Abstract
The objective is to observe the changes in plasma adiponectin (APN) and its predictive capacity for disease severity and prognosis of hemorrhagic fever with renal syndrome (HFRS).
One hundred and five patients who were treated at our center between October 2011 and December 2012 were randomly enrolled in this study. The patients were divided into a mild-type group, a moderate-type group, a severe-type group, and a critical-type group according to the HFRS criteria for clinical classification. Ninety-three plasma samples from the patients in the acute stage and 78 samples from the patients in the convalescent stage were obtained, and 28 samples from healthy subjects were obtained as controls. The concentrations of APN were detected using the enzyme-linked immunosorbent assay. The levels of white blood cells, platelets, hematocrit, albumin, blood urea nitrogen, serum creatinine, and uric acid in the samples were routinely tested. The levels of APN among the different types were compared; the correlation between APN and the laboratory parameters was analyzed. The predictive effectiveness for prognosis of APN and the laboratory parameters as mentioned above were evaluated using the receiver operating characteristic curve analysis.
The levels of APN in the mild- and moderate-type patients in the acute stage were significantly higher than the severe-type and control (P < 0.05) and decreased with the severity of the disease, while there were no obvious difference among severe-, critical-type and control groups. The levels of APN in patients in the convalescent stage were higher than the control group (P < 0.05), and the APN levels of the critical-type group were higher compared with the mild-type groups (P < 0.05). Adiponectin was negatively correlated with white blood cells and hematocrit and positively correlated with platelets, albumin, and uric acid (P < 0.001). Adiponectin showed no statistical significance for predicting prognosis, with the area under the curve equal to 0.609 (95% CI: 0.237–0.745, P = 0.215).
Adiponectin can be considered as a novel biomarker for disease severity in patients with HFRS, while it seems to have no predictive capacity for prognosis of HFRS.
INTRODUCTION
Hemorrhagic fever with renal syndrome (HFRS) is a rodent-borne disease that is caused by Hantavirus, with major clinical characteristics of fever, hemorrhage, hypotension, and renal damage.1,2 The typical disease progresses through five phases: febrile, hypotensive, oliguric, diuretic, and convalescent.3,4 China is the most severe endemic area of HFRS in the world, with a high incidence rate in the last 10 years.5 Xi’an city is the center of Shaanxi province and one of the most severely affected regions in China.6 The HFRS patients in this district display more severe manifestations, usually accompanied with refractory shock, acute respiratory distress syndrome, encephalopathy, disseminated inravascular coagulation and multiple organ dysfunction syndrome, which leads to a greatly increased fatality rate.
Until now, there are nonspecific parameters routinely tested clinically that can evaluate severity and predict the outcome because of the complicated clinical courses and pathophysiology of the disease. So, exploring novel biomarker to determine the disease severity early and exactly is still very important which would be beneficial for clinician to take timely and systematic treatment. In this study, we observed the changes in plasma adiponectin (APN) and explore its predictive capacity for disease severity and prognosis of HFRS.
METHODS
Ethics Statement
The observational perspective study was approved by the Institutional Review Board of Tangdu Hospital. Before inclusion, the patients were informed about the objectives of this study; they or their direct relatives agreed and signed the informed consent form so that blood samples and medical records could be obtained.
Study Participants
One hundred and five patients with HFRS that were treated at our center between October 2011 and December 2012 were randomly enrolled in this study. The demographic characteristics of the patients were collected from medical records. Patients who had other kidney diseases, diabetes, cardiovascular disease, hematological disease, autoimmune disease, viral hepatitis, and other liver diseases were excluded.
Specific IgM and IgG against Hantaan virus in serum during acute phase were detected by enzyme-linked immunosorbent assay for diagnosis of HFRS, which was same with our previous clinical study.4 The assay was analyzed by an autoanalyzer (BIORAD-680, United States).4
According to the HFRS criteria of clinical classification, which has been defined in our previous studies,4,7 the severity of HFRS was classified into the following types: patients who had kidney injury without obvious hypotension and oliguria were defined as mild-type; patients who had hypotension, skin and mucous membranes hemorrhage, bulbar conjunctiva, uremia, acute kidney injury (AKI) with typical oliguria were defined as moderate-type; patients who had severe uremia, hypotension, skin and mucous membranes hemorrhage, bulbar conjunctiva and either peritoneum or pleura, and AKI with urine output of 50–500 mL/day for ≤5 days or urine output of <100 mL/day for ≤2 days were defined as severe-type; and patients who usually had one or more of the following complications based upon the basic clinical feature of the severe patients were defined as critical type: visceral hemorrhage, pulmonary and brain edema, refractory shock (≥2 days), severe AKI with urine output of 50–500 mL/day for >5 days or urine output of <100 mL/day for >2 days, severe secondary infection and heart failure.
In this study, the acute stage was defined as the period that included the febrile, hypotensive, and oliguric phases and the early 3 days of the diuretic phase, considering the clinical conditions that a majority of the survival patients had been discharged before the convalescent phase and the degree of AKI that was still severe during the early stage of the diuretic phase. The convalescent stage was defined as the diuretic and convalescent phase except the early 3 days of the diuretic phase. Furthermore, the prognosis (death) in this study was defined as patient death during hospitalization or within the 28 days following discharge.
Blood Samples and Detection
Ninety-three venous blood samples were drawn randomly from the patients during the acute stage, and 78 samples were drawn randomly during the convalescent stage. Twenty-eight blood samples from healthy subjects were obtained as controls. All of the samples were stored in EDTA tubes and were centrifuged at 2000 rpm for 10 min at 4 °C within 2 hours after drawing. The plasma supernatant was pipetted carefully and transferred to polypropylene tubes and then stored at –80 °C prior to APN analysis.
Adiponectin levels were measured with commercially available enzyme-linked immunosorbent assay kits (Quantikine, XiTang, Inc, Shanghai, China) and were tested using a multifunctional autoanalyzer (BIORAD-680, United States) according to the manufacturer's instructions. Each sample was detected in duplicate and the sensitivity of the minimum concentration of APN was below 61 pg/mL.
Seven laboratory parameters, including white blood cells (WBC), platelets (PLT), hematocrit (HCT), albumin (ALB), blood urea nitrogen, serum creatinine, and uric acid were routinely tested using autoanalyzers (Sysmex XT-4000i, Japan; Hitachi 7600–100, Japan). All the laboratory parameters mentioned above and APN were measured in the same time frame.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc, Chicago, IL). Tables and figures were created using Excel 2003 (Microsoft) and GraphPad Prism 5 (GraphPad Software, San Diego, CA), respectively. The basic statistical principle was same with our previous clinical studies on critical HFRS patients.4,7 The frequencies and percentages are given for qualitative variables, and the differences among the 4 types were tested using the Pearson χ2 test. The non-normally distributed variables are presented as medians with interquartile ranges (IQR) and were compared by the nonparametric Kruskal–Wallis H test. The Nemenyi Rank test was used to compare the differences among the 4 types. Continuous variables are presented as the mean ± SD and were analyzed by Kolmogorov–Smirnov test for normal distribution and by Levene test (ANOVA) for the homogeneity of variance. The variables among the 4 types were compared by SNK test for normally distributed variables. Furthermore, the relationship between APN and the laboratory parameters as mentioned above was determined by Pearson correlation coefficient. The predictor values of APN for disease prognosis were tested using receiver operating characteristic (ROC) curves and quantified by calculating the area under the ROC curve (AUC) and the 95% confidence interval (CI). A 2-tailed P < 0.05 was considered statistically significant.
RESULTS
Clinical Typing and Demographic Characteristics for Patients With Hemorrhagic Fever With Renal Syndrome
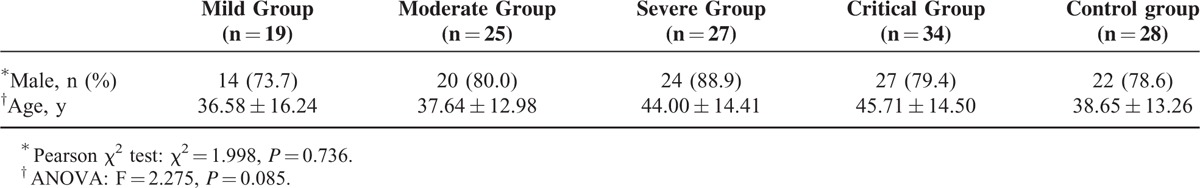
Of the enrolled patients, 19 cases were classified as mild, 25 cases were classified as moderate, 27 cases were classified as severe and 34 cases were classified as critical according to the HFRS criteria of clinical classification. Twelve critical individuals died during the acute stage with a hospital mortality rate of 11.42%. There was no significant difference in the sex or age distribution among the groups (P > 0.05) (Table 1).
TABLE 1.
Demographic Characteristics for Patients With Hemorrhagic Fever With Renal Syndrome
Levels of Adiponectin in Patients With Hemorrhagic Fever With Renal Syndrome
The duration from disease onset to sample collection among the groups was not significantly different (P > 0.05) (Table 2). The levels of APN in the mild- and moderate-type patients in the acute stage were significantly higher than the severe-type and control (P < 0.05) and decreased with the severity of the disease, while there were no obvious difference among severe-, critical-type, and control groups. The levels of APN in patients in the convalescent stage were higher than the control group (P < 0.05), and the APN levels of the critical-type group were higher compared with the mild-type groups (P < 0.05) (Table 2; Figure 1).
TABLE 2.
Levels of Adiponectin and Time Frame of Sample Collection in Patients With Hemorrhagic Fever With Renal Syndrome
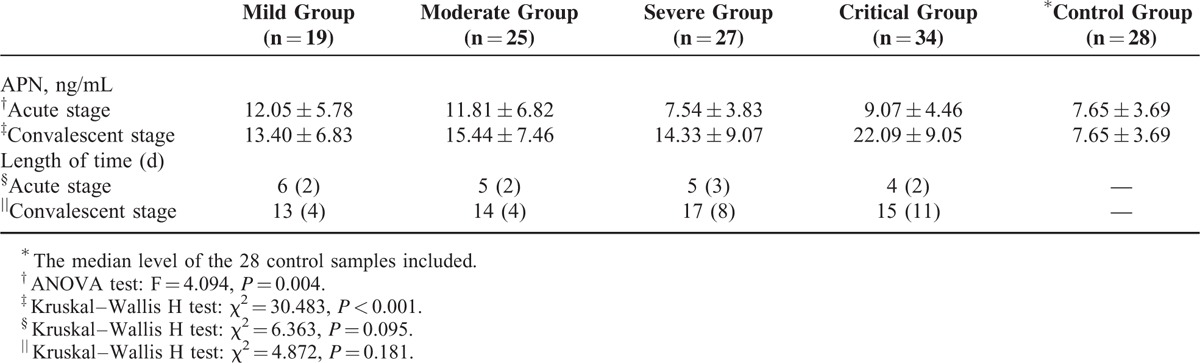
FIGURE 1.
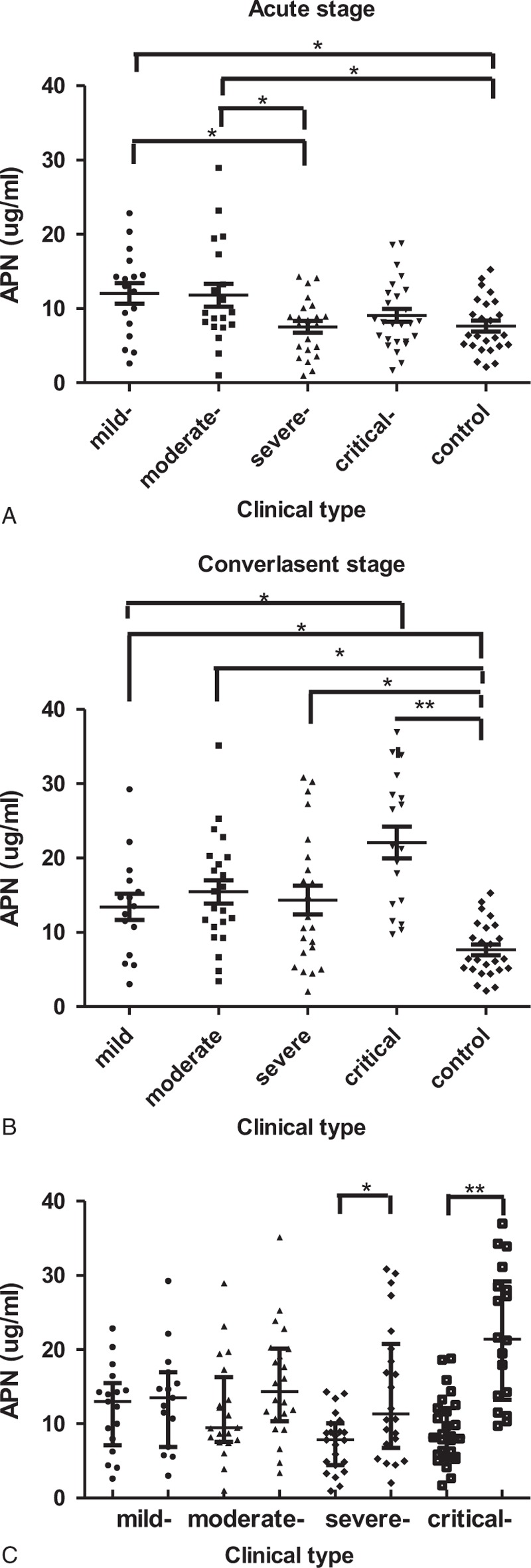
Levels of APN during the clinical course in patients with hemorrhagic fever with renal syndrome. The concentrations of APN were presented as medians with IQR and were compared by the Nemenyi Rank test among the 5 groups (A and B). The concentrations of APN were presented as medians with IQR and were compared by a Mann–Whitney U test for the acute stage and convalescent stage (C). APN = adiponectin. ∗P < 0.05; ∗∗P < 0.001.
Pearson Correlation Analysis and Receiver Operating Characteristic Curves
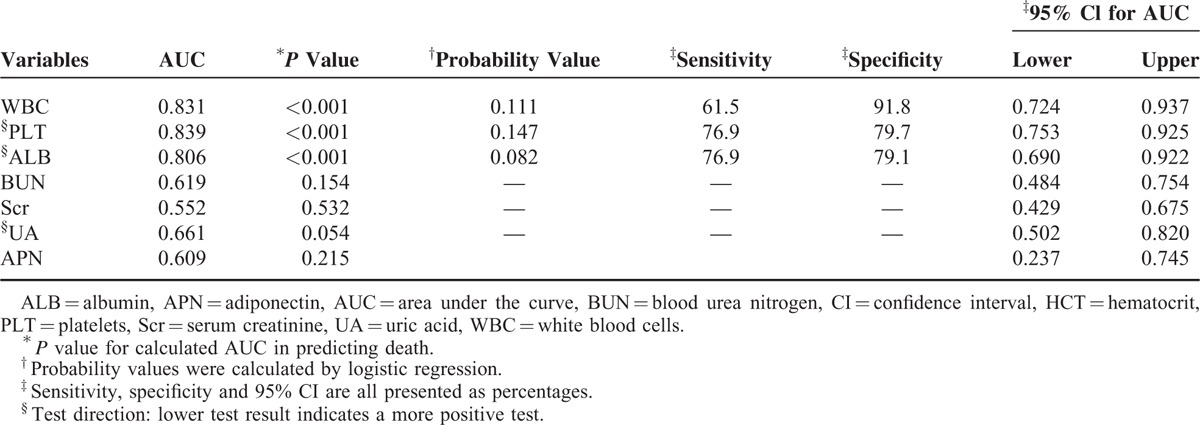
Pearson correlation analysis revealed that APN was negatively correlated with WBC and HCT and positively correlated with PLT, ALB, and uric acid (P < 0.001) (Table 3; Figure 2). Receiver operating characteristic analysis revealed that APN showed no statistical significance for predicting prognosis, with the AUC equal to 0.609 (95% CI: 0.237–0.745, P = 0.215) (Table 4).
TABLE 3.
Pearson Correlation Analysis in Patients With Hemorrhagic Fever With Renal Syndrome
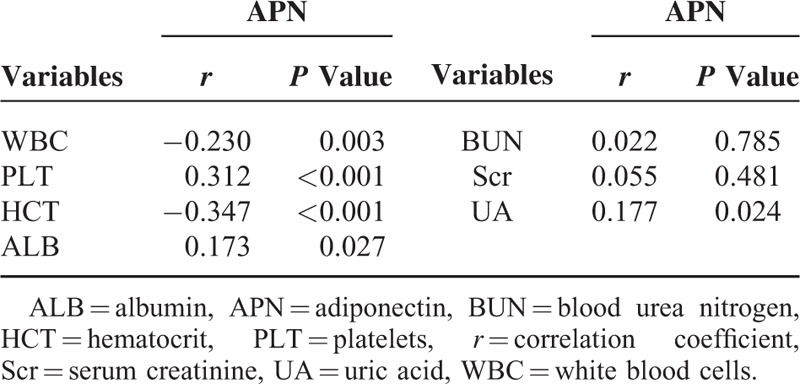
FIGURE 2.
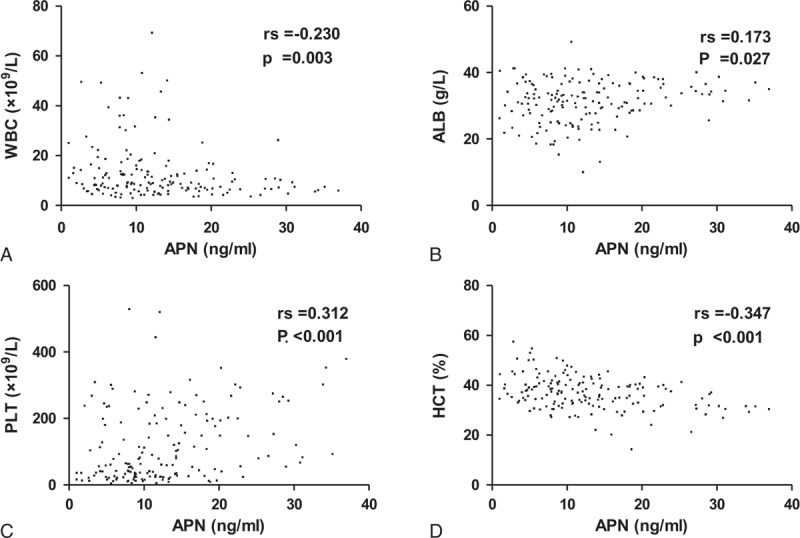
Correlation between adiponectin and white blood cells (A), albumin (B), platelets (C), and hematocrit (D) in patients with hemorrhagic fever with renal syndrome. FRT = ferritin, BUN = blood urea nitrogen, UA = uric acid.
TABLE 4.
Predictive Values for Prognosis With Adiponectin and Laboratory Parameters in Patients With Hemorrhagic Fever With Renal Syndrome
DISCUSSION
Adiponection (APN) is an adipocyte-derived hormone, which is closely associated with atherosclerosis, coronary artery disease, diabetes mellitus, and obesity.8–10 Apart from its insulin-sensitizing and antiatherosclerotic effects, APN also exerts anti-inflammatory activity in some diseases with acute infection.11–13 It has been proved that APN can suppress inflammation cytokine production by macrophages and phagocytic activity stimulated by lipopolysaccharide.14–16 Adiponectin-knockout mice could increase sepsis-related mortality and promote endothelial activation.13 Furthermore, recombinant APN could effectively reduce the mortality of septic mice and inhibit the production of some proinflammatory factors, such as high mobility group box 1, tumor necrosis factor-α, and interleukin-6.17
In the past 10 years, more and more research focused on observing the predictive role of APN on disease severity and prognosis in animals and patients with sepsis. Asta et al15 found that APN was decreased and behaved as a negative acute phase protein after acute endotoxaemia in dogs, and the declining degree of APN might reflect the severity of the disease. Another animal study18 also suggested a protective role of APN in diminishing microvascular organ-specific endothelial cell activation during sepsis. Unlike the consistent result on animal studies, there were three different conclusions focus on the early prediction of severity and prognosis of sepsis in patients. The first viewpoint is APN can be considered as a novel biomarker on early prediction of severity and prognosis. Behens et al19 investigated APN expression in cultured human adipocytes within an inflammatory model and in patients with severe sepsis and evaluated the treatment of drotrecogin α (activated, DAA). They found that stimulation of human adipocytes with TNF-α over 6 and 24 hour resulted in a significant decrease in APN mRNA transcripts; DAA patients revealed significantly higher APN serum levels compared to controls with moderate clinical course and better outcome. The second viewpoint is APN can be used to predict prognosis, whereas not disease severity. Alexander et al20 investigated the potential of pathogenic role and prognostic value of circulating APN levels in 170 critically ill patents (122 with sepsis and 48 without sepsis) and 60 healthy controls. The found that APN concentrations did not differ between healthy controls and critically ill patients, neither in patients with nor in patients without sepsis, whereas low APN levels at intensive care unit (ICU) admission were an independent positive predictor of short term and overall survival. The third viewpoint is APN cannot be used to predict severity and prognosis. Dimitra et al21 observed the variation in APN levels in 41 mechanically ventilated patients, diagnosed as having sepsis. The study revealed that the patients had higher APN levels on admission of ICU compared with the controls, and APN increased over time in the entire cohort with no correlation with the Acute Physiology and Chronic Health Evaluaton II and Sequential Organ Failure Assessment scores.
It is believed that HFRS has the basic clinical characteristics of sepsis, but it also has a unique pathophysiologic feature. The hypotensive phase of HFRS (eg, low blood pressure and circulation collapse) usually occurs between day 3 and day 7 of the clinical course, and grave HFRS patients can manifest more severe leukemoid reaction,22 plasma leakage, and coagulation disorders23,24 compared with septic patients, which would lead to massive bleeding, profound shock, severe tissue hypoperfusion and severe hypoxia, potentially rendering renal, cardiac, cerebellar, and hepatic injury.25,26 Considering the undefinite role of APN on sepsis and no study data obtained in patients of HFRS, we observed the changes of APN in different clinical phases of HFRS and explored its predictive value on disease severity and prognosis. As far as we know, this is the first study to observe the changes of APN and evaluate its predictive capacity for the severity and prognosis of HFRS.
Unlike the results from the research in sepsis patients as mentioned above,19–21 our study demonstrated that APN could reflect the disease severity in different clinical type patients with HFRS, while it seems have no predictive capacity for prognosis. This study demonstrated that the levels of APN in the mild- and moderate-type patients in the acute stage were significantly higher than the severe type and control (P<0.05) and decreased with the severity of the disease, there were no obvious difference among severe-, critical-type, and control groups (Table 2, Figure 1), which indicated that APN played an essential role in the pathogenesis of HFRS as an anti-inflammatory marker. Adiponectin can be considered a novel biomarker to evaluate disease severity in HFRS patients, especially during the acute stage, with potential for applications in clinical practice. In our study, we also observed that the levels of APN in patients in the convalescent stage were higher than the control group (P < 0.05), and the APN levels of the critical-type group were higher compared with the mild-type groups (P < 0.05) (Table 2, Figure 1), which also illustrated that lower expression of APN during the acute phase in HFRS patients usually is accompanied with more reactive and increasing tendency to maintain its anti-inflammatory and protective characteristics in the late clinical course. Our previous study3 analyzed the levels of 12 routinely tested laboratory parameters in HFRS patients and demonstrated that WBC, PLT, and ALB can be beneficial as early indicators of severity and prognosis in HFRS patients. High WBC can reflect the degree of inflammatory reaction, destruction, or dysfunction of PLT (low PLT)27 and the loss of vascular integrity (low ALB) can increase risk of hemorrhage and degree of vascular permeability.26,28 In this study, the correlation analysis revealed that APN was negatively correlated with WBC and positively correlated with PLT and ALB (P < 0.001) (Table 3, Figure 2), which further revealed that the dynamic changes of APN could also reflect the disease severity to a degree.
Unlike WBC, PLT, ALB, and high mobility group box 1,2,3,7 in this study, the ROC analysis revealed that APN showed no statistical significance for predicting prognosis (AUC = 0.609, P = 0.215) (Table 4), which indicates APN seems to have no predictive capacity for prognosis. There are 2 possible reasons causing the finding: one is APN cannot predict the prognosis of HFRS indeed; another is the small number of nonsurvivors enrolled in this study relatively, which may make the statistical power small. Enlargement of samples and further illustrating detailed mechanism of APN on HFRS are quite necessary to explore the value of predicting prognosis in the future.
From this observational perspective study, we got a meaningful conclusion that APN can be considered as a novel biomarker for disease severity in HFRS patients and it seems to have no predictive capacity for prognosis of HFRS, whereas there were still some limitations. First, this study was conducted at a single center for infectious diseases. The length of time form collection of the blood samples from the patients was not unified or precise, considering the different clinical conditions and phases on admission, and we can only define 2 periods, the acute and convalescent stages. Although there was no significant difference in the median collection time of the samples, the dynamic change of the levels of APN can also be influenced by this variation. Second, as we mentioned above, the relatively small number of cases made the statistical power small relatively. There were only 12 patients who died, which would influence the result of the ROC curve analysis. Third, analysis the correlation between the body mass index (BMI) of HFRS patients and APN would provide additional useful information. Body mass index is known to be associated with plasma APN and it have be considered as an independent risk factor for predicting prognosis of sepsis patients.29,30 However, we cannot be able to collect precise data of BMI because it is not routinely measured or it is difficult to obtain in critical HFRS patients, and we have to consider multiple factors, which may also influence the changes of BMI, including body edema, volume change of fluid resuscitation, energy expenditure, and acute metabolic disequilibrium. Finally, the clinical outcomes and classifications of the HFRS patients might be biased due to the lack of a more standardized protocol for the management of patients with HFRS until now.
Footnotes
Abbreviations: AKI = acute kidney injury, ALB = albumin, APN = adiponectin, AUC = area under the ROC curve, BMI = body mass index, CI = confidence interval, DAA = drotrecoginα (activated), DIC = disseminated intravascular coagulation, HCT = hematocrit, HFRS = hemorrhagic fever with renal syndrome, HV = Hantavirus, ICU = intensive care unit, IgG = immunoglobulin G, IgM = immunoglobulin M, PLT = platelets, WBC = white blood cells.
HD, XB, and JL contributed equally to this study.
This work was supported by the Substantial Development Program of Technology Innovation and Clinical Research in Tangdu Hospital (No. 2013–4241370U2), the National Basic Research Program of China (973 Program) (No. 2012CB518905), and the National Natural Science Foundation of China (No. 81373118).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jonsson CB, Figueiredo LT, Vapalahti O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 2010; 23:412–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du H, Li J, Yu H, et al. HMGB-1 as a novel predictor of disease severity and prognosis in patients with hemorrhagic fever with renal syndrome. Mediators Inflamm 2015; 2015:696248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du H, Li J, Yu HT, et al. Early indicators of severity and construction of a risk model for prognosis based upon laboratory parameters in patients with hemorrhagic fever with renal syndrome. Clin Chem Lab Med 2014; 52:1667–1675. [DOI] [PubMed] [Google Scholar]
- 4.Du H, Li J, Jiang W, et al. Clinical study of critical patients with hemorrhagic fever with renal syndrome complicated by acute respiratory distress syndrome. PLoS One 2014; 9:e89740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo CW, Chen HX. Epidemiological characteristics and the strategy of vaccination on hemorrhagic fever with renal syndrome in the last 10 years, in China. Zhonghua Liu Xing Bing Xue Za Zhi 2008; 29:1017–1019. [PubMed] [Google Scholar]
- 6.Ma C, Yu P, Nawaz M, et al. Hantaviruses in rodents and humans, Xi’an, PR China. J Gen Virol 2012; 93 (Pt 10):2227–2236. [DOI] [PubMed] [Google Scholar]
- 7.Du H, Wang PZ, Li J, et al. Clinical characteristics and outcomes in critical patients with hemorrhagic fever with renal syndrome. BMC Infect Dis 2014; 14:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spranger J, Kroke A, Möhlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003; 361:226–228. [DOI] [PubMed] [Google Scholar]
- 9.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie 2012; 94:2143–2149. [DOI] [PubMed] [Google Scholar]
- 10.Lau WB, Tao L, Wang Y, et al. Systemic adiponectin malfunction as a risk factor for cardiovascular disease. Antioxid Redox Signal 2011; 15:1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson K, Prins J, Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Critical Care 2011; 15:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf AM, Wolf D, Avila MA, et al. Up-regulation of the anti-inflammatory adipokine adiponectin in acute liver failure in mice. J Hepatol 2006; 44:537. [DOI] [PubMed] [Google Scholar]
- 13.Teoh H, Quan A, Bang KW, et al. Adiponectin deficiency promotes endothelial activation and profoundly exacerbates sepsis-related mortality. Am J Physiol Endocrinol Metab 2008; 295:E658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti- inflammatory Phenotype. J Biol Chem 2010; 285:6153–6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tvarijonaviciute A, Eralp O, Kocaturk M, et al. Adiponectin and IGF-1 are negative acute phase proteins in a dog model of acute endotoxaemia. Vet Immunol Immunopathol 2011; 140:147–151. [DOI] [PubMed] [Google Scholar]
- 16.Uji M, Yamamoto H, Maeda K, et al. Adiponectin deficiency promotes the production of inflammatory mediators while severely exacerbating hepatic injury in mice with polymicrobial sepsis. J Surg Res 2010; 161:301–311. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Bao HG, Han L, et al. Effects of adiponectin on mortality and its mechanism in a sepsis mouse model. J Invest Surg 2012; 25:214. [DOI] [PubMed] [Google Scholar]
- 18.van Meurs M, Castro P, Shapiro NI, et al. Adiponectin diminishes organ-specific microvascular endothelial activation associated with sepsis. Shock 2012; 37:392–398. [DOI] [PubMed] [Google Scholar]
- 19.Behnes M, Brueckmann M, Lang S, et al. Alternations of adiponection in the course of inflammation and severe sepsis. Shock 2012; 38:243–248. [DOI] [PubMed] [Google Scholar]
- 20.Koch A, Sanson E, Voigt S, et al. Serum adiponectin upon admission to the intensive care unit may predict mortality in critically ill patients. J Crit Care 2011; 26:166–174. [DOI] [PubMed] [Google Scholar]
- 21.Vassiliadi DA, Tzanela M, Kotanidou A, et al. Serial changes in adiponectin and resistin in critically ill patients with sepsis: associations with sepsis phase, severity, and circulating cytokine levels. J Crit Care 2012; 27:400–409. [DOI] [PubMed] [Google Scholar]
- 22.Dellinger RP, Lew MM, Rhodes A, et al. Surviving sepsis campaign: International Guideline for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41:580–637. [DOI] [PubMed] [Google Scholar]
- 23.Rasche FM, Uhel B, Kruger DH, et al. Thrombocytopenia and acute renal failure in Puumala hantavirus infections. Emerg Infect Dis 2004; 10:1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae JS. Effects of lower concentration thrombin on high-mobility group box 1 protein-mediated inflammatory responses. Inflammation 2011; 35:1078–1086. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Sung SH, An HR, et al. A case report of crescentic glomerulonephritis associated with hantaan virus infection. Nephrol Dial Transplant 2010; 25:2790–2792. [DOI] [PubMed] [Google Scholar]
- 26.Kim YO, Yoon SA, Ku YM, et al. Serum albumin level correlates with disease severity in patients with hemorrhagic fever with renal syndrome. J Korean Med Sci 2003; 18:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi M, Löwenberg EC. Thrombocytopenia in critically ill patients. Semin Thromb Hemost 2008; 34:417–424. [DOI] [PubMed] [Google Scholar]
- 28.Cosgriff TM, Lewis RM. Mechanisms of disease in hemorrhagic fever with renal syndrome. Kidney Int 1991; 40:72–79. [PubMed] [Google Scholar]
- 29.Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflam 2011; 2011:376909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaegashi M, Jean R, Zurigat M, et al. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med 2005; 20:147–154. [DOI] [PubMed] [Google Scholar]


