment could have advantages beyond a “watch and wait” policy.16 The results of these trials are promising.
In an RCT which compared homeopathic and conventional treatment in 33 children diagnosed with OME, 75% in the homeopathic group had a normal tympanogram after 12 months, compared to 31% in the conventional group. A higher proportion of children receiving homeopathic treatment had a hearing loss <20 dB at follow-up, though the difference was not statistically significant. In another prospective observational study of 230 children receiving homeopathic treatment for AOM, pain control was achieved in ∼40% of patients after 6 hours, and in further 33% of patients after 12 hours.17 The rate of AOM resolution in the homeopathic group was 2.4 times faster, without complications.
According to homeopathy, there is an “effectiveness gap” in the conventional approach for OM. Thus, it is argued that homeopathy should be integrated into the treatment strategy for OM.18 Nevertheless, other authors who published in esteemed journals considered homeopathy to be no more effective than placebo, and essentially dismissed the need for further RCTs.19,20
Herbal Medicine/Phytotherapy
Herbal medicine and homeopathy are interchangeable practiced together and sometimes confused. Herbal medicine is the use of plants for medicinal purposes.1 Herbal products are generally considered as safe, though efficacy is unclear and side effects may vary.
Phytotherapy is the study of the use of extracts of natural origin as medicines or health-promoting agents. Although standard pharmacology isolates an active compound from a given plant, phytotherapy aims to preserve the complexity of substances from a given plant. Phytotherapy avoids mixing plant ingredients with synthetic substances.
Phytotherapy has been reported to be effective in the management of ear pain in OM. Otic solutions, such as Otikon (Healthy-On, Israel), which contains extracts of garlic bulb, mullein flower, calendula flower and St. John's wort herb in olive oil, or Mullein Garlic (Equinox Botanicals, Rutland, OH, USA), which contains extract of mullein flowers, garlic, yarrow, calendula flowers, and vitamin E, were shown to be as effective as oral amoxicillin and topical anesthetics due to their presumed antimicrobial, antiinflammatory, immunostimulating effects, and good penetration through the tympanic membrane.21,22 Yet, phytotherapy has been heavily criticized by others, since the alleged antiinflammatory properties could not be tested or confirmed in vitro.23,24
OSTEOPATHY
Osteopathy is a noninvasive manual medicine that focuses on total body health by treating and strengthening the musculoskeletal framework.1 Its aim is to positively affect the body's nervous, circulatory, and lymphatic systems, leading to “’balance” and providing overall good health and well-being.
Osteopathic manipulative treatments (OMTs) are occasionally used for acute and recurrent cases of OM. The 2 most common OMTs for OM include: “Galbreath” maneuver, a movement of the mandible aimed to indirectly generate a pumping action on the Eustachian tube (ET);25 and “Muncie” and “modified Muncie” techniques, the placement of a fingertip on the Rosenmuller's fossa to open the ET.26
In the largest study so far, combinations of OMTs with antibiotics decreased the frequency of AOM episodes and the insertion of tympanostomy tubes in otitis-prone children, when compared to antibiotics without OMTs.27 Children who received weekly treatments had fewer episodes of AOM (P = 0.04), and fewer required tympanostomy tubes (P = 0.03). Yet, there were no differences in the overall antibiotic use, tympanometry measurements, behavioral parameters, and hearing results between both groups. When considering the large drop-out rate (∼25%), these conclusions are questionable. Other studies have shown that OMTs administered adjunctively with standard care for children with AOM resulted in faster resolution of middle ear effusion following AOM, there are no serious adverse effects, and that OMTs may change the progression of recurrent OM cases.14,28–30 The methodology of these studies is lacking; the study groups were small with high drop-out rates and lacked a control group.
Chiropratic
Chiropractic focuses on the diagnosis and treatment of mechanical disorders of the musculoskeletal system, especially the spine. Chiropractic medicine believes that disorders of the musculoskeletal system affect the general health, via the nervous system.2 The main techniques involve manipulations of the spine, joints, and soft tissues.
It is hypothesized that spinal manipulation therapy (SMT) mediates changes in the sympathetic and parasympathetic neural activity via the biomechanical changes produced in the spine during treatment. Another hypothesis suggests that cervical SMT reduces tension within hypertonic muscles, increasing both lymphatic drainage and ET opening.31 Chiropractic is thought to prevent recurrent infections by correcting “misalignments,” and allowing fluid drainage from the middle ear (illustrating maneuvers are shown in Figure 2).
FIGURE 2.

Common chiropractic maneuvers for otitis media (OM).
A systematic report found only a limited quality of evidence for SMT use in children with OM.31 Although there were no serious adverse effects of SMT, there was no clear evidence to support using SMT.
Xylitol
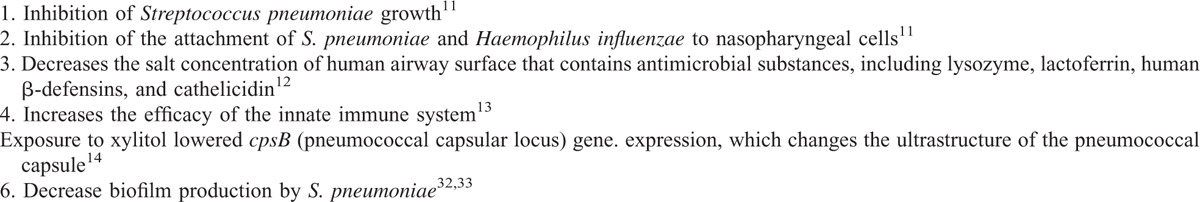
Xylitol is a 5-carbon sugar alcohol, which is naturally found in low concentrations in the fibers of fruits and vegetables. Fair evidence found that xylitol reduced the incidence of AOM episodes in healthy children.1 The alleged properties of xylitol to prevent OM are summarized in Table 3.
TABLE 3.
Protective Characteristics of Xylitol
In 1996, Uhari first published an RCT, in which xylitol reduced AOM occurrence by 41%, and fewer children receiving xylitol required antibiotics.34 Later, the same group showed that xylitol was effective in AOM prevention among daycare toddlers.35 Although ≥1 AOM episode(s) was observed in 41% of the children who received control syrup, only 29% of the children who received xylitol had AOM episode(s) (30% decrease, 95% confidence interval [CI] 4.6–55.4). AOM incidence decreased by 40% compared with control subjects in the children who received xylitol chewing gum, and by 20% in the lozenges group.
These encouraging results initiated additional studies. In RCTs in which different xylitol remedies were used yielded less convincing results. Xylitol was shown to be ineffective in children with indwelling tympanostomy tubes. When xylitol mixture, control mixture, control chewing gum, xylitol chewing gum, and xylitol lozenges were given during an active upper respiratory tract infection (URTI), there was no preventive effect for any of the xylitol mixtures.36 Recent Cochrane review examined the evidence gathered for the use of xylitol in preventing recurrent OM, and found 4 RCT studies that met the criteria for analysis.37 Overall, it demonstrated a statistically significant reduction (25%) in the risk of occurrence of OM among healthy children in the xylitol group, compared with the control placebo group (relative risk [RR] 0.75; 95% CI: 0.65–0.88; 95% CI: −0.12 to −0.03). Chewing gum and lozenges containing xylitol appeared to be more effective than syrup; however, it emphasized that children <2 years who are at the greatest risk of developing OM cannot safely use lozenges or chewing gum. Most studies report a 5 times-per-day dosing schedule, which lowers the compliance in most children. Concomitant increase of quantity in each dose reduced the number of xylitol doses to 3-times-per-day, but resulted in various side effects.
A recent National Institute of Health-funded study examined if viscous xylitol solution at a dose of 5 g 3-times-per-day could reduce the occurrence of clinically diagnosed AOM among otitis-prone children 6 months through 5 years.32 Unfortunately, the results were discouraging, as there were no significant differences in the occurrence of AOM and total antibiotic use between the xylitol group versus the placebo group. Therefore, the use of xylitol was not opted by many national guidelines as a means to prevent OM.
Ear Candling
Ear candling, also known as ear coning or thermal-auricular therapy, consists of placing a hollow candle in the ear canal and lighting the other end33 (Figure 3). Ear coning has its roots in the traditional healing practices of China, Greece, Egypt, Tibet, and North America.
FIGURE 3.
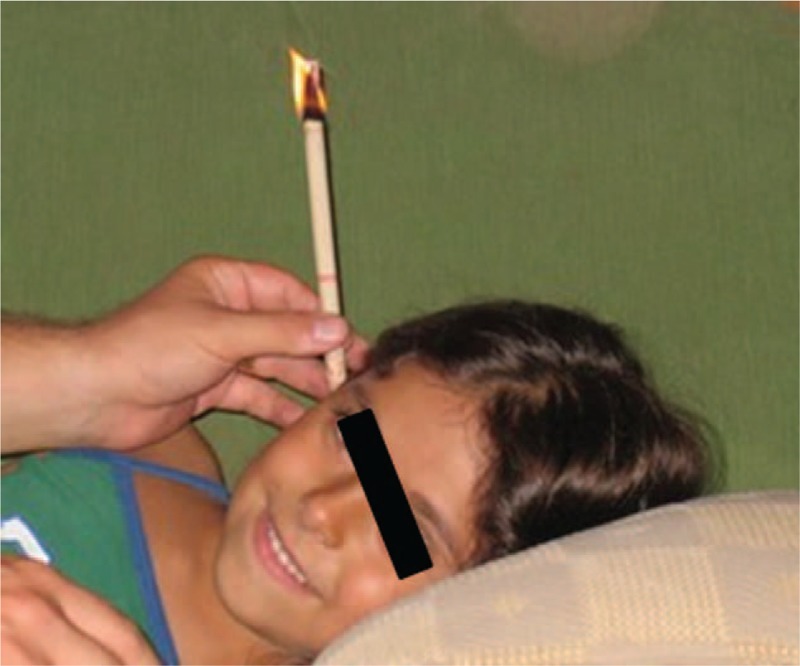
Ear candling.
Ear candling claims to “purify the blood” and heal children with OM through “’cleaning” of the middle ear cleft by creating a “negative pressure.” Little research has been performed on ear candling. Seely reported that ear candling is implausible and demonstrably wrong, leading to deposit of candle residue in the ear canal with no therapeutic effect on extraction of cerumen or the middle ear.33 Furthermore, the authors stated that this therapy may be harmful, causing ear injuries (burns, occlusions of the ear canal, and tympanic membrane perforation), as well as otitis externa.
Vitamin D Supplement
In addition to its role in bone metabolism and calcium homeostasis, vitamin D plays a role in immunity and infection.38–43 In particular, it has been postulated that 25-hydroxyvitamin D [25(OH)D], the isoform that reflects the individual's vitamin D status, acts as an immunomodulator of both innate and adaptive immune systems, by shifting the T-helper cell pool toward Th2 status, inducing antimicrobial peptide synthesis, that is, cathelicidin and β-defensins, and inhibiting the production of pro-inflammatory cytokines.38,40–43 Moreover, vitamin D is involved in the modulation of macrophages and dendritic cells activities, and in regulation of toll-like receptor mediated events in neutrophils. Therefore, vitamin D status may influence the incidence and severity of some bacterial and viral infections, as indicated by previous clinical studies performed in patients with tuberculosis, respiratory tract infections, and AOM.39,44,45
Cayir et al46 published a longitudinal cross-sectional study conducted in 84 children aged 1 to 5 years with RAOM and in 108 comparable healthy controls. He found significantly reduced mean serum 25(OH)D levels in children with RAOM compared to controls (11.4 ± 9.8 vs 29.2 ± 13.9 ng/mL; P < 0.05), and an increased percentage of children with serum 25(OH)D levels <20 ng/mL in the study group compared to controls (69% vs 30%; P < 0.05). When vitamin D was given to children with RAOM who also had vitamin D deficiency, the occurrence of AOM and RAOM significantly dropped during the 1-year follow-up period. In the authors’ opinion, vitamin D quantities may play a role in the susceptibility to OM. These data were confirmed by the same group,47 which has recently reported in a single-blind, case–control study significantly reduced serum 25(OH)D levels in 88 children with AOM compared to 81 healthy controls (20.6 ± 10.2 vs 23.8 ± 10.3 ng/mL; P < 0.05).
Marchisio et al45 evaluated the relationship between decreased vitamin D levels and the increased risk of RAOM. They studied the possible effect of vitamin D supplementation in reducing the number of AOM episodes in 116 otitis-prone children (58 receiving vitamin D supplementation and 58 receiving placebo). They found that the number of children experiencing at least 1 AOM episode was significantly lower in the treatment group, when compared with the placebo group (26/58 vs 38/58; P = 0.03), and that the mean number of global AOM episodes (P = 0.03) and uncomplicated AOM episodes (P < 0.001) occurring in the vitamin D group was significantly lower, when compared to the control group. The likelihood of AOM occurrence was significantly reduced in patients with serum 25(OH) D levels ≥30 ng/mL. This study concluded that vitamin D deficiency is frequent in otitis-prone children, and that blood 25(OH)D concentrations ≥30 ng/mL are protective.
Despite these data, there is not enough evidence to support a causative effect of vitamin D deficiency on the etiology and pathogenesis of AOM, and to suggest a protective effect of vitamin D supplementation in children with RAOM; further controlled clinical trials are needed to solve these questions.
PROBIOTICS
Oral Probiotics
Probiotics are live microorganisms that offer health benefits by modulating the microbial community and enhancing host immunity.2 These effects can be obtained through inhibition of pathogen colonization, production of bacteriocins, and enhancement of both mucosal and systemic immunity.48
Commercial probiotics preparations are based on single or multiple bacteria. Most of the data regarding preventive efficacy of probiotics against infections have been obtained in patients with gastrointestinal diseases, in whom it was demonstrated that administration of probiotics can significantly reduce the risk of development of antibiotic-associated diarrhea.49
Data regarding the use of probiotics on OM have gathered in the last few years, showing variable efficacy.50–52 In general, the reduction in OM incidence in treated children was limited. Hatakka et al53 evaluated the possibility that probiotics could reduce the occurrence and duration of AOM episodes or the nasopharyngeal carriage of otopathogens in otitis-prone children. The study involved 309 children, aged 10 months to 6 years, who were randomised to consume for 24 weeks a probiotic daily or a placebo capsule. The probiotic treatment did not reduce the occurrence (probiotic vs placebo 72% vs 65%) or the recurrence (≥3 episodes) of AOM (probiotic vs placebo 18% vs 17%), while a reduction in the occurrence of recurrent URTIs was noticed in the probiotic group (OR for ≥4 URTIs = 0.56, OR for ≥6 URTIs = 0.59). The administration of probiotics did not modify the nasopharyngeal carriage of Streptococcus pneumoniae or Haemophilus influenzae, but increased the carriage of Moraxella catarrhalis (OR = 1.79), confirming from a microbiological point of view the basis for the negative results in prevention of AOM. These data are in agreement with the work of Tapiovaara et al,54 who demonstrated that Lactobacillus GG is able to penetrate the middle ear, but that its presence is not associated with a reduction in the presence of pathogenic bacteria or viruses.
Rautava et al55 enrolled 81 infants requiring formula feeding, who were randomized to receive either infant formula supplemented with the probiotics Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb-12 or placebo until the age of 12 months. During the first 7 months of life, the proportion of AOM episodes was significantly lower (treated: 22% vs placebo: 50%, P = 0.01), and antibiotics were significantly less prescribed (treated: 31% vs placebo: 60%, P = 0.01). However, when considering the whole 1st year of life, the prevalence of AOM was not statistically different (treated: 13% vs 25%).
In a double-blind, placebo-controlled trial, Cohen et al56 assessed whether follow-up formula supplemented with probiotics and prebiotics could reduce the risk of AOM. A total of 224 healthy infants aged 7 to 13 months were randomly assigned to follow-up formula supplemented with probiotics and prebiotics (Raftilose/Raftiline), or follow-up formula alone. During the 12 months study period, the treatment and the control groups did not differ in the incidence of AOM (incidence rate ratio, 1.0, 95% CI: 0.8–1.2), lower URIs incidence (IRR 0.9, 95% CI: 0.7–1.2), or number of antibiotic treatment courses (RR 1.0, 95% CI: 0.8–1.2), which were mainly prescribed for AOM (82%). The nasopharyngeal flora composition did not differ in the 2 groups at any time during the follow-up.
Topical Probiotics
Topical administration of probiotics has been considered as a method to reduce the risk of recurrent AOM in children when administered by nasal spray. The most largely studied microorganism has been α-hemolytic Streptococcus (AHS), taking into account that the presence in the nasopharynx could interfere with survival and multiplication of pathogens more frequently associated with AOM development.57
Roos et al58 enrolled 108 otitis-prone children and, after a 10-day antibiotic course, randomized them to receive a nasal spray containing 5 AHS strains (selected among those colonizing the ETs opening, because of their superior inhibitory activities against otopathogens) or a placebo solution. Both streptococcal and placebo solutions were sprayed for a first 10-day period and then resumed for 10 days starting from day 60 of the study. During the 3-months follow-up, children who were given AHS-supplemented spray experienced significantly more cure from AOM (42% vs 22%, P = 0.02) and less recurrences (40% vs 51%, P = 0.04). The author's conclusions favored the use of AHS to protect against RAOM.
Subsequently, Tano59 randomized 43 children to receive with a nasal spray daily for 4 months a suspension of 10% skim milk and 0.9% NaCl containing 5 selected AHS strains with very good in vitro inhibitory activity on otopathogens, or skim milk with 0.9% NaCl. The proportion of children with recurrences was similar in the 2 groups (treatment group: 44%; placebo group: 40%) and no significant changes in the nasopharyngeal colonization of otopathogens was detected.
Skovbjerg et al60 studied the topical use of a nasal spray containing S. sanguinis, L. rhamnosus, or placebo in children with long-lasting OME before the insertion of tympanostomy tubes. Complete or significant clinical recovery occurred in 7/19 patients treated with S. sanguinis compared to 1/17 patients in the placebo group (P = 0.05). In the L. rhamnosus treatment group, no significant difference in cure rates was detected. It should be taken into consideration that the study population was small.
The negative results, in association with the potential risk of infections directly due to the bacteria used for topical treatment, have led to halting of research with these strains. More recently, Streptococcus salivarius, an AHS isolated from the pharynx of healthy subjects, has received attention. It is a potential nasopharyngeal probiotic, thanks to its immunomodulatory and antiinflammatory skills, its production of plasmin-encoded bacteriocins and its good safety profile.57,61,62 Di Pierro et al63 evaluated the role of S. salivarius K12 in preventing recurrent streptococcal pharyngitis and AOM in 82 children aged 3 to 12 years with a recent history of recurrent oral streptococcal pathology, who were randomized to be administered an oral slow-release tablet containing 5 billion colony-forming units of S. salivarius K12 (Bactoblis) and to a control group. The 41 children who completed the 90-days treatment had significantly fewer episodes of streptococcal pharyngeal infections (−92.2%) and/or of reported AOM (−40%) during the 90-day probiotic intake compared to the previous 12 months (but the difference was not significant for AOM if adjusted for the time period). A reduction in the reported incidence of pharyngeal and middle ear infections by 65.9% was also registered in the treatment group in the 6 months follow-up after the treatment.
Marchisio et al64 recently reported the results of the 1st study in which Streptococcus salivarius 24SMB, with significant activity against AOM pathogens, was intranasally administered in otitis-prone children. Children aged 1 to 5 years with RAOM history were randomized 1:1 to receive an intranasal S. salivarius 24SMB or placebo twice daily for 5 days each month for 3 consecutive months and followed up for 6 months. The number of children who did not experience any AOM was higher among the children treated with the S. salivarius 24SMB preparation than among those in the placebo group (30.0% vs 14.9%; P = 0.076) and among children colonized by S. salivarius 24SMB after treatment compared to the noncolonized (42.8% vs 13.6%; P = 0.03). Similar results were observed when the children treated with antibiotics for AOM were analyzed (67.8% vs 95.5%; P = 0.029).
Probiotics indeed seem a promising method in the prevention of AOM and URI but, because of the contrasting results of the available studies, further clinical evaluation is needed in order to assess their true potential.
CONCLUSIONS
Despite the conservative therapeutic nature of CAM therapies for OM, which do not include drugs or surgery, CAM is currently not considered a treatment option for OM in the medical community, due to the limited and confusing supporting scientific evidence. In our opinion, there may be some benefits using homeopathy, phytotherapy, xylitol, vitamin D, and probiotics for the prevention, and treatment of AOM. For RAOM, we have noticed scant benefit for the use of probiotics and vitamin D. For OME, a mild-moderate benefit was demonstrated for the use of probiotics and xylitol. At this time, we recommend that further studies should be conducted in order to establish the additive value of the of CAM therapies for OM. We propose RCTs in pediatric mild-moderate AOM cases, in which antibiotics can be deferred or withheld, so the tested CAM therapy will be evaluated versus placebo/no treatment. We further suggest that trials should be conducted in which infants fed with probiotic-enriched formulas will be evaluated against others fed with standard formula, in terms of age of 1st AOM episode and RAOM prevalence.
Acknowledgements
The authors thank Richard Niemtzow, MD, PhD, MPH, for reviewing this paper and his helpful comments.
Footnotes
Abbreviations: AHS = α-hemolytic Streptococcus, AOM = acute otitis media, CAM = complementary and alternative medicine, CI = confidence interval, ET = Eustachian tube, 25(OH)D = 25-hydroxyvitamin D, OM = otitis media, OMT = osteopathic manipulative treatment, RAOM = recurrent AOM, RCT = randomized controlled trial, SMT = spinal manipulation therapy, URTI = upper respiratory tract infection.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Levi JR, O’Reilly R. Complementary and integrative treatments: otitis media. Otolaryngol Clin North Am 2013; 46:309–327. [DOI] [PubMed] [Google Scholar]
- 2.Levi JR, Brody RM, McKee-Cole K, et al. Complementary and alternative medicine for pediatric otitis media. Int J Pediatr Otorhinolaryngol 2013; 77:926–931. [DOI] [PubMed] [Google Scholar]
- 3.Yussman SM, Ryan SA, Auinger P, et al. Visits to complementary and alternative medicine providers by children and adolescents in the United States. Ambul Pediatr 2004; 4:429–435. [DOI] [PubMed] [Google Scholar]
- 4.National Institutes of Health. National Center for Complementary and Alternative Medicine Web site. Available from http://nccam.nih.gov [Accesssed September 12, 2015]. [Google Scholar]
- 5.Lee HJ, Park SK, Choi KY, et al. Korean clinical practice guidelines: otitis media in children. J Korean Med Sci 2012; 27:835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura K, Iino Y, Kamide Y, et al. Clinical practice guidelines for the diagnosis and management of acute otitis media (AOM) in children in Japan – 2013 update. Auris Nasus Larynx 2015; 42:99–106. [DOI] [PubMed] [Google Scholar]
- 7.Karkos PD, Leong SC, Arya AK, et al. ‘Complementary ENT’: a systematic review of commonly used supplements. J Laryngol Otol 2007; 121:779–782. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZM. [Acupuncture with blood-letting puncture for 52 cases of bullous myringitis]. Zhongguo Zhen Jiu 2013; 33:988. [PubMed] [Google Scholar]
- 9.Tian ZM. Acupuncture treatment for aerotitis media. J Tradit Chin Med 1985; 5:259–260. [PubMed] [Google Scholar]
- 10.Bikbaeva AI, Gabbasova NG, Tsyglin AA. [Cerebral and peripheral hemodynamics after complex therapy, including acupuncture, of patients with chronic suppurative mesotympanitis]. Vestn Otorinolaringol 1987; 23–26. [PubMed] [Google Scholar]
- 11.Sánchez-Araujo M, Puchi A. Acupuncture prevents relapses of recurrent otitis in dogs: a 1-year follow-up of a randomised controlled trial. Acupunct Med 2011; 29:21–26. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Araujo M, Puchi A. Acupuncture enhances the efficacy of antibiotics treatment for canine otitis crises. Acupunct Electrother Res 1997; 22:191–206. [DOI] [PubMed] [Google Scholar]
- 13.Friese KH, Feuchter U, Moeller H. [Homeopathic treatment of adenoid vegetations. Results of a prospective, randomized double-blind study]. HNO 1997; 45:618–624. [DOI] [PubMed] [Google Scholar]
- 14.Posadzki P, Lee MS, Ernst E. Osteopathic manipulative treatment for pediatric conditions: a systematic review. Pediatrics 2013; 132:140–152. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs J, Springer DA, Crothers D. Homeopathic treatment of acute otitis media in children: a preliminary randomized placebo-controlled trial. Pediatr Infect Dis J 2001; 20:177–183. [DOI] [PubMed] [Google Scholar]
- 16.Sinha MN, Siddiqui VA, Nayak C, et al. Randomized controlled pilot study to compare homeopathy and conventional therapy in acute otitis media. Homeopathy 2012; 101:5–12. [DOI] [PubMed] [Google Scholar]
- 17.Frei H, Thurneysen A. Homeopathy in acute otitis media in children: treatment effect or spontaneous resolution? Br Homeopath J 2001; 90:180–182. [DOI] [PubMed] [Google Scholar]
- 18.Fixsen A. Should homeopathy be considered as part of a treatment strategy for otitis media with effusion in children? Homeopathy 2013; 102:145–150. [DOI] [PubMed] [Google Scholar]
- 19.Shang A, Huwiler-Müntener K, Nartey L, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet 2005; 366:726–732. [DOI] [PubMed] [Google Scholar]
- 20.Altunç U, Pittler MH, Ernst E. Homeopathy for childhood and adolescence ailments: systematic review of randomized clinical trials. Mayo Clin Proc 2007; 82:69–75. [DOI] [PubMed] [Google Scholar]
- 21.Sarrell EM, Cohen HA, Kahan E. Naturopathic treatment for ear pain in children. Pediatrics 2003; 111 (5 Pt 1):e574–e579. [DOI] [PubMed] [Google Scholar]
- 22.Sarrell EM, Mandelberg A, Cohen HA. Efficacy of naturopathic extracts in the management of ear pain associated with acute otitis media. Arch Pediatr Adolesc Med 2001; 155:796–799. [DOI] [PubMed] [Google Scholar]
- 23.Fay DL, Schellhase KG, Wujek D. Naturopathic ear drops minimally effective for acute otitis media. J Fam Pract 2003; 52:673.6. [PubMed] [Google Scholar]
- 24.Ciuman RR. Phytotherapeutic and naturopathic adjuvant therapies in otorhinolaryngology. Eur Arch Otorhinolaryngol 2012; 269:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratt-Harrington D. Galbreath technique: a manipulative treatment for otitis media revisited. J Am Osteopath Assoc 2000; 100:635–639. [PubMed] [Google Scholar]
- 26.Channell MK. Modified Muncie technique: osteopathic manipulation for eustachian tube dysfunction and illustrative report of case. J Am Osteopath Assoc 2008; 108:260–263. [PubMed] [Google Scholar]
- 27.Mills MV, Henley CE, Barnes LL, et al. The use of osteopathic manipulative treatment as adjuvant therapy in children with recurrent acute otitis media. Arch Pediatr Adolesc Med 2003; 157:861–866. [DOI] [PubMed] [Google Scholar]
- 28.Steele KM, Carreiro JE, Viola JH, et al. Effect of osteopathic manipulative treatment on middle ear effusion following acute otitis media in young children: a pilot study. J Am Osteopath Assoc 2014; 114:436–447. [DOI] [PubMed] [Google Scholar]
- 29.Prakash L, Michalik DE. Brief report of a clinical trial on the duration of middle ear effusion in young children using a standardized osteopathic manipulative medicine protocol. J Am Osteopath Assoc 2010; 110:738–739.author reply 9-40. [PubMed] [Google Scholar]
- 30.Degenhardt BF, Kuchera ML. Osteopathic evaluation and manipulative treatment in reducing the morbidity of otitis media: a pilot study. J Am Osteopath Assoc 2006; 106:327–334. [PubMed] [Google Scholar]
- 31.Pohlman KA, Holton-Brown MS. Otitis media and spinal manipulative therapy: a literature review. J Chiropr Med 2012; 11:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vernacchio L, Corwin MJ, Vezina RM, et al. Xylitol syrup for the prevention of acute otitis media. Pediatrics 2014; 133:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seely DR, Quigley SM, Langman AW. Ear candles–efficacy and safety. Laryngoscope 1996; 106:1226–1229. [DOI] [PubMed] [Google Scholar]
- 34.Uhari M, Kontiokari T, Koskela M, et al. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. BMJ 1996; 313:1180–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics 1998; 102 (4 Pt 1):879–884. [DOI] [PubMed] [Google Scholar]
- 36.Tapiainen T, Luotonen L, Kontiokari T, et al. Xylitol administered only during respiratory infections failed to prevent acute otitis media. Pediatrics 2002; 109:E19. [DOI] [PubMed] [Google Scholar]
- 37.Azarpazhooh A, Limeback H, Lawrence HP, et al. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database Syst Rev 2011; CD007095. [DOI] [PubMed] [Google Scholar]
- 38.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 2008; 4:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergman P, Lindh AU, Björkhem-Bergman L, et al. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One 2013; 8:e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charan J, Goyal JP, Saxena D, et al. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother 2012; 3:300–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005; 19:1067–1077. [DOI] [PubMed] [Google Scholar]
- 42.Barlow PG, Svoboda P, Mackellar A, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One 2011; 6:e25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 2013; 136:321–329. [DOI] [PubMed] [Google Scholar]
- 44.Esposito S, Baggi E, Bianchini S, et al. Role of vitamin D in children with respiratory tract infection. Int J Immunopathol Pharmacol 2013; 26:1–13. [DOI] [PubMed] [Google Scholar]
- 45.Marchisio P, Consonni D, Baggi E, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J 2013; 32:1055–1060. [DOI] [PubMed] [Google Scholar]
- 46.Cayir A, Turan MI, Ozkan O, et al. Serum vitamin D levels in children with recurrent otitis media. Eur Arch Otorhinolaryngol 2014; 271:689–693. [DOI] [PubMed] [Google Scholar]
- 47.Cayir A, Turan MI, Ozkan O, et al. Vitamin D levels in children diagnosed with acute otitis media. J Pak Med Assoc 2014; 64:1274–1277. [PubMed] [Google Scholar]
- 48.Iannitti T, Palmieri B. Therapeutical use of probiotic formulations in clinical practice. Clin Nutr 2010; 29:701–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szajewska H, Ruszczyński M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr 2006; 149:367–372. [DOI] [PubMed] [Google Scholar]
- 50.Niittynen L, Pitkäranta A, Korpela R. Probiotics and otitis media in children. Int J Pediatr Otorhinolaryngol 2012; 76:465–470. [DOI] [PubMed] [Google Scholar]
- 51.John M, Dunne EM, Licciardi PV, et al. Otitis media among high-risk populations: can probiotics inhibit Streptococcus pneumoniae colonisation and the risk of disease? Eur J Clin Microbiol Infect Dis 2013; 32:1101–1110. [DOI] [PubMed] [Google Scholar]
- 52.Esposito S, Rigante D, Principi N. Do children's upper respiratory tract infections benefit from probiotics? BMC Infect Dis 2014; 14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatakka K, Blomgren K, Pohjavuori S, et al. Treatment of acute otitis media with probiotics in otitis-prone children-a double-blind, placebo-controlled randomised study. Clin Nutr 2007; 26:314–321. [DOI] [PubMed] [Google Scholar]
- 54.Tapiovaara L, Lehtoranta L, Swanljung E, et al. Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlled oral administration. Int J Pediatr Otorhinolaryngol 2014; 78:1637–1641. [DOI] [PubMed] [Google Scholar]
- 55.Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy – a randomised, double-blind, placebo-controlled study. Br J Nutr 2009; 101:1722–1726. [DOI] [PubMed] [Google Scholar]
- 56.Cohen R, Martin E, de La Rocque F, et al. Probiotics and prebiotics in preventing episodes of acute otitis media in high-risk children: a randomized, double-blind, placebo-controlled study. Pediatr Infect Dis J 2013; 32:810–814. [DOI] [PubMed] [Google Scholar]
- 57.Santagati M, Scillato M, Patanè F, et al. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol Med Microbiol 2012; 65:23–31. [DOI] [PubMed] [Google Scholar]
- 58.Roos K, Håkansson EG, Holm S. Effect of recolonisation with “interfering” alpha streptococci on recurrences of acute and secretory otitis media in children: randomised placebo controlled trial. BMJ 2001; 322:210–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tano K, Grahn Håkansson E, Holm SE, et al. A nasal spray with alpha-haemolytic streptococci as long term prophylaxis against recurrent otitis media. Int J Pediatr Otorhinolaryngol 2002; 62:17–23. [DOI] [PubMed] [Google Scholar]
- 60.Skovbjerg S, Roos K, Holm SE, et al. Spray bacteriotherapy decreases middle ear fluid in children with secretory otitis media. Arch Dis Child 2009; 94:92–98. [DOI] [PubMed] [Google Scholar]
- 61.Power DA, Burton JP, Chilcott CN, et al. Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Eur J Clin Microbiol Infect Dis 2008; 27:1261–1263. [DOI] [PubMed] [Google Scholar]
- 62.Santagati M, Scillato M, Muscaridola N, et al. Colonization, safety, and tolerability study of the Streptococcus salivarius 24SMBc nasal spray for its application in upper respiratory tract infections. Eur J Clin Microbiol Infect Dis 2015; 34:2075–2080. [DOI] [PubMed] [Google Scholar]
- 63.Di Pierro F, Donato G, Fomia F, et al. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med 2012; 5:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchisio P, Santagati M, Scillato M, et al. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur J Clin Microbiol Infect Dis 2015; 34:2377–2383. [DOI] [PubMed] [Google Scholar]


